Abstract
Intra-abdominal infections (IAI) continue to be a serious clinical problem. Bacterial synergism is an important factor that influences the shift from contamination to IAI, leading to the development of lesions and abscess formation. Escherichia coli and Bacteroides fragilis are particularly abundant in IAI. The underlying molecular mechanisms of this pathogenic synergy are still unclear. The role of the hemoglobin protease (Hbp) autotransporter protein from E. coli in the synergy of IAI was investigated. Hbp is identical to Tsh, a temperature-sensitive hemagglutinin associated with avian pathogenic E. coli. Clinical isolates from miscellaneous extraintestinal infections were phenotypically and genotypically screened for Hbp. The presence of Hbp was significantly associated with E. coli isolated from IAI and other extraintestinal infections. In a murine infection model, Hbp was shown to contribute to the pathogenic synergy of abscess development. Mice immunized with Hbp were protected against mixed infections and did not develop abscess lesions. Furthermore, an E. coli wild-type strain that did not induce abscess formation in the synergy model was transformed with a plasmid encoding the hbp gene, and mixed infections with this strain lead to increased growth of B. fragilis and induction of abscess lesions. Growth-promoting studies showed that purified Hbp is able to deliver heme to B. fragilis strain BE1. In conclusion, results suggest the synergy of abscess formation by E. coli and B. fragilis can be partly explained by the capacity of B. fragilis to intercept Hbp and iron from heme to overcome the iron restrictions imposed by the host.
Intra-abdominal infections (IAI) in humans due to an abdominal trauma or surgery are a serious clinical problem (1, 4, 9, 11). The mortality rate lies between 5 and 50%, depending on the anatomic origin of bacterial contamination. IAI may spread to adjacent tissues or become loculated with abscess formation. In particular, the abscesses form a serious threat to the patient. Bacterial leakage from or the rupture of an abscess can result the sudden entry of a huge number of microorganisms into the host’s circulation. This bacteremia may progress to sepsis, septic shock, and multisystem organ failure (1, 9). The mortality of these complications is very high (70%). Infected patients often need surgery to stop the advancing spread of the infection, remove loculated pus, and reestablish sufficient blood flow to deliver appropriate antimicrobial agents to the infected site. In some cases, abscesses can be drained in combination with antibiotic treatment.
Since normal intestinal flora consists of many bacterial species, the initial microflora at the site of an IAI is polymicrobial. Despite the complexity of this microflora, only a limited number of different bacterial species are recovered from an abscess. In particular, Escherichia coli and the strictly anaerobic Bacteroides fragilis are often isolated from these abscesses (3, 4, 9, 11). The frequent presence of these two bacterial species in abscesses has led to the concept of pathogenic synergy. The mechanism of the pathogenic synergy between E. coli and B. fragilis during mixed IAI is poorly understood. Bacterial factors that may be involved are adherence, interference with host resistance factors, mutual growth stimulation, cytotoxic substances, and extracellular enzymes (15). Data from experimental peritoneal infections showed that the addition of heme or hemoglobin strongly enhances bacterial growth and mortality rates (2, 5, 23). This indicates that iron limitation in the peritoneal cavity of the host is an important defense mechanism. Recently, a novel heme-binding protein, termed the “hemoglobin-binding protease” (Hbp), has been characterized in an E. coli strain isolated from a wound infection (21). Hbp is identical to Tsh, a hemagglutinin associated with avian pathogenic E. coli (8, 22), and represents the first described member of the SPATE group of the autotransporter proteins (14). Autotransporters comprise a special group of virulence-associated proteins in gram-negative bacteria that exhibit diverse biological functions. (13). Following export to the periplasm via what is though to be a Sec-dependent mechanism, these proteins probably use an autosecretion mechanism for presentation at the bacterial cell surface and in some cases are released into the extracellular milieu. Hbp scavenges heme from its environment, interacts specifically with human hemoglobin, degrades it, and subsequently binds the released heme (21). This specific heme-binding protein could make heme accessible for bacterial growth, not only for E. coli, but also possibly for B. fragilis during mixed infections.
