Abstract
The protozoan pathogen Giardia is an important cause of parasitic diarrheal disease worldwide. It colonizes the lumen of the small intestine, suggesting that effective host defenses must act luminally. Immunoglobulin A (IgA) antibodies are presumed to be important for controlling Giardia infection, but direct evidence for this function is lacking. B-cell-independent effector mechanisms also exist and may be equally important for antigiardial host defense. To determine the importance of the immunoglobulin isotypes that are transported into the intestinal lumen, IgA and IgM, for antigiardial host defense, we infected gene-targeted mice lacking IgA-expressing B-cells, IgM-secreting B-cells, or all B-cells as controls with Giardia muris or Giardia lamblia GS/M-83-H7. We found that IgA-deficient mice could not eradicate either G. muris or G. lamblia infection, demonstrating that IgA is required for their clearance. Furthermore, although neither B-cell-deficient nor IgA-deficient mice could clear G. muris infections, IgA-deficient mice controlled infection significantly better than B-cell-deficient mice, suggesting the existence of B-cell-dependent but IgA-independent antigiardial defenses. In contrast, mice deficient for secreted IgM antibodies cleared G. muris infection normally, indicating that they have no unique functions in antigiardial host defense. These data, together with the finding that B-cell-deficient mice have some, albeit limited, residual capacity to control G. muris infection, show that IgA-dependent host defenses are central for eradicating Giardia spp. Moreover, B-cell-dependent but IgA-independent and B-cell-independent antigiardial host defenses exist but are less important for controlling infection.
Giardia infection is an important cause of parasitic diarrheal disease worldwide (15). Symptomatic infection is characterized by various degrees of abdominal pain, diarrhea, nausea, vomiting, malabsorption, fatigue, and weight loss (15). Although symptoms can be severe and protracted, a significant proportion of infected persons are asymptomatic. Infection is initiated by ingestion of cysts from contaminated drinking water or, occasionally, contaminated food or by person-to-person contact (15). Cysts excyst and release trophozoites that colonize the lumen of the small intestine but do not invade the epithelium or deeper layers of the mucosa (1, 16). To avoid removal with the bulk flow of the lumen, Giardia trophozoites attach to the intestinal epithelium or reside in the mucus layer overlying the epithelium or can actively move against the bulk flow by means of four pairs of flagella (1, 16).
Infections with Giardia spp. are usually self-limited in immunocompetent individuals, indicating the presence of effective host defense mechanisms against this strictly luminal parasite, although chronic giardiasis with continued cyst excretion occurs in some individuals with no apparent immunodeficiency (15, 25). Secretory antibodies against Giardia spp. are presumed to play a central role in clearance of this parasite from the intestinal tract (1, 14). However, direct evidence is lacking, and very little is known about the physiologic functions of specific isotypes in clearing Giardia infection. Even the overall role of B cells in antigiardial defense has not been defined unequivocally, as suggested by the conflicting reports on their importance in immunity against Giardia spp.
Several studies suggest an important role for B cells in clearing Giardia infection. For example, infections of humans with Giardia lamblia or of mice with Giardia muris result in the production of antigiardial antibodies of the immunoglobulin A (IgA), IgM, and IgG isotypes in mucosal secretions and serum, and specific antibody production correlates with giardial clearance (11, 14, 18, 25, 38). Such antibodies reach their targets in vivo, since antigiardial IgA and IgG antibodies coat trophozoites in Giardia-infected mice (18). Mice depleted of B cells by treatment with anti-IgM antibodies and mice with X-linked immunodeficiency, which have a defect in B-cell development and function, are unable to clear G. muris infection (36, 37). B-cell-deficient mice generated by gene targeting appear to be unable to completely clear infections with the human pathogen, G. lamblia (41).
In contrast, other data suggest that B cells have only a limited role in antigiardial immunity. For example, mice with X-linked immunodeficiency can develop acquired immunity against secondary challenge with G. muris (35). Moreover, a recent study reported little difference in parasite clearance between wild-type littermates and B-cell-deficient mice infected with either G. lamblia or G. muris (33). Patients deficient for the production of IgA, the major immunoglobulin isotype in mucosal secretions, appear to have an only slightly increased incidence of Giardia infections (22). These data suggest that B-cell-independent host defenses against Giardia may play an important role in controlling and clearing infection.
