Abstract
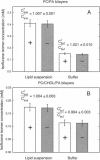
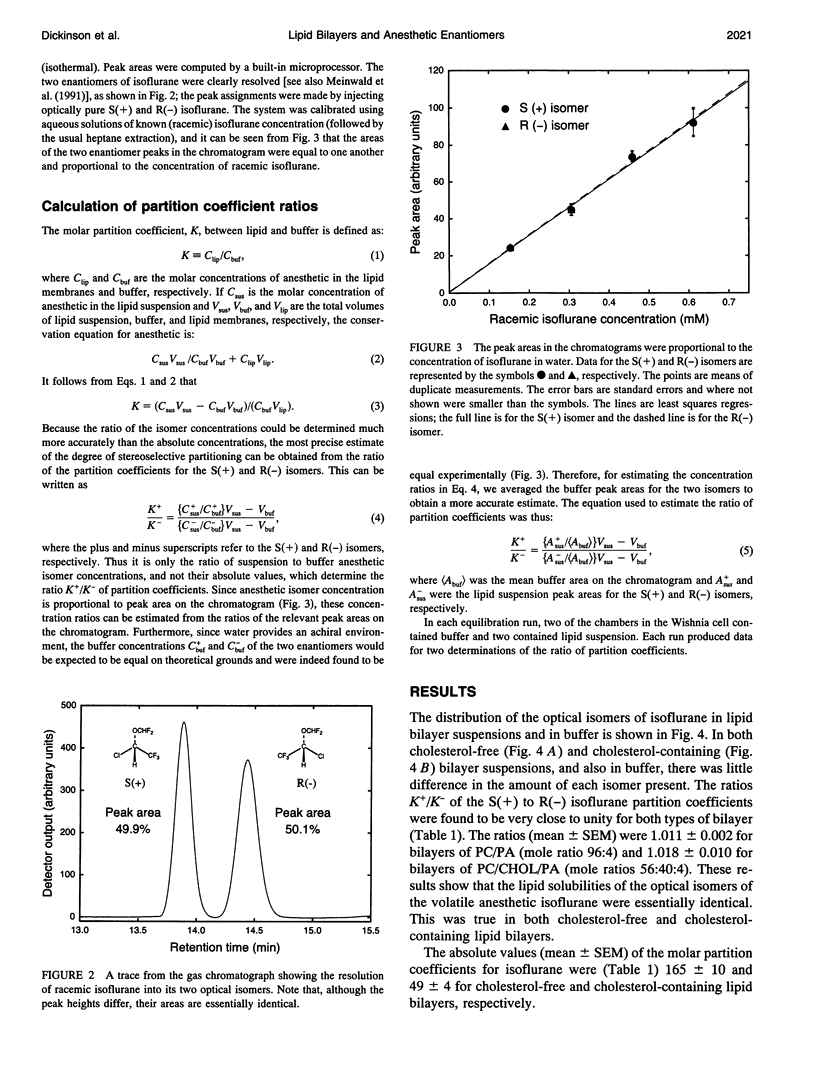
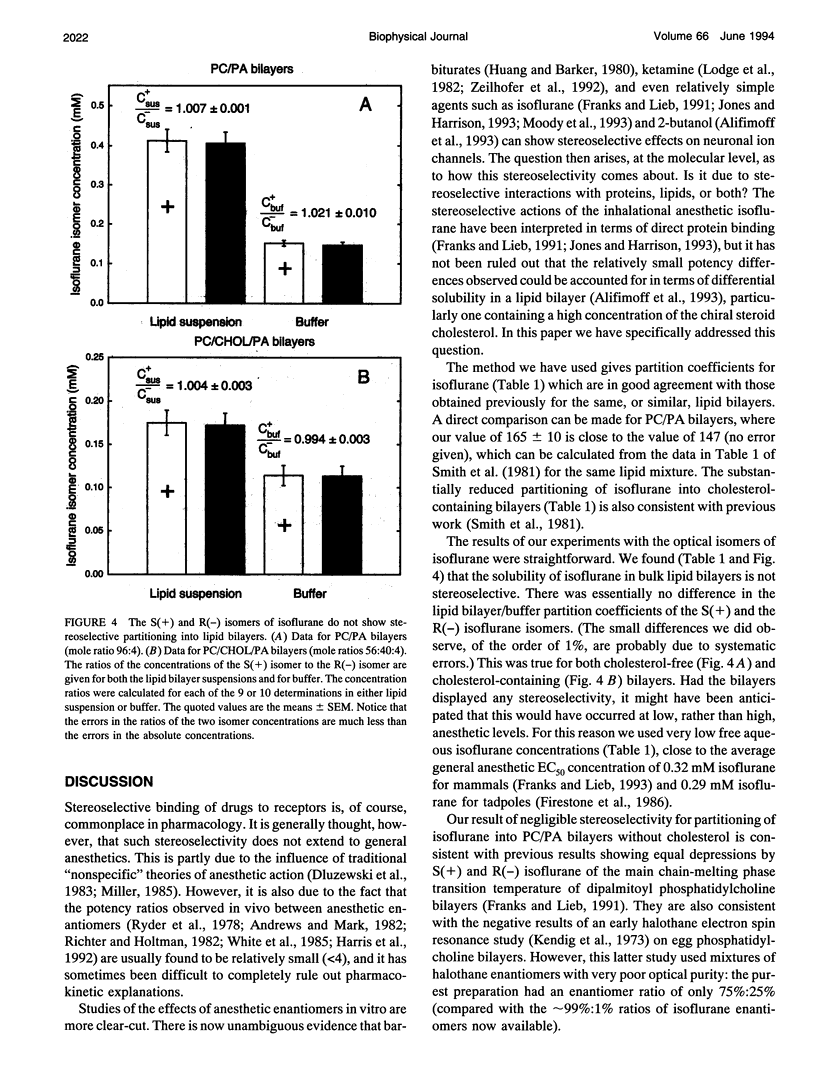
Isoflurane is an inhalational general anesthetic widely used in surgical operations as a racemic mixture of its two optical isomers. The recent availability of pure enantiomers of isoflurane has encouraged their use in experimental studies, and stereoselective effects have now been observed on anesthetic-sensitive neuronal ion channels. Although it has been assumed that such chiral effects demonstrate direct interactions with proteins, it is possible that they could be due to stereoselective interactions with chiral membrane lipids. We have determined the partition coefficients of the two optical isomers of isoflurane between lipid bilayers and water, using racemic isoflurane and gas chromatography with a chiral column. For lipid bilayers of phosphatidylcholine (PC) and 4 mol% phosphatidic acid (PA), both with and without cholesterol (CHOL), we found equal partitioning of the isoflurane optical isomers. The ratios of the S(+) to R(-) isoflurane partition coefficients were (mean +/- SEM): 1.018 +/- 0.010 for bilayers of PC/CHOL/PA (mole ratios 56:40:4) and 1.011 +/- 0.002 for bilayers of PC/PA (mole ratio 96:4). Molar partition coefficients for racemic isoflurane were 49 +/- 4 and 165 +/- 10, respectively. These findings support the view that the stereoselective effects on ion channels observed with isoflurane are due to direct actions on proteins rather than lipids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alifimoff J. K., Bugge B., Forman S. A., Miller K. W. Stereoselectivity of channel inhibition by secondary alkanol enantiomers at nicotinic acetylcholine receptors. Anesthesiology. 1993 Jul;79(1):122–128. doi: 10.1097/00000542-199307000-00018. [DOI] [PubMed] [Google Scholar]
- Andrews P. R., Mark L. C. Structural specificity of barbiturates and related drugs. Anesthesiology. 1982 Oct;57(4):314–320. doi: 10.1097/00000542-198210000-00014. [DOI] [PubMed] [Google Scholar]