Increased knowledge of the factors that mediate the synergy between E. coli and B. fragilis and that contribute to abscess formation could lead to strategies to prevent and treat IAI and abscesses. This study demonstrates the important role of Hbp in the pathogenic synergy between E. coli and B. fragilis in IAI. We propose that the capacity of B. fragilis to intercept the heme-saturated Hbp overcomes iron restrictions imposed by the host and hence contributes to this pathogenic synergy.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli strains isolated from feces and miscellaneous extraintestinal sources were obtained from the Academic Hospital of the Vrije Universiteit. The E. coli strains were identified by the API 20E system (API S.A., Montalieu Vercieu, France), and the serotypes were determined at the National Institute for Public Health and Environmental Hygiene (RIVM), Bilthoven, The Netherlands. E. coli K-12 strain DH5α (GIBCO BRL) and the plasmids pACYC184 (6) and pBRHbp (21) were used in routine cloning procedures (27). The characteristics of B. fragilis strain BE1 and the E. coli strains used in experimental infections are listed in Table 1. The clinical isolates were maintained on 5% horse blood agar plates (no. 2 agar; Oxoid, Ltd., London, England) at 4°C and stored as glycerol stocks at −30°C. E. coli and B. fragilis BE1 were routinely grown in Luria-Bertani medium (17) and in BM medium (29), respectively. B. fragilis was grown in an anaerobic jar in an atmosphere of 80% N2, 10% H2, and 10% CO2. If required, antibiotics were added to the culture media.
TABLE 1.
Bacterial strains used in experimental infections
| Strains | Origin or source | Hbpa | Characteristic |
|---|---|---|---|
| E. coli | |||
| EB1 (O8:K43) | IAI (29) | + | Synergism with BE1 |
| EB4 (O2:K−) | Intra-abdominal abscess (29) | + | Synergism with B. fragilis |
| χ7122 (O78:K80) | Colisepticemia (avian) (22) | +b | Expression similar to EB1 |
| F274 (O111:K−) | Normal feces | − | |
| EB19 (O18:K5) | Colon lavage fluid | − | |
| EB19(pACYCHbp) | EB19 carrying pACYCHbp | + | Overexpression of Hbp |
| LG1522 | K-12 strain AN1937 carrying pColV-K30 (31) | + | Expression similar to EB1 |
| B. fragilis BE1 | IAI (29) | − | Synergism with EB1 |
Detection of hbp and its product was verified by Southern blotting and immunoblotting, respectively.
This strain produces Tsh, a protein that is identical to Hbp.
General methods.
Recombinant DNA techniques were carried out as described previously (27). For Western blotting, anti-Hbp antiserum SN-165 raised against the NH2-terminal part of Hbp was used (21). Another polyclonal anti-Hbp antiserum (J40) was raised in rabbit against purified Hbp. Polyclonal immunoglobulin G (IgG) was purified from this antiserum by protein A affinity chromatography (Pharmacia Biotech). The J40 IgG antiserum was used in the growth experiments with B. fragilis BE1. Colicin V expression of the E. coli strains was determined as described previously (21).
Cloning of the hbp gene and DNA manipulation.
The hbp gene, including its own HindIII and BamHI restriction sites, was cloned into pACYC184. The obtained plasmid pACYCHbp was introduced into E. coli DH5α and wild-type strain EB19. A DNA probe specific for hbp (21) was used for the detection of hbp in E. coli strains in Southern blot hybridization experiments.
Experimental infections.
A skin infection model in mice (29) was used to investigate the possible role of Hbp in the pathogenic synergism. E. coli strains and B. fragilis BE1 (Table 1) were cultured and subsequently injected into mice as described previously (29). The inoculum size for E. coli was 5 × 106 CFU, and that for B. fragilis BE1 was 2 × 108 CFU. Six days after the inoculation, animals were killed and examined for pus formation. Only mice with pus-containing abscesses were regarded as positive. Viable bacterial counts from the inoculation sites were made to determine the clearance of bacteria. Mice were immunized with purified Hbp before challenge by intraperitoneal injection of 50 μg of Hbp in Freund’s complete adjuvant. After 4 weeks, the mice were boosted with 50 μg of Hbp in Freund’s incomplete adjuvant. Another group of mice was only treated with adjuvant as a control. A week after the booster, the mice from both groups were challenged as described above. The titer against Hbp was 1:80,000 in the Hbp-immunized mice at the time of the challenge.
Growth promotion assays.