Potential candidates for such defenses have been identified by in vitro studies. For example, defensins, small antimicrobial peptides produced by Paneth cells in the small intestine, can kill G. lamblia trophozoites in vitro (2). Nitric oxide, which can be produced by intestinal epithelial cells, inhibits proliferation and differentiation of G. lamblia trophozoites in vitro (13). However, the role of these potential host defenses against Giardia infection in vivo is not known.
Taken together, the preponderance of evidence suggests that B cells are needed for effective Giardia clearance (36, 37, 41). However, no direct evidence has been reported on the physiologic role of specific immunoglobulin isotypes in clearing Giardia infection, particularly of IgA and IgM, which are normally secreted into the intestinal lumen. To determine the physiologic importance of secretory IgA and IgM antibodies in antigiardial host defense, we used gene-targeted mice lacking IgA-expressing B cells, IgM-secreting B cells, or all B cells as controls and challenged the mice with G. muris or G. lamblia. The studies show that IgA is the major host defense mechanism against Giardia infection. B-cell-dependent but IgA-independent and B-cell-independent antigiardial defenses exist but play less important roles in controlling Giardia infection. In contrast, secreted IgM antibodies play no unique role in clearing Giardia infection.
MATERIALS AND METHODS
Mice.
The following strains of mutant mice were obtained from the Jackson Laboratory (Bar Harbor, Maine), unless otherwise specified: C57BL/6-Igh-6tm1Cgn mice (B-cell knockout [KO] mice) have an insertional mutation in the membrane exons of the immunogobulin μ chain gene, which renders these mice deficient in expression of membrane IgM and, therefore, all mature B cells (21). IgA−/− mice (IgA KO mice, obtained from J. Nedrud, Case Western Reserve University, Cleveland, Ohio) have a deletion in the Iα exon, the Sα switch region, exon 1, and part of exon 2 of the immunoglobulin α chain gene (17). These mice are completely deficient in IgA production but have modestly increased serum levels of IgM and IgG (17). Mice deficient in secreted IgM (secreted IgM KO mice) were generated by deleting the μs exon and its three downstream poly(A) sites in the immunoglobulin μ chain gene and replacing it with a cDNA fragment encoding the μm exons already spliced to the Cμ4 exon (6). These mice do not secrete IgM but still express surface IgM and IgD and undergo class switching to express other isotypes (6).
As controls for B-cell KO mice, we used C57BL/6J mice (C57 mice). As controls for IgA KO mice and secreted IgM KO mice, we used wild-type littermate controls with a similar B6 × 129 genetic background as the KO mice, as well as (B6129F1/J)F2 mice, which are F2 hybrids of B6129F1/J mice derived by mating C57BL/6 and 129/J mice. No significant differences in clearing G. muris infection were observed between these two strains of mice, and the data from these mice are reported together (under the designation B6129 mice). Mice were bred and maintained at the University of California-San Diego (UCSD) animal facilities under specific-pathogen-free conditions. All animal studies were approved by the UCSD Animal Subjects Committee.
To confirm the phenotype of the different KO mice, we determined plasma immunoglobulin levels by enzyme-linked immunosorbent assay (ELISA). The data from male and female mice did not differ significantly and were combined for these studies. As expected, the B-cell KO mice had undetectable levels (<5 μg/ml) of plasma IgM, IgG, or IgA, while the C57 control mice had 271 ± 55 μg of IgM, 1,476 ± 382 μg of IgG, and 73 ± 11 μg of IgA per ml in the plasma (all values are means ± standard error of the mean [SEM], n ≥ 6). The IgA KO mice had 137 ± 14 μg of IgM, 3,802 ± 437 μg of IgG, and undetectable levels (<5 μg/ml) of plasma IgA per ml. The secreted IgM KO mice had undetectable levels (<5 μg) of IgM, 2,673 ± 473 μg of IgG, and 229 ± 31 μg of IgA per ml. The B6129 control mice had 66 ± 17 μg of IgM, 913 ± 204 μg of IgG, and 122 ± 50 μg of IgA per ml of plasma. These data show that the plasma levels of IgM and IgG were 2.1- and 4.2-fold higher in IgA KO mice compared to B6129 controls (P < 0.001 by Student’s t test), which confirms previous observations in IgA KO mice (17), whereas the plasma levels of IgG and IgA were 2.9- and 1.9-fold higher in secreted IgM KO mice compared to B6129 controls (P < 0.001 by Student’s t test).
Infections.
