Abstract
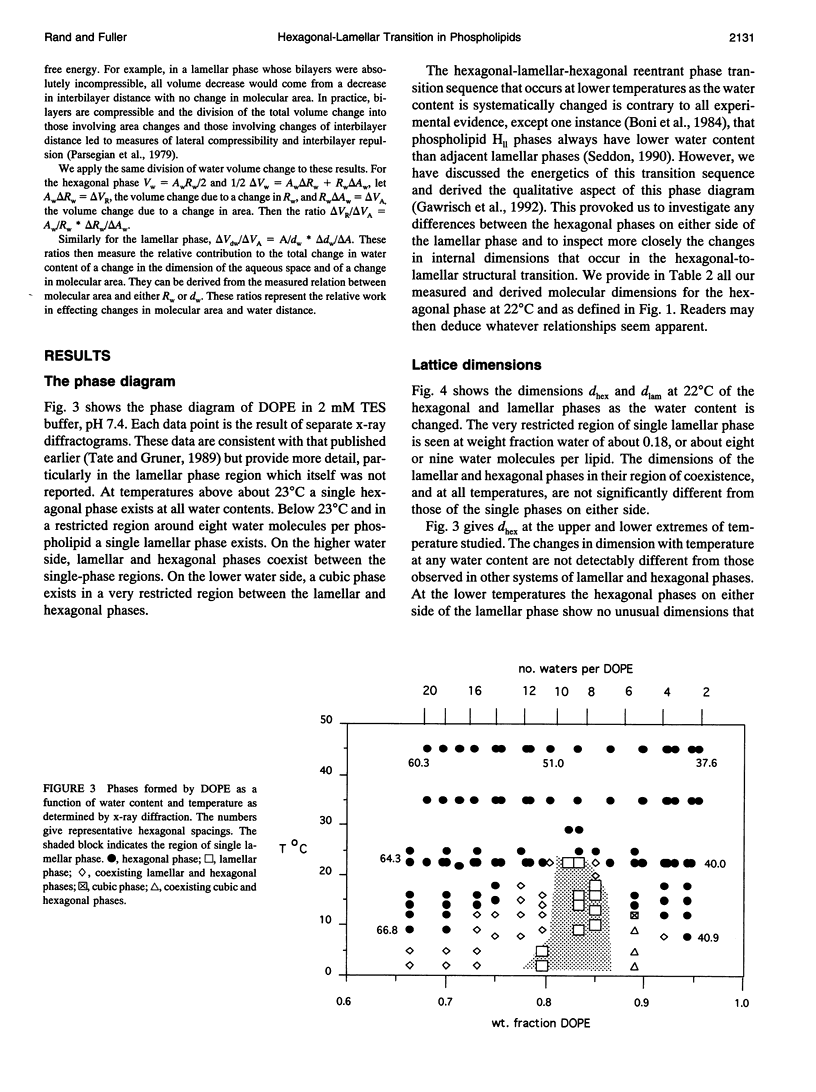
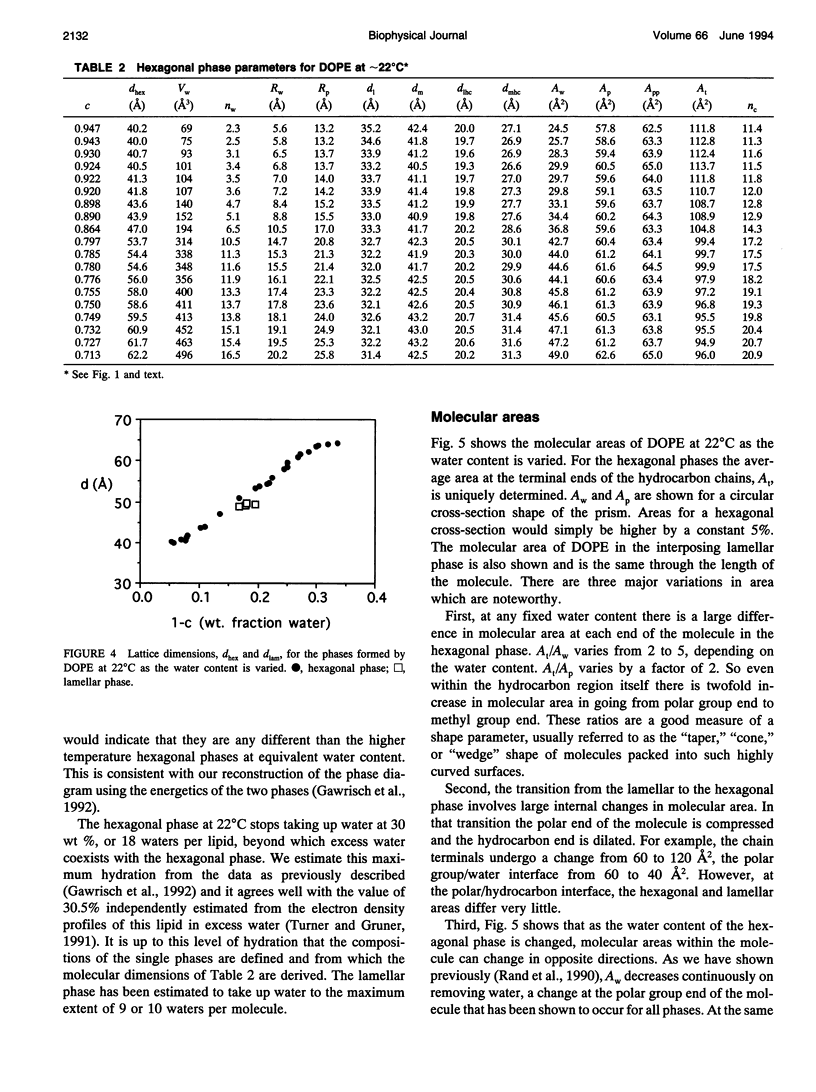
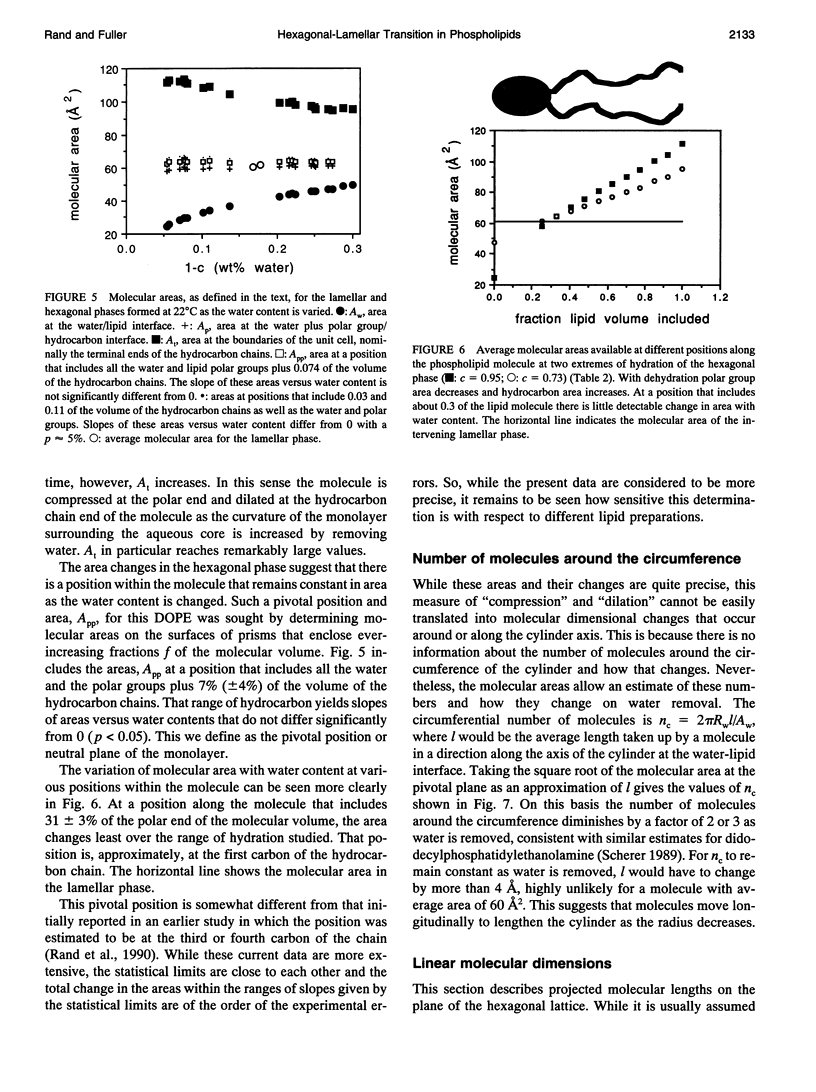
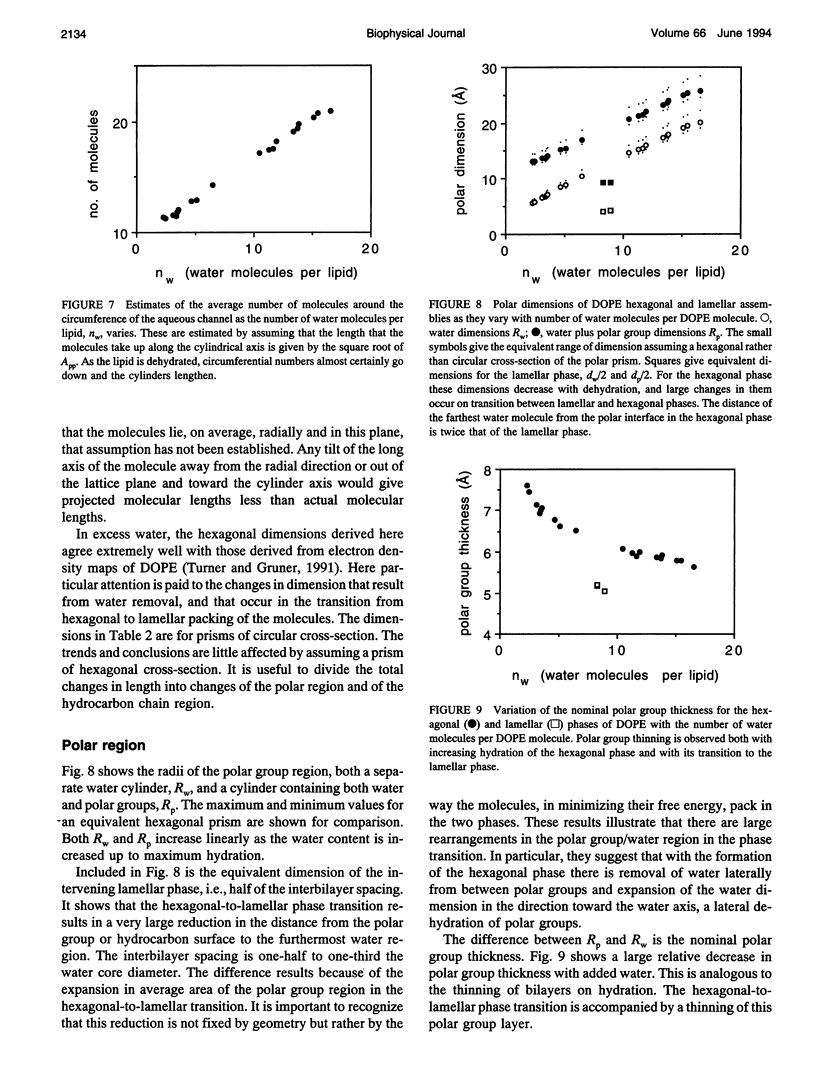
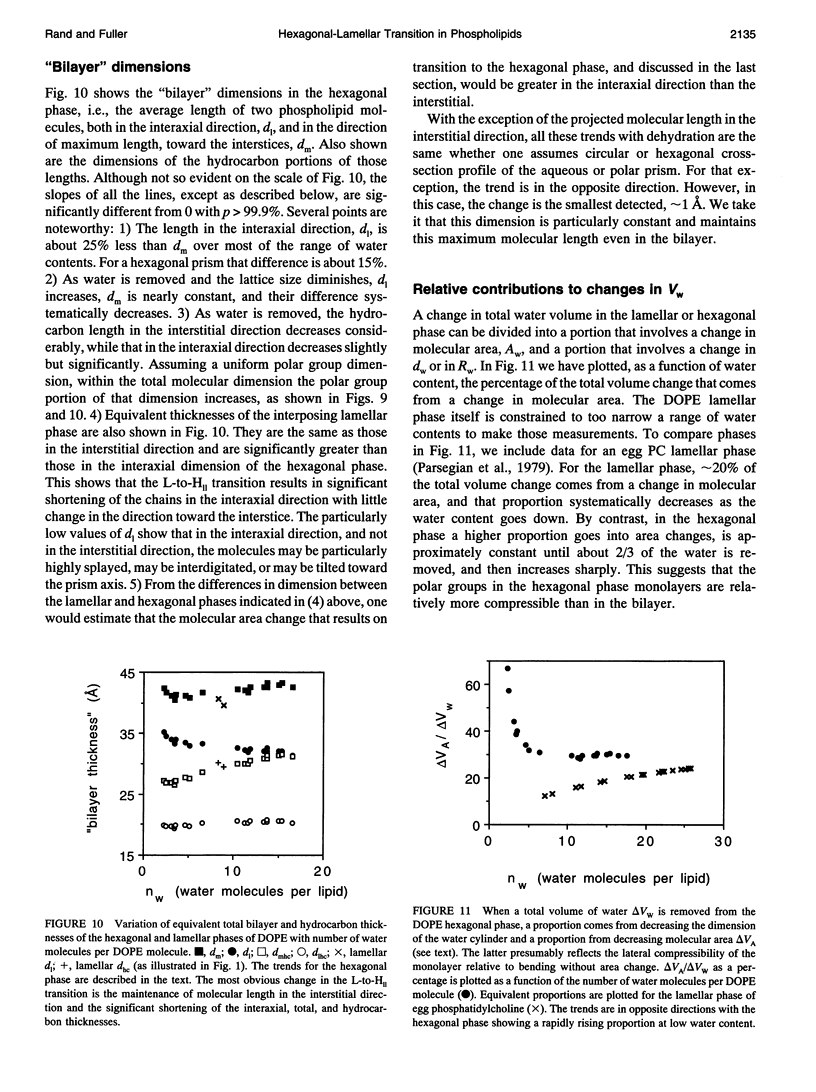
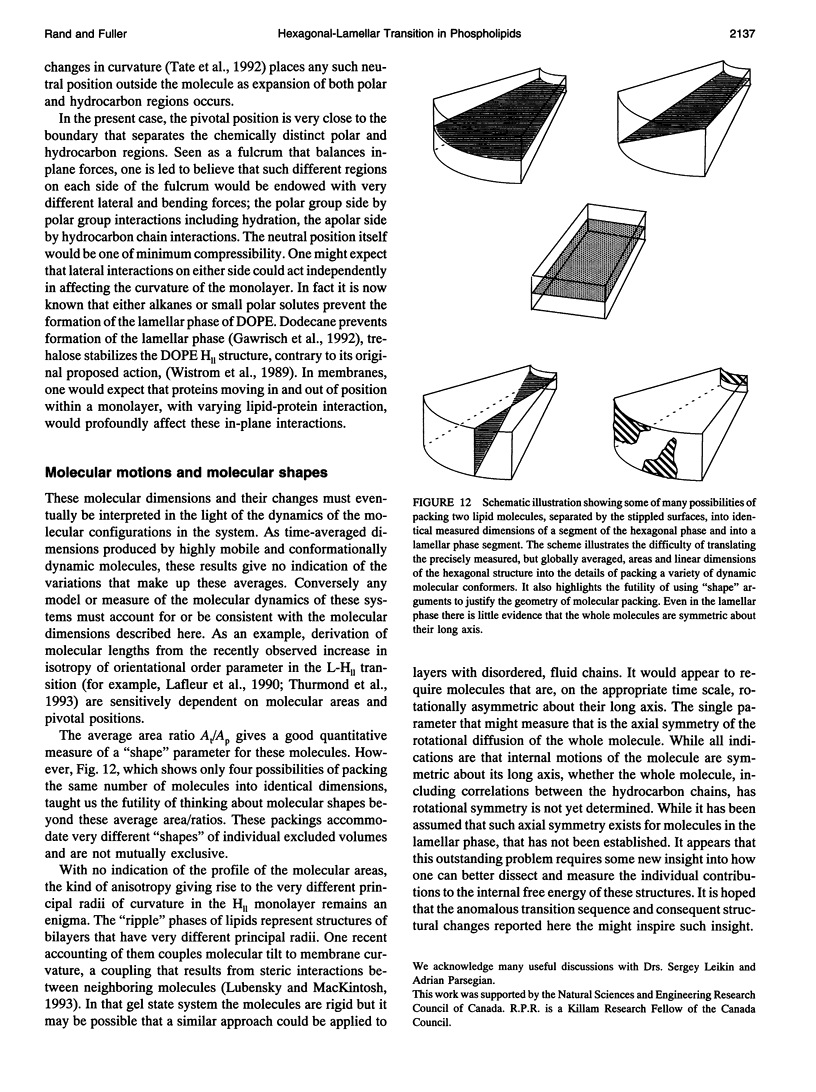
A hexagonal-lamellar-hexagonal (HII-L-HII) reentrant phase transition sequence on dehydration of dioleoylphosphatidylethanolamine occurs below 22 degrees C. This provides an unusual opportunity to measure how several structural dimensions change during this transition. Using x-ray diffraction, we have measured these dimensions with a hope of gaining some clue about the accompanying internal stresses. The principal dimensions described are molecular areas and molecular lengths projected onto the hexagonal lattice. In contrast with large changes in average area at the polar and hydrocarbon ends of