A preculture of the anaerobic bacterium B. fragilis BE1 in MA medium (21) supplemented with 0.3% tryptone (MAT-0.3%) and 7.7 μM hemin was subcultured in MAT-0.3% for 7 h. This was done to exhaust the internal heme pool of the cells. In a liquid assay, 25 μl of heme-depleted cells was inoculated in 2 ml of MAT-0.3%. Hemin, apo-Hbp, or holo-Hbp was added, and after 20 h of anaerobic growth, the optical density at 660 nm (OD660) was measured. Holo-Hbp was prepared by incubating purified apo-Hbp with hemin under anaerobic conditions. Under these conditions, Hbp binds heme in a 1:1 molar ratio (unpublished data). Hemin is the oxidized form of heme and tends to form dimers or larger aggregates in an aerobic environment (10). This was a rate-limiting factor for Hbp to bind heme. Therefore, reduction of hemin was necessary for an optimal binding of this molecule to Hbp. The obtained holo-Hbp was used in the growth studies. In the growth-promoting studies in which hemin was added, the anaerobic environment readily converted hemin to heme, in this way making the molecule accessible for apo-Hbp. In a filter assay, MAT-0.3% agar plates were overlaid with 4 ml of MAT-0.3% soft agar (0.6% agar) containing 107 CFU of heme-depleted B. fragilis BE1 cells. Paper discs, loaded with 50 μl of a putative growth-promoting agent, were placed on the agar plates. After 24 h of anaerobic incubation, the surfaces of the growing zones were calculated by photoimaging with a Fluor-S MultiImager (Bio-Rad Laboratories).
RESULTS
Hbp delivers heme to the anaerobic bacterium B. fragilis.
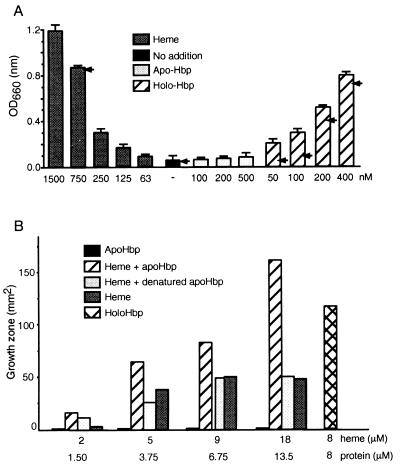
A possible mechanism that contributes to the synergy is the provision of essential growth factors by one microbe to the other (25). In preliminary studies, the culture supernatants of E. coli EB1 and B. fragilis BE1 were tested for their growth-promoting capabilities. The culture supernatant of E. coli EB1 promoted the growth of B. fragilis in a heme-restricted medium (data not shown). Further analysis of the culture filtrate revealed that a high-molecular-weight heat-labile factor induced the growth promotion. In addition, a previous study (21) showed that Hbp is a heme-binding protein. Therefore, the most likely candidate for the growth-promoting activity of B. fragilis in vitro is Hbp. To determine whether Hbp delivers heme to B. fragilis BE1, growth-promoting studies under anaerobic conditions with purified apo- and holo-Hbp were carried out. Hbp binds heme in a 1:1 molar ratio (see Materials and Methods). A special culture medium (MAT-0.3%) was developed with heme as the growth-restricting factor for the growth-promoting studies. In control experiments, B. fragilis was not able to grow in this medium unless a heme source was added (Fig. 1A). Increasing amounts of holo-Hbp or heme clearly stimulated the growth of B. fragilis. Roughly twice the amount of heme in comparison to holo-Hbp was needed to obtain a similar growth promotion of the BE1 cultures (Fig. 1A). Apo-Hbp, even in higher molar concentrations than holo-Hbp, did not promote the growth of B. fragilis. An anti-Hbp antiserum, J40, was used in the growth experiments to determine if this antiserum has an effect on the growth promotion of B. fragilis by holo-Hbp. Addition of holo-Hbp together with 175 nM anti-Hbp antiserum reduced the growth of BE1 cells considerably. In contrast, the anti-Hbp antiserum did not induce or inhibit the growth of BE1 in MAT-0.3% medium alone or supplemented with heme (Fig. 1A). Levels of 100 and 80% inhibition of growth were achieved with antiserum added to the cultures supplemented with 50 or 100 nM holo-Hbp, respectively (Fig. 1A). With larger amounts of holo-Hbp (200 and 400 nM) growth was reduced 22 to 13%, respectively. Apparently, heme is not spontaneously released from the protein during the incubation period. Also, it implies the need for a specific interaction between BE1 cells and holo-Hbp in order to release heme from the protein. One remarkable finding is the 80% inhibition of growth at an approximately 2:1 ratio of antibody to Hbp, indicating that already two molecules of antibody could inhibit 1 molecule of protease. At antibody/Hbp ratios of 1:1 and 1:2, 20 and 10%, respectively, inhibition was achieved. Extrapolation of these data should give 40% inhibition instead of the 80% inhibition at the 2:1 ratio of antibody to Hbp. An explanation for the strong inhibitory effect of the antibody could be the stressful growth conditions for B. fragilis in MAT-0.3% medium, especially at low heme concentrations (≤200 pmol), like those in the cultures supplemented with 50 or 100 nM holo-Hbp. Therefore, small changes in the heme availability in these cultures could have a dramatic effect on the growth of bacteria.