G. muris cysts were obtained from M. Belosevic (University of Alberta, Edmonton, Canada), and maintained by passage through mice every 4 to 8 weeks. Prior to infection, cysts were purified from fresh stool samples collected no more than 2 days before infection. Mice were infected orally by gavage with 104 G. muris cysts in 0.2 ml of water. Before secondary infections, all mice were treated orally with 8 mg/mouse/day of metronidazole for three consecutive days to clear any G. muris infection remaining from the primary challenge. Clearance was confirmed by the absence of G. muris cysts in fecal cyst counts. Mice had free access to food and water during the experiments.
G. lamblia GS/M-83-H7 was obtained from the American Type Culture Collection (ATCC 50581) and grown in TYI-S-33 medium as described (2, 10). Prior to infection, trophozoites from confluent cultures in tissue culture flasks were detached by chilling on ice for 10 min, washed, and resuspended in TYI-S-33 medium at 5 × 107/ml. Mice were starved overnight, infected orally by gavage with 107 G. lamblia trophozoites in a 0.2-ml volume, and returned to solid foods 2 to 3 h later. To render the different mouse strains equally infectible with G. lamblia (34), mice were given 1.5 mg of neomycin per ml in the drinking water for 24 h before infection and throughout the entire infection period.
G. muris cyst preparation and counts.
Feces were collected from individual mice over a 2- to 3-h period, weighed, and homogenized in 20 ml of phosphate-buffered saline (PBS). G. muris cysts were enriched from homogenized stool samples by centrifugation over a 1 M sucrose gradient (3, 32). Briefly, 20 ml of homogenized stool was layered on top of a 10-ml cushion of a 1 M sucrose solution, and samples were centrifuged at 500 × g for 15 min at 4°C. The interface containing the G. muris cysts was collected in a 10-ml volume, diluted with 25 ml of PBS, and centrifuged at 500 × g for 15 min at 4°C. The pellet was resuspended in 1 ml of PBS, and cysts were counted in a counting chamber using a phase-contrast microscope. The detection limit of the G. muris cyst assay was 2 × 103 cysts/g of feces.
Trophozoite counts.
For trophozoite counts in G. muris-infected mice, the small intestine was removed and cut into seven equal-sized pieces, numbered 1 to 7 from orad to caudad. Each segment was opened longitudinally in 4 ml of PBS, placed on ice for 10 min, and agitated on a Vortex mixer to detach trophozoites from the mucosa. Counts of viable G. muris trophozoites were performed without further purification in a hemacytometer using a phase-contrast microscope. Viability was apparent as tumbling whole-cell movements for unattached trophozoites or flagellar movements for attached trophozoites. Total trophozoite load in the small intestine was determined by adding the numbers of trophozoites in each of the seven individually counted segments. The detection limit of the assay was 2 × 103 G. muris trophozoites/small intestine.
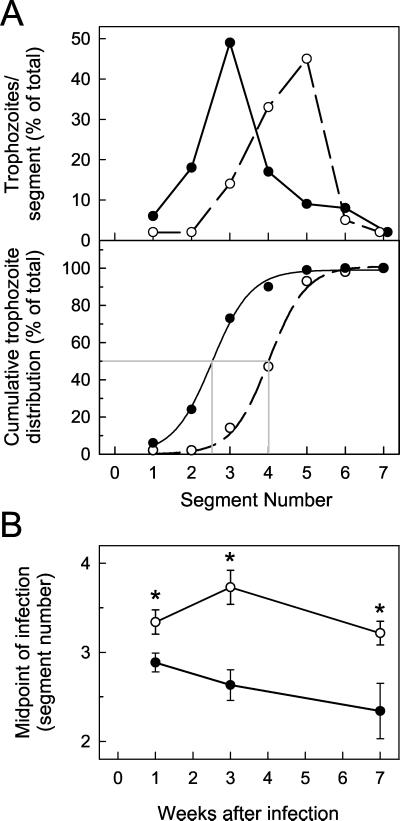
To evaluate G. muris trophozoite distribution along the orad-caudad axis of the small intestine, we first expressed the number of trophozoites in each of the seven equally spaced segments along the small intestine as a percentage of the total trophozoite load in the small intestine. We then calculated, for each segment, the cumulative percentage of trophozoite load, i.e., the sum of the percentages of the specific segment and all the segments orad of that segment. Subsequently, the midpoint of the infection was determined mathematically, i.e., the segment number (or fraction thereof) for which 50% of all trophozoites were on the orad side and 50% were on the caudad side of the small intestine. To calculate this, we fit a curve to the data points representing segment numbers versus cumulative trophozoite percentages by iterative approximation using the three-parameter sigmoid function y = a/{1 + exp[−([x − xo]/b)]} and the Fit Curve function of the computer program Sigmaplot 4.0 (SPSS Inc., Chicago, Ill.). Based on the sigmoid function describing the best curve fit for a specific set of data points, we determined the x value (i.e., the segment number representing the midpoint of infection) for which y = 50% (i.e., a cumulative G. muris trophozoite distribution of 50%).