- Dluzewski A. R., Halsey M. J., Simmonds A. C. Membrane interactions with general and local anaesthetics: a review of molecular hypotheses of anaesthesia. Mol Aspects Med. 1983;6(6):461–573. doi: 10.1016/0098-2997(83)90001-8. [DOI] [PubMed] [Google Scholar]
- Eckenhoff R. G., Shuman H. Halothane binding to soluble proteins determined by photoaffinity labeling. Anesthesiology. 1993 Jul;79(1):96–106. doi: 10.1097/00000542-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Firestone L. L., Sauter J. F., Braswell L. M., Miller K. W. Actions of general anesthetics on acetylcholine receptor-rich membranes from Torpedo californica. Anesthesiology. 1986 Jun;64(6):694–702. doi: 10.1097/00000542-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br J Anaesth. 1993 Jul;71(1):65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991 Oct 18;254(5030):427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Harris B., Moody E., Skolnick P. Isoflurane anesthesia is stereoselective. Eur J Pharmacol. 1992 Jul 7;217(2-3):215–216. doi: 10.1016/0014-2999(92)90875-5. [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Barker J. L. Pentobarbital: stereospecific actions of (+) and (-) isomers revealed on cultured mammalian neurons. Science. 1980 Jan 11;207(4427):195–197. doi: 10.1126/science.7350656. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Buttress N. The osmotic insensitivity of sonicated liposomes and the density of phospholipid-cholesterol mixtures. Biochim Biophys Acta. 1973 Apr 25;307(1):20–26. doi: 10.1016/0005-2736(73)90021-7. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Harrison N. L. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993 Oct;70(4):1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- Kendig J. J., Trudell J. R., Cohen E. N. Halothane stereoisomers: lack of stereospecificity in two model systems. Anesthesiology. 1973 Nov;39(5):518–524. [PubMed] [Google Scholar]
- Lawrence D. K., Gill E. W. The effects of delta1-tetrahydrocannabinol and other cannabinoids on spin-labeled liposomes and their relationship to mechanisms of general anesthesia. Mol Pharmacol. 1975 Sep;11(5):595–602. [PubMed] [Google Scholar]