FIG. 1.
Growth stimulation of B. fragilis BE1 by Hbp under heme-restrictive conditions. (A) Growth of BE1 cells in 2-ml cultures. Heme-depleted cells were inoculated in MAT-0.3% medium supplemented with apo-Hbp or holo-Hbp. Cultures supplemented with only heme or with no extra additions were used as positive and negative growth controls, respectively (see Materials and Methods for details). The arrows indicate the obtained growth of the 2-ml cultures in the presence of 175 nM anti-Hbp antiserum J40. The OD660 was measured after 20 h. Data represent means ± standard deviations of five independent experiments. (B) Ability of Hbp to donate heme to B. fragilis. Paper discs were loaded with an increasing concentration of heme and apo-Hbp. The 50-μl samples on the discs were placed on agar plates inoculated with heme-depleted BE1 cells. The surfaces of the growth zones around the discs were measured after 24 h of growth. Apo-Hbp alone and holo-Hbp were used as negative and positive growth controls, respectively. The values of the growth zones around the discs are expressed in square millimeters. Four independent growth measurements were conducted, producing essentially identical results.
The results of the liquid assay showed that B. fragilis was able to use holo-Hbp very efficiently as a source of heme. A filter assay was used to test the ability of apo-Hbp to capture heme and subsequently donate it to B. fragilis during the growth of this microorganism. A dose-dependent growth promotion was observed with apo-Hbp plus heme in the paper discs (Fig. 1B). Holo-Hbp-loaded discs also induced growth in this assay. Apo-Hbp alone at the same molar concentrations induced no growth at all, while only heme-loaded paper discs resulted in a moderate growth. A mixture of denatured apo-Hbp and heme provided a growth-promoting activity that was similar to growth in the presence of heme alone, indicating that a native conformation of Hbp is required for efficient heme transfer to B. fragilis.
Hbp is involved in the formation of abscesses.
Bacterial synergism in abscess formation has been demonstrated in several animal models (4, 20, 29). The role of Hbp in bacterial synergism was investigated by screening E. coli strains (Table 1) for their capacity to induce a synergistic interaction with B. fragilis in a mouse model (29). In this model, it is possible to test the role of bacterial factors that in vitro experiments suggest are relevant. A synergy between the organisms is demonstrated when abscesses are present 6 days after inoculation. In addition, high numbers (≥106) of bacteria of both species are typically isolated from the abscesses in a ratio of approximately 1:1.
Infections with any of the E. coli strains or B. fragilis BE1 alone failed to induce abscesses, and only low numbers of bacteria (<5,000 CFU) were recovered from the sites of infection 6 days after inoculation (data not shown). Mixed infections with either E. coli strain EB1 or EB4 and B. fragilis BE1 resulted in abscess formation, and large numbers of bacteria were present in these abscesses (Fig. 2A). These Hbp-positive E. coli strains were previously isolated from IAI (Table 1). The avian pathogenic strain χ7122 produces Tsh, a protein that is identical to Hbp (22). This strain was able to induce the formation of abscesses, although the number of bacteria recovered from these abscesses was lower than the number of bacteria isolated for strain EB1 or EB4 (Fig. 2A). Thus, only the combination of B. fragilis and Hbp-positive E. coli strains induced the formation of abscesses. However, among the Hbp-positive strains, a significant difference in the number of bacteria recovered from the abscesses was observed (Fig. 2A). This is due to the fact that these E. coli strains induced the formation of abscesses of different sizes. E. coli EB1 together with B. fragilis BE1 induced the formation of abscesses of approximately 84 mm2 in size, while mixed infections with EB4 or χ7122 revealed abscesses of 26 or 18 mm2 in size, respectively. Larger abscesses contain higher numbers of bacteria than smaller abscesses. These results indicate that, besides Hbp, other strain-specific properties of E. coli play a role in the formation and final size of an abscess. No bacterial synergy between B. fragilis strain BE1 and either of the Hbp-negative E. coli strains F274 or EB19 was detected. E. coli K-12 strains carrying hbp or pColV-K30, which encodes Hbp (Table 1), were also tested in this model. None of these strains showed a synergistic interaction with B. fragilis, and only low numbers of either bacterial species (<5,000 CFU) were recovered from the site of inoculation (data not shown). Thus, neither Hbp nor any of the virulence-associated properties encoded on pColV-K30 significantly enhanced abscess formation in mice by E. coli K-12.