For G. lamblia counts, the entire small intestine was removed and opened longitudinally in 10 ml of PBS, placed on ice for 10 min, and agitated on a Vortex mixer to detach trophozoites from the mucosa. The intestinal contents were allowed to settle for 5 min on ice, and the supernatant (without the intestine and large pieces of debris) was removed and centrifuged at 1,000 × g for 15 min at 4°C. The pellet was resuspended in 1 ml of PBS, and G. lamblia trophozoites were counted in a hemacytometer using a phase-contrast microscope. The detection limit of the assay was 5 × 102 G. lamblia trophozoites/small intestine.
Data analysis.
Cyst and trophozoite counts were log10 transformed, and means and SEM were calculated from the log values. Samples without detectable cysts or trophozoites were assigned a log value equivalent to half of the detection limit for each assay. Differences between groups of mice were compared by the Mann-Whitney rank sum test or Student’s t test, as appropriate. Differences with a P value of <0.05 were considered significant. Results from males and females were combined for all experiments, since no significant differences were found in stool cyst output (G. muris) or trophozoite numbers in the small intestine (G. muris and G. lamblia) at 1 and 7 weeks after infection.
RESULTS
B cells are required for clearing primary G. muris infection in mice.
To determine the role of B-cell-dependent and -independent mechanisms in antigiardial host defense and to establish a baseline for the studies using immunoglobulin isotype KO mice, we infected B-cell KO mice and genetically matched C57 control mice with cysts of the mouse parasite G. muris. In C57 control mice, G. muris cyst output and trophozoite numbers in the small intestine peaked at 1 week after infection (Fig. 1). Subsequently, the mice began to clear infection, and stool cyst output decreased >100-fold and trophozoite numbers >1,000-fold from 1 to 7 weeks, at which time parasite load was close to or below the detection limit for cysts and trophozoites (Fig. 1).
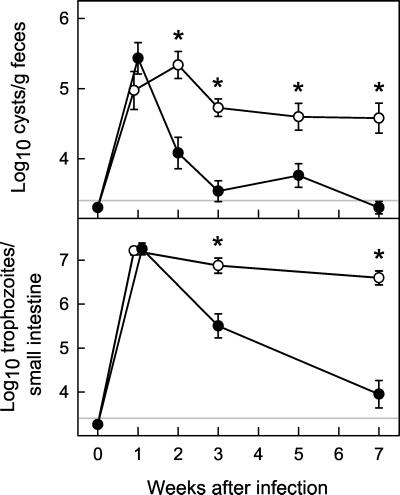
FIG. 1.
Primary G. muris infections of B-cell KO mice. B-cell KO mice (○) and wild-type littermate C57 control mice (•) were infected orally with 104 G. muris cysts. Infection intensity was assessed at the indicated times after infection by determining stool cyst output (top panel) and total trophozoite numbers in the small intestine (bottom panel). All data are means ± SEM from nine or more mice for each data point. The detection limits of the assays are indicated by the gray line in each panel. Asterisks indicate values of B-cell KO mice that are significantly different from controls at the same time point (P < 0.01 by rank sum test).
In contrast, B-cell KO mice failed to clear G. muris infection, with high numbers of cysts in the stool and trophozoites in the small intestine at 7 weeks after infection (Fig. 1). Even over extended periods (>1 year), infected B-cell KO mice continued to shed high levels of cysts in the stool (e.g., log10 cyst number/g of feces of 5.84 by 77 weeks after infection). Despite their inability to clear infection, B-cell KO mice showed a significant, albeit limited, ability to reduce the infectious load, since mean trophozoite numbers in the small intestine were 4.1-fold lower at 7 weeks compared to the maximum of infection at 1 week (P < 0.01 by rank sum test).
Efficient clearance of primary G. muris infection depends on IgA.
