Abstract
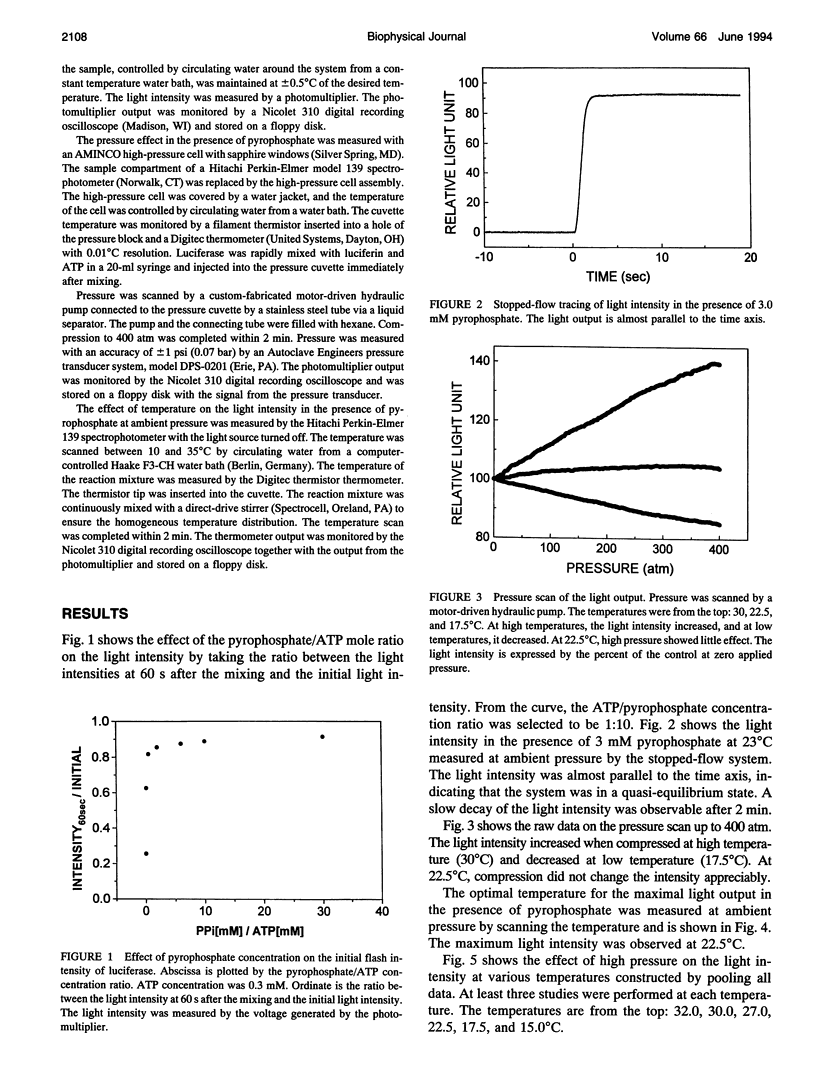
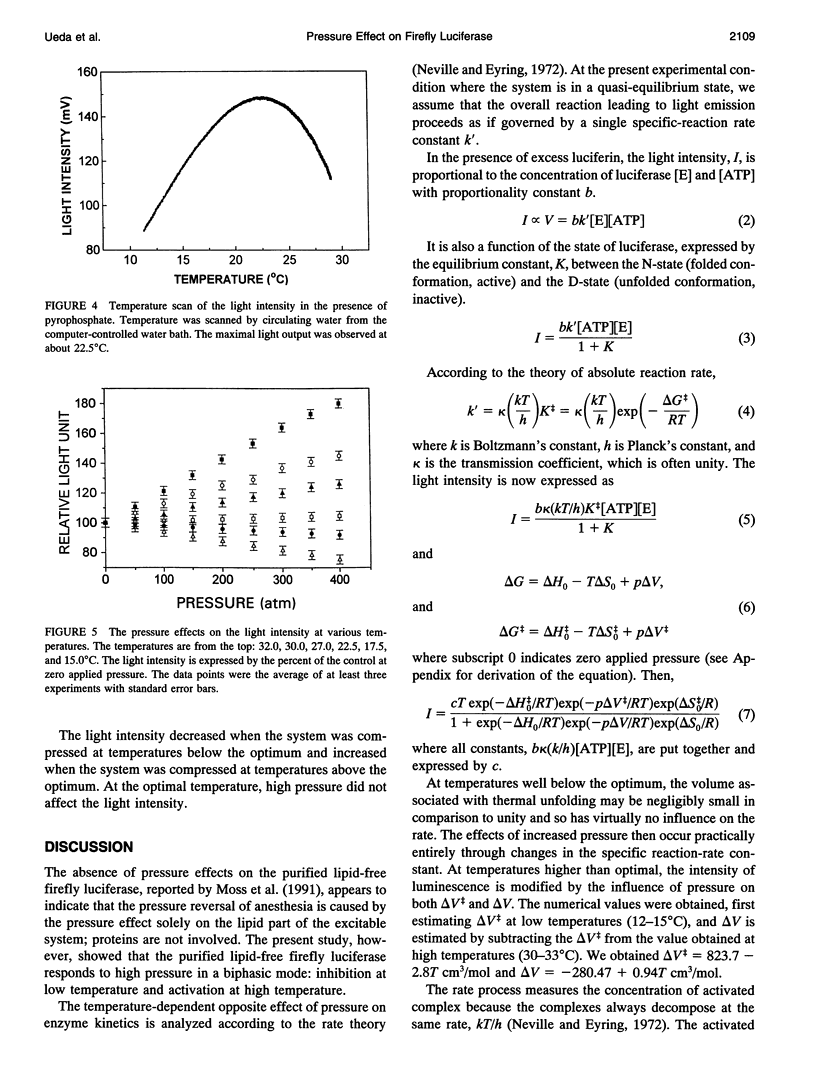
This study measured the effect of high pressure on the enzyme kinetics of firefly luciferase. When firefly luciferase is mixed with luciferin and ATP, a transient flash of light is produced, followed by a weak light, lasting hours. The first stage reaction produces an enzyme-luciferin-AMP complex and pyrophosphate. Addition of pyrophosphate to the reaction mixture decelerated the reaction rate, and the initial flash was prolonged to a plateau, showing a quasi-equilibrium state. The effects of temperature and pressure were analyzed at the plateau. The temperature scan showed that the maximum light intensity was observed at about 22.5 degrees C. When pressurized below the temperature optimum, pressure decreased the light intensity, while increasing it above the temperature optimum. According to the theory of absolute reaction rate, the following values were obtained for the bioluminescent reaction: delta V++ = 823.7 - 2.8 T cm3/mol and delta V = -280.47 + 0.94T cm3/mol, where T is the absolute temperature, delta V++ and delta V are, respectively, activation volume and the volume change due to thermal unfolding. The optimal temperature for the maximum light output occurs because the reaction rate increases with the temperature elevation at low temperature range, but the thermal unfolding of the enzyme decelerates the reaction velocity when the temperature exceeds a critical value. The intensity of luminescence is modified by the influence of pressure on both delta V++ and delta V. So long as the volume of the activated complex (V++) exceeds the average volume of the nonactivated complex (VN), pressure will slow down the