FIG. 2.
Experimental infections in mice. (A) Numbers of E. coli and B. fragilis BE1 cells at the sites of infection 6 days postinoculation with a mixture of both species. (Inset) Significance levels among the numbers of bacteria of the different strains. (B) Numbers of EB1 and BE1 after a challenge in immunized or sham-immunized mice and the numbers of EB19, EB19(pACYCHbp) and BE1 6 days postinoculation with a mixture of both species. The sham-immunized and Hbp-immunized groups of mice are indicated as ψ and γ, respectively. The significance levels among the numbers of EB1 or BE1 cells from immunized or sham-immunized mice and the levels between the number of EB19 or EB19(pACYCHbp) cells together with BE1 were determined. Each column represents the mean of at least six samples, and bars indicate the standard error of the mean. The significance of the difference is indicated as * at 0.01 < P ≤ 0.05, ** at 0.001 < P ≤ 0.01, and *** at P ≤ 0.001, as tested by analysis of variance. Abscess formation in mice on day 6 following bacterial inoculation is indicated by minus signs for no abscesses and plus signs for abscesses.
To determine whether Hbp has a critical role in abscess formation, mice were immunized with Hbp and were then challenged with a mixed infection of BE1 and EB1. Another group of mice was only treated with adjuvant before the challenge. Abscess formation was prevented by this immunization (Fig. 2B). Following the challenge, a significant decrease in the number of both bacterial species was observed at the site of inoculation in Hbp-vaccinated mice (Fig. 2B). To examine the influence of Hbp on abscess formation in mixed infections, the Hbp-negative wild-type E. coli strain EB19 was transformed to an Hbp-positive phenotype (Table 1). As shown in Fig. 2B, the combination of strain EB19(pACYCHbp) with BE1 induced abscesses, in contrast to the combination of strain EB19 with B. fragilis BE1. Remarkably, a significant increase in the number of B. fragilis cells in mixed infections with strain EB19(pACYCHbp) was observed, whereas similar levels of E. coli cells were recovered from the inoculation sites following infection with either strain EB19 or strain EB19(pACYCHbp). It appears that strain EB19(pACYCHbp) cannot use Hbp for its own benefit, possibly due to the lack of a specific Hbp receptor. These results indicate that Hbp is a contributing factor for the growth of B. fragilis in vivo and for the synergistic development of abscess formation in the mouse model.
Prevalence of Hbp-positive E. coli among strains isolated from mixed IAI.
If Hbp contributes to the pathogenesis of IAI, then this would predict a high prevalence of Hbp-positive strains among E. coli isolated from IAI should be expected. To test this hypothesis, clinical isolates from extraintestinal infections, including IAI, urinary tract infections, and bacteremia, were phenotypically and genotypically screened for Hbp. For comparison, E. coli isolates from feces of healthy individuals were also examined for Hbp. This screening clearly revealed a high frequency of Hbp-positive E. coli among strains from IAI (Table 2). The overall incidence of Hbp-positive strains isolated from extraintestinal infections was 31%; it varied from 20% for blood, to 17% for urine, to 57% for strains isolated from IAI. In contrast, only 1 of 22 strains isolated from normal enteric flora was Hbp positive, indicating that Hbp is associated with extraintestinal infections, especially with IAI (Table 2). There was no prevalent serotype among the Hbp-positive E. coli strains isolated from IAI (data not shown).
TABLE 2.