Several immunoglobulin isotypes could mediate the role of B cells in host defense against Giardia, since antigiardial antibodies of the IgA, IgM, and IgG isotypes are detectable in Giardia-infected mice (11, 18, 38). Since IgA is the most abundant immunoglobulin isotype in mucosal secretions in the intestine, we tested the role of IgA in clearing G. muris infection, using IgA KO mice (17) and B6129 littermate controls. Like C57 controls, B6129 control mice had peak stool cyst output and trophozoite numbers at 1 to 2 weeks after infection and began to clear the infection thereafter (Fig. 2). By 5 to 7 weeks after infection, cysts were undetectable in the stool, which represented a ≈100-fold decrease compared with maximal levels. Mean trophozoite numbers in the small intestine 7 weeks after infection were >1,000-fold lower than the maximal levels at 1 week, and a substantial proportion (5 of 12, 42%) of B6129 mice had no detectable trophozoites.
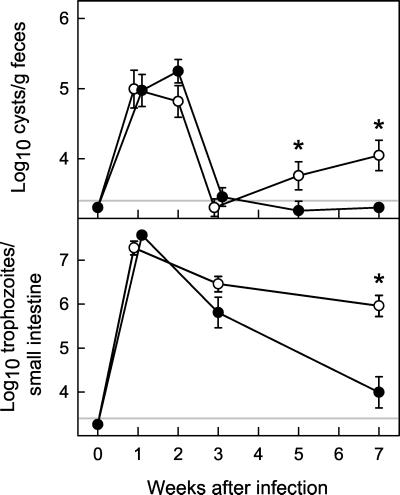
FIG. 2.
Primary G. muris infections of IgA KO mice. IgA KO mice (○) and wild-type littermate B6129 control mice (•) were infected orally with G. muris cysts, and infection severity was assessed at the indicated times after infection as described for Fig. 1. All data are means ± SEM from 11 or more mice for each data point. The detection limits of the assays are indicated by the gray line in each panel. Asterisks indicate values of IgA KO mice that are significantly different from controls at the same time point (P < 0.05 by rank sum test).
In contrast, IgA KO mice could not clear infection with G. muris (Fig. 2). IgA KO and control mice showed very similar peak levels of stool cyst output and trophozoite numbers in the small intestine 1 to 2 weeks after infection. Like B6129 mice, IgA KO mice began to reduce G. muris numbers by 3 weeks after infection, but unlike the controls, IgA KO mice subsequently failed to clear infection. By 7 weeks, IgA KO mice had ≈100-fold-higher trophozoite numbers and ≈10-fold-higher stool cyst output compared to B6129 controls (Fig. 2) and continued to shed cysts in the stool over at least 4 months (e.g., log10 cyst number/g of feces of 5.59 by 17 weeks after infection). Nonetheless, mean trophozoite numbers were 21-fold lower in IgA KO mice at 7 weeks compared to maximal numbers (P < 0.001 by rank sum test). This indicates that although IgA KO mice could not eradicate G. muris infection, they had some ability to control the infection, which was significantly greater than the residual ability of B-cell KO mice to control infection (log10 trophozoite numbers of 6.59 ± 0.12 in B-cell KO mice versus 5.96 ± 0.24 in IgA KO mice at week 7; P < 0.05 by rank sum test).
Secreted IgM is not required for clearing primary G. muris infection.
Since IgA KO mice showed a significantly greater ability to control G. muris infection than B-cell KO mice, we investigated whether another immunoglobulin, secreted IgM, may play a role in controlling Giardia infection in mice. Secreted IgM, like IgA, is transported into the intestinal lumen by the polymeric immunoglobulin receptor on intestinal epithelial cells. The course of G. muris infection in mice deficient in secreted IgM but not membrane IgM or other isotypes (6) did not differ significantly from that in B6129 littermate controls (Fig. 3), indicating that secreted IgM has no unique role in clearing G. muris infection.
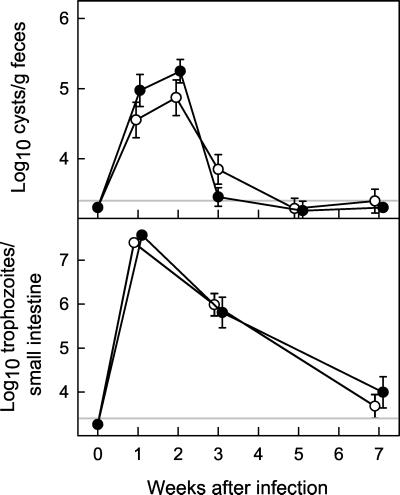
FIG. 3.
