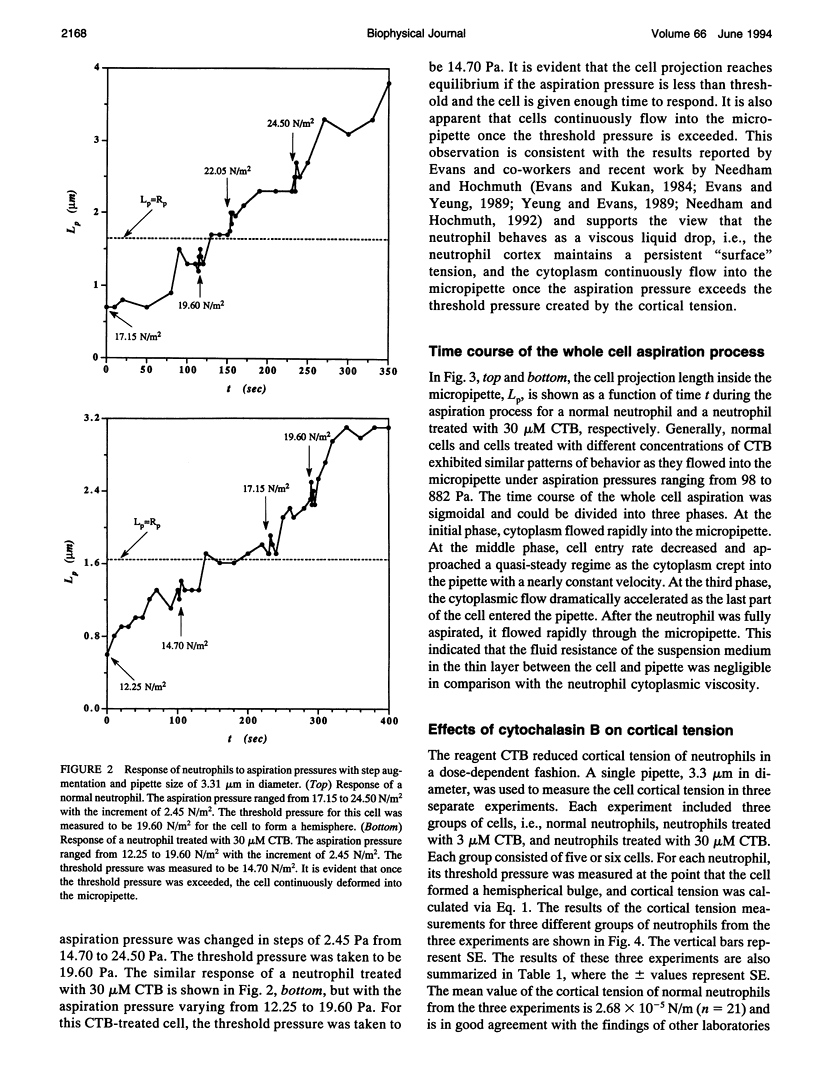
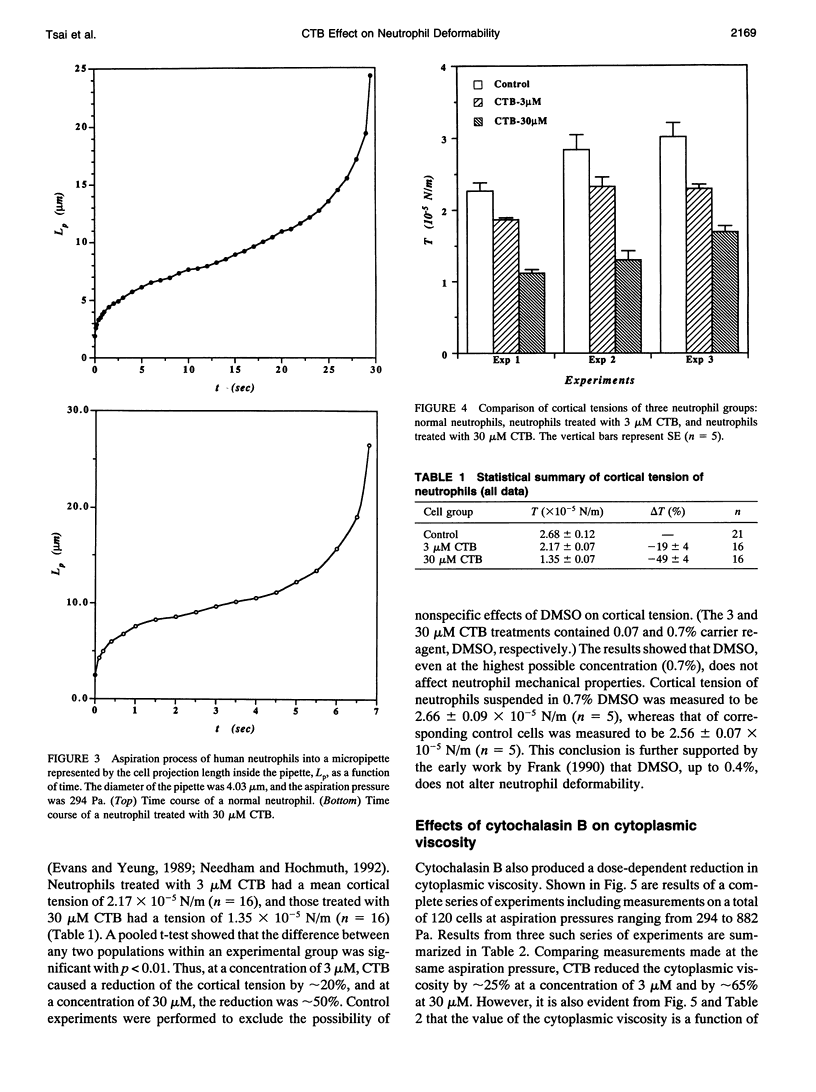
Abstract
Actin is a ubiquitous protein in eukaryotic cells. It plays a major role in cell motility and in the maintenance and control of cell shape. In this article, we intend to address the contribution of actin to the passive mechanical properties of human neutrophils. As a framework for assessing this contribution, the neutrophil is modeled as a simple viscous fluid drop with a constant cortical ("surface") tension. The reagent cytochalasin B (CTB) was used to disrupt the F-actin structure, and the neutrophil cortical tension and cytoplasmic viscosity were evaluated by single-cell micropipette aspiration. The cortical tension was calculated by simple force balance, and the viscosity was calculated according to a numerical analysis of the cell entry into the micropipette. CTB reduced the cell cortical tension in a dose-dependent fashion: by 19% at a concentration of 3 microM and by 49% at 30 microM. CTB also reduced the cytoplasmic viscosity by approximately -25% at a concentration of 3 microM and by approximately 65% at a concentration of 30 microM when compared at the same aspiration pressures. All three groups of neutrophils, normal cells, and cells treated with either 3 or 30 microM CTB, exhibited non-Newtonian behavior, in that the apparent viscosity decreased with increasing shear rate. The dependence of the cytoplasmic viscosity on deformation rate can be described empirically by mu = mu c(gamma m/gamma c)-b, where mu is cytoplasmic viscosity, gamma m is mean shear rate, mu c is the characteristic viscosity at the characteristic shear rate gamma c, and b is a material coefficient. The shear rate dependence of the cytoplasmic viscosity was reduced by CTB treatment. This is reflected by the changes in the material coefficients. When gamma c was set to 1 s-1, pc = 130 +/- 23 Pa.s and b = 0.52 +/- 0.09 for normal neutrophils and pc = 54 +/- 15 Pa.S and b = 0.26 +/- 0.05 for cells treated with 30 micro M CTB. These results provide the first quantitative assessment of the role that Pa-s-actin structure plays in the passive mechanical properties of human neutrophils.