Incidence of Hbp, colicin V, and hemolysin among E. coli strains
| Source of strainsa (no. tested) | % of strains producing:
|
||
|---|---|---|---|
| Hbpb | Colicin V | Hemolysin | |
| Blood (25) | 20 | 40 | 44 |
| Urine (18) | 17 | 22 | 33 |
| Intra-abdomen (IAI) (21) | 57 | 38 | 24 |
| Total extraintestinal (64) | 31 | 34 | 34 |
| Normal feces (22) | 5 | 18 | 14 |
Blood included E. coli isolates from sepsis, septic shock, carcinomas, and acute myeloid leukemia. Urine included urinary tract infections and urosepsis. Intra-abdomen included abscesses, wounds, appendicitis, and peritonitis.
Significant difference in the incidence of Hbp between E. coli strains isolated from extraintestinal infections and those isolated from normal feces (Fisher’s exact test, P = 0.016), and in the prevalence of Hbp from IAI versus other extraintestinal infections (P = 0.005).
All strains were evaluated on blood agar plates for hemolytic activity and were also tested for colicin V. The incidences of hemolytic strains from IAI, urine, and blood were 24, 33, and 44%, respectively. In contrast, only 14% of the strains isolated from feces were hemolytic (Table 2). Only 3 out of the 12 Hbp-positive strains from IAI showed hemolytic activity, while the 5 Hbp-positive strains from blood were all positive for hemolysin (data not shown). A higher prevalence for colicin V-producing E. coli was found among strains from blood and IAI compared with strains from urine and feces (Table 2). Six of the 12 Hbp-positive strains from IAI were also positive for colicin V (data not shown), indicating that Hbp is not always associated with the production of colicin V.
DISCUSSION
The results of our study confirm earlier observations (18, 30, 31) that hemolysin and colicin V are associated with E. coli isolates from extraintestinal infections. Moreover, we found that Hbp is clearly correlated with these types of infections and is significantly more prevalent in IAI than in other extraintestinal infections. A clear relationship between Hbp and colicin V or hemolysin in IAI was not found.
The mechanism of the pathogenic synergy between E. coli and B. fragilis in IAI is poorly understood. Factors that might contribute to synergy are anaerobic conditions produced by E. coli, inhibition of chemotaxis, phagocytosis and killing of E. coli by factors from B. fragilis (26), and provision of growth factors (9, 12, 15, 20). Herein, we have demonstrated that Hbp contributes to this synergy, since only the combination of B. fragilis and Hbp-positive E. coli strains induced abscesses. It should be noted that the number of bacteria still present in abscesses and the sizes of the abscesses 6 days after inoculation was dependent on the E. coli strain used. This indicates that besides Hbp, other strain-specific properties of E. coli play a role in the pathogenesis of IAI.
The results of the growth-promoting studies showed that Hbp is capable of delivering heme to B. fragilis and that a native conformation of Hbp is required for an efficient heme transfer. In addition, heme was not spontaneously released from Hbp in the growth-promoting experiments, because 100 to 80% of growth inhibition was obtained with specific anti-Hbp antiserum. A possible leakage of heme from Hbp during these experiments should give growth comparable to that in the cultures supplemented with heme alone (Fig. 1). Therefore, these results suggest the presence of a specific receptor at the B. fragilis cell surface to internalize the heme from Hbp. The use of a heme-binding protein of one bacterial species by another species has not been observed before. Such an interchangeable usage of iron-binding molecules has only been previously described for siderophore-mediated iron uptake systems (7, 16).
Hemolysins can make hemoglobin accessible for Hbp-producing E. coli by lysing erythrocytes, and, therefore, a strong correlation between hemolysin and Hbp was expected among strains from IAI. However, such a correlation was not found. In contrast, among E. coli strains isolated from blood, all Hbp-positive strains produced hemolytic activity, suggesting that hemolysin production is more important for E. coli associated with mono infections of bacteremia than for E. coli strains associated with mixed IAI. Possibly during IAI, B. fragilis makes hemoglobin accessible for Hbp-positive E. coli by lysing erythrocytes. Consistently, it is well known that Bacteroides spp. produce hemolysin (15), including B. fragilis strain BE1 used in this study.