Primary G. muris infections of secreted IgM KO mice. Secreted IgM KO mice (○) and wild-type littermate B6129 control mice (•) were infected orally with G. muris cysts, and infection severity was assessed at the indicated times after infection as described for Fig. 1. All data are means ± SEM from 11 or more mice for each data point. The detection limits of the assays are indicated by the gray line in each panel. None of the values in secreted IgM KO mice were significantly different from controls at the same time point, as determined by rank sum tests.
B cells are key for developing acquired immune protection against secondary G. muris challenge.
Although clearly of limited importance for clearing primary G. muris infection (Fig. 1), B-cell-independent host defenses could play a more important role in the acquired immune protection against secondary challenge. To test this, B-cell KO mice and genetically matched C57 control mice were immunized by primary infection with G. muris and treatment with the antigiardial drug metronidazole at 7 weeks. Immunized mice were then challenged with a secondary G. muris inoculum, and the course of the infection was compared with the primary infection.
At 1 week after infection, immunized C57 controls were almost completely protected from secondary challenge, since trophozoite numbers in the small intestine (Fig. 4, top left panel) and stool cyst output (data not shown) were >100-fold lower in immunized relative to nonimmunized mice. Immune protection in C57 mice was efficient but not complete, as small numbers of cysts in the stool (data not shown) and trophozoites in the small intestine (Fig. 4) were observed occasionally in some of the immunized mice throughout the course of the infection. In contrast to control mice, immunized B-cell KO mice showed only a minor (2.0-fold) difference in the trophozoite numbers in the small intestine after 1 week compared with nonimmunized mice (Fig. 4, bottom left panel). These data demonstrate that B cells are absolutely required for developing acquired immune protection against secondary challenge with G. muris.
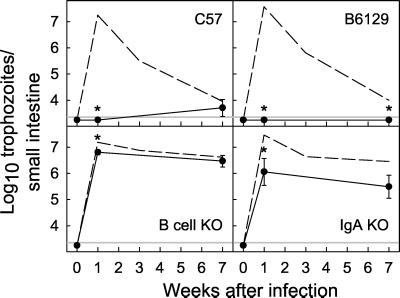
FIG. 4.
Secondary G. muris challenge of immunized B-cell KO and IgA KO mice. C57 control mice (top left), B-cell KO mice (bottom left), B6129 controls (top right), and IgA KO mice (bottom right) were first infected orally with a primary inoculum of 104 G. muris cysts. After 7 weeks, residual infection was cleared by oral metronidazole treatment. Immunized mice were then challenged with a secondary inoculum of 104 G. muris cysts, and infection severity was assessed at the indicated times after secondary challenge by determining total trophozoite numbers in the small intestine. All data are means ± SEM from 10 or more mice for each data point. For comparison, the values from the primary infections of naive mice (from Fig. 1 and 2) are shown in each panel by dashed lines. The detection limits of the assays are indicated by the gray line in each panel. Asterisks indicate values from immunized mice that are significantly different from those in naive mice of the same mouse strain at the same time point (P < 0.01 by rank sum test).
IgA is important for acquired immunity to G. muris, but other host defenses also play a role.
Since B-cell-dependent host defenses are key for developing acquired immunity, we next focused on the role of IgA in acquired immune protection against secondary G. muris challenge. Immunized B6129 control mice were completely protected from a secondary challenge with G. muris, since no trophozoites in the small intestine (Fig. 4, top right panel) or cysts in the stool (data not shown) could be detected at 1 or 7 weeks after infection. Immunized IgA KO mice showed partial protection against secondary G. muris challenge, as trophozoite numbers in the small intestine were significantly (16.6-fold) lower at 1 week after challenge of immunized compared to naive IgA KO mice (Fig. 4, bottom right panel). However, immunized IgA KO mice, like naive IgA KO mice, failed to clear the infection at later times, with continued moderate trophozoite numbers in the small intestine (Fig. 4) and low but detectable stool cyst output (data not shown) for at least 7 weeks. These data show that IgA is important for efficient acquired immune protection against secondary G. muris challenge, but they also suggest that other host defenses exist that can control G. muris infection after secondary challenge. In contrast to IgA KO mice, immunized secreted IgM KO mice were fully protected against secondary G. muris challenge (data not shown).