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien S., Sung K. L. Effect of colchicine on viscoelastic properties of neutrophils. Biophys J. 1984 Sep;46(3):383–386. doi: 10.1016/S0006-3495(84)84034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Doherty D. E., Schwab B., 3rd, Elson E. L., Henson P. M., Worthen G. S. Retention of leukocytes in capillaries: role of cell size and deformability. J Appl Physiol (1985) 1990 Nov;69(5):1767–1778. doi: 10.1152/jappl.1990.69.5.1767. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Worthen G. S. Neutrophil retention in model capillaries: deformability, geometry, and hydrodynamic forces. J Appl Physiol (1985) 1988 Oct;65(4):1861–1871. doi: 10.1152/jappl.1988.65.4.1861. [DOI] [PubMed] [Google Scholar]
- Erzurum S. C., Downey G. P., Doherty D. E., Schwab B., 3rd, Elson E. L., Worthen G. S. Mechanisms of lipopolysaccharide-induced neutrophil retention. Relative contributions of adhesive and cellular mechanical properties. J Immunol. 1992 Jul 1;149(1):154–162. [PubMed] [Google Scholar]
- Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984 Nov;64(5):1028–1035. [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Frank R. S. Time-dependent alterations in the deformability of human neutrophils in response to chemotactic activation. Blood. 1990 Dec 15;76(12):2606–2612. [PubMed] [Google Scholar]
- Hartwig J. H., Niederman R., Lind S. E. Cortical actin structures and their relationship to mammalian cell movements. Subcell Biochem. 1985;11:1–49. doi: 10.1007/978-1-4899-1698-3_1. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Varani J., Smolen J. E. Neutrophil activation and function in health and disease. Immunol Ser. 1992;57:1–46. [PubMed] [Google Scholar]
- Keller H. U., Naef A., Zimmermann A. Effects of colchicine, vinblastine and nocodazole on polarity, motility, chemotaxis and cAMP levels of human polymorphonuclear leukocytes. Exp Cell Res. 1984 Jul;153(1):173–185. doi: 10.1016/0014-4827(84)90459-2. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. A sensitive measure of surface stress in the resting neutrophil. Biophys J. 1992 Jun;61(6):1664–1670. doi: 10.1016/S0006-3495(92)81970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. M., Padmanabhan J., Cohen H. J. Cytochalasins induce actin polymerization in human leukocytes. Cell Motil Cytoskeleton. 1992;21(1):58–64. doi: 10.1002/cm.970210107. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Hopkins C. R. Transmembrane linkage between surface glycoproteins and components of the cytoplasm in neutrophil leukocytes. J Cell Biol. 1981 Sep;90(3):743–754. doi: 10.1083/jcb.90.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Boothroyd B., Richards R. C. Phorbol ester induces rapid actin assembly in neutrophil leucocytes independently of changes in [Ca2+]i and pHi. J Muscle Res Cell Motil. 1986 Oct;7(5):405–412. doi: 10.1007/BF01753583. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Involvement of the cortical actin filament network of neutrophil leucocytes during phagocytosis. Biochem Soc Trans. 1984 Dec;12(6):983–987. doi: 10.1042/bst0120983. [DOI] [PubMed] [Google Scholar]
- Tsai M. A., Frank R. S., Waugh R. E. Passive mechanical behavior of human neutrophils: power-law fluid. Biophys J. 1993 Nov;65(5):2078–2088. doi: 10.1016/S0006-3495(93)81238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace P. J., Packman C. H., Wersto R. P., Lichtman M. A. The effects of sulfhydryl inhibitors and cytochalasin on the cytoplasmic and cytoskeletal actin of human neutrophils. J Cell Physiol. 1987 Aug;132(2):325–330. doi: 10.1002/jcp.1041320218. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Schwab B., 3rd, Elson E. L., Downey G. P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989 Jul 14;245(4914):183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- Yahara I., Harada F., Sekita S., Yoshihira K., Natori S. Correlation between effects of 24 different cytochalasins on cellular structures and cellular events and those on actin in vitro. J Cell Biol. 1982 Jan;92(1):69–78. doi: 10.1083/jcb.92.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A., Evans E. Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys J. 1989 Jul;56(1):139–149. doi: 10.1016/S0006-3495(89)82659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]