We have demonstrated a role for Hbp in the formation of abscesses. A previous study has shown that the capsular polysaccharide of B. fragilis promotes the formation of abscesses (28). We hypothesize that in the presence of Hbp B. fragilis can more readily acquire iron from heme and grow at the site of infection. Hbp is likely used for iron acquisition by B. fragilis during the early stage of a mixed infection. Due to improved growth, increased amounts of capsular polysaccharide may be produced by this anaerobe and could promote the formation of abscesses. In addition, short-chain fatty acids secreted by B. fragilis inhibit the killing of E. coli by neutrophils (26). The inhibition of neutrophil function by the growing B. fragilis in its turn benefits E. coli, accelerating in this way the process of abscess formation. The abscesses shelter the microorganisms from circulating host defense mechanisms (24).
Tsh, a protein that is identical to Hbp, was identified as a hemagglutinin of an avian pathogenic E. coli strain recently isolated (22). In a recent report (8), there was a clear correlation between the presence of tsh, and the virulence of avian pathogenic E. coli strains as well as an association and linkage between tsh and colicin V genes encoded on plasmids. The results of this report also suggested that Tsh might contribute to the pathogenesis of early stages of colisepticemia within the avian respiratory tract. We have screened 26 isolates from affected hearts of diseased chickens with E. coli septicemia phenotypically and genotypically for Hbp and colicinV, and 65% of the strains appeared to be positive for both of these properties (data not shown). It seems that Hbp (Tsh) contributes to at least two different infectious diseases: IAI in humans and respiratory tract infections in poultry.
Identification of molecular interactions in host-microbe and microbe-microbe relationships in IAI is a crucial first step in order to develop novel antimicrobial strategies, especially at a time that resistance to antibiotics is a significant and growing clinical problem (1, 19). The results presented here define mechanisms that could be used to develop novel therapeutic targets at the level of protein interactions between infecting pathogens and their host in IAI. In the present study, mice immunized with Hbp were protected against the formation of abscesses following challenge with an Hbp-positive E. coli strain and B. fragilis strain that synergize and readily induce abscesses in naive unimmunized mice. Possible modes of this inhibition of abscess formation may be a general neutralization of Hbp, just like what was observed in the in vitro growth assays with B. fragilis, or the E. coli bacterium may be killed by an immune response to Hbp associated with the bacterial cell surface. The presence of cell surface-associated Hbp in E. coli was tested (21). It appears that the wild-type strain EB1 very efficiently processed Hbp, and no cell surface-associated Hbp was detected. K-12 strains that overexpress Hbp are less efficient in the processing of this protein, and cell surface-exposed Hbp can be detected, especially at lower growth temperatures (data not shown). Furthermore, the results of the experimental infections with strain EB19(pACYCHbp) clearly suggest that the induction of abscess formation is due to the presence of the hbp gene encoded on a plasmid (Fig. 2A). These results support a role for Hbp in the synergy of abscess formation in vivo that correlates with the increased growth of B. fragilis BE1 in vitro under heme-limiting conditions. In conclusion, it is likely that a general neutralization of Hbp by antibodies is responsible for the protection against the formation of abscesses.
Further studies will be directed at the possible targets for the development of new treatments against IAI, which include the proteolytic, heme-binding, and receptor domains of Hbp, as well as the putative cell surface-exposed Hbp receptors of E. coli and B. fragilis.
Acknowledgments
We thank W. Schouten for assistance with mouse experiments, T. Hekker for the gift of clinical blood isolates, J. van den Bosch for the gift of avian E. coli strains, and J. Tame for comments on the manuscript.
This work was supported by The Netherlands Organization for Technology (STW).