B-cell-dependent host defenses affect G. muris trophozoite distribution along orad-caudad axis of small intestine.
Further analysis of the trophozoite counts showed that host immune defenses influenced the distribution of G. muris trophozoites along the orad-caudad axis of the small intestine. For both groups of control mice, C57 (Fig. 5) and B6129 (data not shown), the calculated midpoint of infection was in segment 2.7 to 2.9 early in the infection (week 1) and subsequently moved progressively orad. In contrast, in B-cell KO mice, the midpoint of the infection was significantly more caudad compared to C57 controls at 1 week after infection and moved further caudad at 3 weeks after infection (Fig. 5). The orad-caudad trophozoite distribution in IgA KO mice followed a pattern similar to that in B-cell KO mice, whereas the distribution in secreted IgM KO mice was comparable to that in the B6129 control mice (data not shown). Taken together, these data show that B-cell-dependent immune defenses not only control total G. muris trophozoite load, but also significantly affect the trophozoite distribution along the orad-caudad axis of the small intestine.
FIG. 5.
G. muris trophozoite distribution along the orad-caudad axis of the small intestine. B-cell KO mice (○) and wild-type littermate C57 control mice (•) were infected orally with 104 G. muris cysts, and the number of trophozoites was determined in each of seven equally spaced segments along the small intestine, with segment 1 being the most orad segment. The numbers were then expressed as a percentage of the total trophozoite load in the small intestine, as shown in a representative example in panel A (top) for two individual mice at 3 weeks after infection. The cumulative percent trophozoite load, i.e., the sum of the percentages of the specific segment and all the segments orad of that segment, was determined for each intestinal segment, as depicted by the symbols in panel A (bottom) for the same mice shown in panel A (top). Subsequently, a sigmoid function was calculated that describes a curve with the best fit to each set of data points, as depicted by the solid and dashed lines in panel A (bottom). Based on this sigmoid function, the midpoint of the infection, i.e., the segment number (or fraction thereof) for which 50% of all trophozoites were on the orad side and 50% on the caudad side of the small intestine, was determined, as shown by the gray lines in panel A (bottom). In the example shown, the midpoint of infection was at segment 2.5 for the C57 control mouse and segment 4.0 for the B-cell KO mouse. Panel B shows a summary of the results from different times after infection. Values are means ± SEM from seven or more mice for each data point. Asterisks indicate values from B-cell KO mice that are significantly different from controls at the same time point (P < 0.05 by Student’s t test).
IgA is required for clearance of G. lamblia in mice.
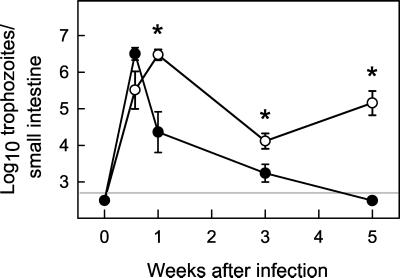
To determine the relevance of our findings in the G. muris model for host defense against the human pathogen, G. lamblia, we used strain GS/M-83-H7, which, unlike most G. lamblia strains, can infect adult mice (10). Since our studies had shown that IgA is key for controlling G. muris infection and previous studies with G. lamblia in mice had suggested a role of B cells (41), we focused on the role of IgA in host defense against G. lamblia. B6129 control mice could be reliably infected with G. lamblia GS/M-83-H7, with peak trophozoite numbers in the small intestine by 4 days and complete clearance by week 5 (Fig. 6). IgA KO mice had comparable numbers of trophozoites early after infection (day 7), but could not clear infection at 5 weeks, with >100-fold-higher trophozoite numbers compared to controls (Fig. 6). Therefore, IgA is required for clearance of both G. muris and G. lamblia in mice.
FIG. 6.
Primary G. lamblia infections of IgA KO mice. IgA KO mice (○) and wild-type littermate B6129 control mice (•) were infected orally with 107 G. lamblia trophozoites. Infection severity was assessed at the indicated times after infection by determining total trophozoite numbers in the small intestine. All data are means ± SEM from six to eight mice for each data point. The detection limits of the assays are indicated by the gray line in each panel. Asterisks indicate values of IgA KO mice that are significantly different from controls at the same time point (P < 0.05 by rank sum test).