the molecule, a position near the polar group/hydrocarbon interface is one of constant molecular area. It remains constant both as the monolayers curl from changing water content and in the transition from one structure to the other. In the L-to-HII transition, the most obvious change in molecular length is a 25% decrease in the distance between aqueous cylinders, the interaxial direction. There is little change in the interstitial direction, the direction toward the interstice equidistant from three aqueous cylinders. As the hexagonal phase is dehydrated, a number of internal changes in molecular lengths are described. Increases in the interaxial direction are much larger than in the interstitial. Simultaneously however, hydrocarbon chain lengths decrease, and polar group lengths increase. It is likely that molecules move axially and the cylinders become longer with dehydration. These dimensions and their changes might be used in the search for a better understanding of the energetics of molecular packing, of the interpretation of spectroscopic measurements of these phases, and of the mechanics of lipid layers.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boni L. T., Stewart T. P., Hui S. W. Alterations in phospholipid polymorphism by polyethylene glycol. J Membr Biol. 1984;80(1):91–104. doi: 10.1007/BF01868693. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys J. 1993 Nov;65(5):1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K., Parsegian V. A., Hajduk D. A., Tate M. W., Graner S. M., Fuller N. L., Rand R. P. Energetics of a hexagonal-lamellar-hexagonal-phase transition sequence in dioleoylphosphatidylethanolamine membranes. Biochemistry. 1992 Mar 24;31(11):2856–2864. doi: 10.1021/bi00126a003. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Hydrocarbon chain conformation in the HII phase. Biophys J. 1989 Nov;56(5):1045–1049. doi: 10.1016/S0006-3495(89)82751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Fine B., Bloom M. Comparison of the orientational order of lipid chains in the L alpha and HII phases. Biochemistry. 1990 Sep 11;29(36):8325–8333. doi: 10.1021/bi00488a018. [DOI] [PubMed] [Google Scholar]
- Lubensky TC, MacKintosh FC. Theory of "Ripple" Phases of Lipid Bilayers. Phys Rev Lett. 1993 Sep 6;71(10):1565–1568. doi: 10.1103/PhysRevLett.71.1565. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Scherer J. R. Dependence of lipid chain and head group packing of the inverted hexagonal phase on hydration. Biophys J. 1989 May;55(5):965–971. doi: 10.1016/S0006-3495(89)82895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993 Nov;65(5):2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]
- Thurmond R. L., Lindblom G., Brown M. F. Curvature, order, and dynamics of lipid hexagonal phases studied by deuterium NMR spectroscopy. Biochemistry. 1993 May 25;32(20):5394–5410. doi: 10.1021/bi00071a015. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gruner S. M., Huang J. S. Distribution of decane within the unit cell of the inverted hexagonal (HII) phase of lipid-water-decane systems determined by neutron diffraction. Biochemistry. 1992 Feb 11;31(5):1356–1363. doi: 10.1021/bi00120a010. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gruner S. M. X-ray diffraction reconstruction of the inverted hexagonal (HII) phase in lipid-water systems. Biochemistry. 1992 Feb 11;31(5):1340–1355. doi: 10.1021/bi00120a009. [DOI] [PubMed] [Google Scholar]