Editor: B. B. Finlay
REFERENCES
- 1.Aldridge, K. E. 1995. The occurrence, virulence, and antimicrobial resistance of anaerobes in polymicrobial infections. Am. J. Surg. 169: S2–S7. [PubMed] [Google Scholar]
- 2.Bornside, G. H., P. J. Bouis, Jr., and I. Cohn, Jr. 1968. Hemoglobin and Escherichia coli, a lethal intraperitoneal combination. J. Bacteriol. 95: 1567–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook, I. 1989. A 12 year study of aerobic and anaerobic bacteria in intra-abdominal and postsurgical abdominal infections. Surg. Gynecol. Obstet. 169: 387–392. [PubMed] [Google Scholar]
- 4.Brook, I. 1995. Pathogenesis and management of polymicrobial infections due to aerobic and anaerobic bacteria. Med. Res. Rev. 15: 73–82. [DOI] [PubMed] [Google Scholar]
- 5.Bullen, J. J., and H. J. Rogers. 1969. Bacterial iron metabolism and immunity to Pasteurella septica and Escherichia coli. Nature 224: 380–382. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134: 1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosa, J. H. 1989. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53: 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dozois, C. M., M. Dho-Moulin, A. Brée, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the tsh genetic region. Infect. Immun. 68: 4145–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farthmann, E. H., and U. Schöffel. 1998. Epidemiology and pathophysiology of intraabdominal infections (IAI). Infection 26: 329–334. [DOI] [PubMed] [Google Scholar]
- 10.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial haem capture. Mol. Microbiol. 39: 1–11. [DOI] [PubMed] [Google Scholar]
- 11.Hall, J. C., K. A. Heel, J. M. Papadimitriou, and C. Platell. 1998. The pathobiology of peritonitis. Gastroenterology 114: 185–196. [DOI] [PubMed] [Google Scholar]
- 12.Hau, T. 1990. Bacteria, toxins, and the peritoneum World J. Surg. 14: 167–175. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69: 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6: 370–378. [DOI] [PubMed] [Google Scholar]
- 15.MacLaren, D. M., F. Namavar, A. M. J. J. Verweij-van Vught, W. A. C. Vel, and J. A. Kaan. 1984. Pathogenic synergy: mixed intra-abdominal infections. Anthonie Leeuwenhoek 50: 775–787. [DOI] [PubMed] [Google Scholar]
- 16.Mietzner, T. A., and S. A. Morse. 1994. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu. Rev. Nutr. 14: 471–493. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Minshew, B. H., J. Jorgensen, M. Swanstrum, G. A. Grootes-Reuvecamp, and S. Falkow. 1978. Some characteristics of Escherichia coli strains isolated from extraintestinal infections of humans. J. Infect. Dis. 137: 648–654. [DOI] [PubMed] [Google Scholar]
- 19.Neu, H. C. 1995. Emergence and mechanisms of bacterial resistance in surgical infections. Am. J. Surg. 169: S13–S20. [PubMed] [Google Scholar]
- 20.Onderdonk, A. B., J. G. Bartlett, T. Louie, N. Sullivan-Seigler, and S. L. Gorbach. 1976. Microbial synergy in experimental intra-abdominal abscess. Infect. Immun. 13: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto, B. R., S. J. M. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli EB1. J. Exp. Med. 188: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62: 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruett, T. L., O. D. Rotstein, V. D. Fiegel, J. J. Sorenson, R. D. Nelson, and R. L. Simmons. 1984. Mechanism of the adjuvant effect of hemoglobin in experimental peritonitis. A leukotoxin is produced by E. coli metabolism in hemoglobin. Surgery 96: 375–383. [PubMed] [Google Scholar]
- 24.Rotstein, O. D. 1992. Role of fibrin deposition in the pathogenesis of intraabdominal infection. Eur. J. Clin. Microbiol. Infect. Dis. 11: 1064–1068. [DOI] [PubMed] [Google Scholar]
- 25.Rotstein, O. D., T. L. Pruett, and R. L. Simmons. 1985. Mechanisms of microbial synergy in polymicrobial surgical infections. Rev. Infect. Dis. 7: 151–170. [DOI] [PubMed] [Google Scholar]
- 26.Rotstein, O. D., T. Vittorini, J. Kao, M. I. McBurney, P. E. Nasmith, and S. Grinstein. 1989. A soluble Bacteroides by-product impairs phagocytic killing of Escherichia coli by neutrophils. Infect. Immun. 57: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Tzianabos, A. O., A. B. Onderdonk, B. Rosner, R. L. Cisneros, and D. L. Kasper. 1993. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262: 416–419. [DOI] [PubMed] [Google Scholar]
- 29.Verweij van Vught, A. M. J. J., F. Namavar, W. A. C. Vel, M. Sparrius, and D. M. MacLaren. 1986. Pathogenic synergy between Escherichia coli and Bacteroides fragilis or B. vulgatus in experimental infections: a non-specific phenomenon. J. Med. Microbiol. 21: 43–47. [DOI] [PubMed] [Google Scholar]
- 30.Waters, V. L., and J. H. Crosa. 1991. Colicin V virulence plasmids. Microbiol. Rev. 55: 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, P. H. 1979. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect. Immun. 26: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]