- Lodge D., Anis N. A., Burton N. R. Effects of optical isomers of ketamine on excitation of cat and rat spinal neurones by amino acids and acetylcholine. Neurosci Lett. 1982 Apr 26;29(3):281–286. doi: 10.1016/0304-3940(82)90330-5. [DOI] [PubMed] [Google Scholar]
- Meinwald J., Thompson W. R., Pearson D. L., König W. A., Runge T., Francke W. Inhalational anesthetics stereochemistry: optical resolution of halothane, enflurane, and isoflurane. Science. 1991 Feb 1;251(4993):560–561. doi: 10.1126/science.1846702. [DOI] [PubMed] [Google Scholar]
- Miller K. W. The nature of the site of general anesthesia. Int Rev Neurobiol. 1985;27:1–61. doi: 10.1016/s0074-7742(08)60555-3. [DOI] [PubMed] [Google Scholar]
- Moody E. J., Harris B. D., Skolnick P. Stereospecific actions of the inhalation anesthetic isoflurane at the GABAA receptor complex. Brain Res. 1993 Jun 25;615(1):101–106. doi: 10.1016/0006-8993(93)91119-d. [DOI] [PubMed] [Google Scholar]
- Richter J. A., Holtman J. R., Jr Barbiturates: their in vivo effects and potential biochemical mechanisms. Prog Neurobiol. 1982;18(4):275–319. doi: 10.1016/0301-0082(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Ryder S., Way W. L., Trevor A. J. Comparative pharmacology of the optical isomers of ketamine in mice. Eur J Pharmacol. 1978 May 1;49(1):15–23. doi: 10.1016/0014-2999(78)90217-0. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Porter E. G., Miller K. W. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta. 1981 Jul 20;645(2):327–338. doi: 10.1016/0005-2736(81)90204-2. [DOI] [PubMed] [Google Scholar]
- White P. F., Schüttler J., Shafer A., Stanski D. R., Horai Y., Trevor A. J. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985 Feb;57(2):197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- Zeilhofer H. U., Swandulla D., Geisslinger G., Brune K. Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur J Pharmacol. 1992 Mar 17;213(1):155–158. doi: 10.1016/0014-2999(92)90248-3. [DOI] [PubMed] [Google Scholar]