reaction. At the point where the volumes become equal, there is no change in the rate under pressure. When the volume of the activated complex is less than that of the reactants, pressure will speed up the rate. This study showed that firefly luciferase is not exceptional to other enzymes in responding to high pressure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter J. V., Knox D. G., Rosenberg A. Pressure effects on folded proteins in solution. Hydrogen exchange at elevated pressures. J Biol Chem. 1978 Mar 25;253(6):1947–1953. [PubMed] [Google Scholar]
- JOHNSON F. H., FLAGLER E. A. Hydrostatic pressure reversal of narcosis in tadpoles. Science. 1950 Jul 21;112(2899):91–92. doi: 10.1126/science.112.2899.91-a. [DOI] [PubMed] [Google Scholar]
- McElroy W. D., Seliger H. H., White E. H. Mechanism of bioluminescence, chemiluminescence and enzyme function in the oxidation of firefly luciferin. Photochem Photobiol. 1969 Sep;10(3):153–170. doi: 10.1111/j.1751-1097.1969.tb05676.x. [DOI] [PubMed] [Google Scholar]
- Moss G. W., Lieb W. R., Franks N. P. Anesthetic inhibition of firefly luciferase, a protein model for general anesthesia, does not exhibit pressure reversal. Biophys J. 1991 Dec;60(6):1309–1314. doi: 10.1016/S0006-3495(91)82168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville W. M., Eyring H. Hydrostatic pressure and ionic strength effects on the kinetics of lysozyme. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2417–2419. doi: 10.1073/pnas.69.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Drickamer H. G. The effect of high pressure upon proteins and other biomolecules. Q Rev Biophys. 1983 Feb;16(1):89–112. doi: 10.1017/s0033583500004935. [DOI] [PubMed] [Google Scholar]