DISCUSSION
Giardia trophozoites reside strictly in the lumen of the small intestine, indicating that effective host defense against this pathogen requires effector mechanisms that are active luminally. The present study shows that B-cell-dependent effector mechanisms are key for controlling and eradicating G. muris infection from experimentally infected mice, consistent with the ability of antibodies to reach luminal targets in vivo (18) and to immobilize or kill Giardia trophozoites in vitro (4, 20, 40). This finding confirms and extends other studies, which have suggested an important role of B cells in controlling Giardia infection. For example, mice in which B cells are ablated by treatment with anti-IgM antibodies cannot clear G. muris infection (36), although their B-cell depletion is less complete than in B-cell KO mice with a targeted gene deletion. B-cell-dependent host defenses are also important for clearing G. lamblia infection in mice (41). These experimental findings are consistent with the increased susceptibility to Giardia infection of patients with chronic variable immunodeficiency, who are characterized by defects in B-cell functions, although these patients may also have T-cell defects (22).
The data reported here demonstrate that antibodies of the IgA isotype are required for effective clearance of G. muris and G. lamblia from the murine host. This role of IgA may be expected, given that it is the most abundant immunoglobulin in mucosal secretions and antigiardial IgA antibodies are elicited by infection (18, 38). IgA is likely to exert its antigiardial functions through “immune exclusion” (e.g., immobilization or detachment of trophozoites from epithelium) rather than direct killing, since antigiardial IgA antibodies do not kill G. muris trophozoites in the presence or absence of complement (19), although one study suggested that antigiardial IgA may exert cytotoxic effects on G. lamblia (39).
Clinical reports on the incidence of Giardia infections in patients with IgA deficiency have provided conflicting results, as some studies found an increased incidence of infections, while others found that such patients are not more susceptible (9, 22, 42). Patients with IgA deficiency are usually defined by serum IgA levels of <0.05 g/liter (normal levels are 0.5 to 3.5 g/liter), but many, if not most, IgA-deficient patients have low but detectable levels of serum IgA antibodies (9, 30) and can produce IgA antibodies at near-normal levels in vitro (8, 23). This is in contrast to the complete lack of IgA production in IgA-deficient mice, which lack crucial gene segments for expressing IgA heavy chains (17). The simplest interpretation of both clinical and experimental data would be that although IgA is required for clearing Giardia infection, IgA levels well below normal are sufficient. Alternative interpretations are possible, e.g., host defenses against Giardia in mice and G. lamblia in humans differ in regard to IgA dependency, or IgA-deficient patients but not mice can develop compensatory host defenses against Giardia infection.
We found that secreted IgM (in contrast to membrane IgM, which is required for normal B-cell development and, therefore, antigiardial defense) had no unique role in clearing G. muris infection. This is in contrast to intraperitoneal infection with enteric bacteria after ligation and puncture of the cecum, where secreted IgM plays a critical role in host defense against infection (7). Antibodies of the IgM isotype, particularly natural antibodies, often recognize phylogenetically conserved structures, such as carbohydrates, phospholipids, or nucleic acids (7), suggesting that recognition of such structures may not be important for controlling giardial infection in the intestinal lumen. It is also possible that levels of secreted IgM in the intestinal lumen are too low to be important in the presence of normal IgA levels. Nonetheless, IgM may be able to cooperate with, or possibly compensate for, other host defenses, such as IgA, in antigiardial host defense. This could be particularly important if crucial antigiardial host defenses are not operative, as in IgA-deficient patients. Such a compensatory role of secreted IgM could explain why IgA KO mice have a greater residual capacity to control Giardia infection than B-cell KO mice. However, the latter finding could also be explained by a role of IgG antibodies, since IgG can be transported from the basolateral to the apical side of polarized intestinal epithelial cells in vitro (12) and can be detected in the intestinal lumen in vivo (18, 29, 31).
Our findings of a central role of B cells and IgA in antigiardial host defense differ from those in a recent report by Singer and Nash, in which B-cell KO mice were shown to control G. lamblia infection as well as normal littermate controls after 4 weeks, suggesting that B cells played a limited role, if any, in controlling acute G. lamblia infection in those studies (33). Although it is difficult at this point to reconcile the data, differences in the experimental design may be responsible for the apparent discrepancy. For example, we used higher G. lamblia inocula, which yielded 10- to 100-fold-higher peak infectious loads 1 week after infection (33). It is possible that B cells are required for controlling higher infectious burdens, while they play a less important role in controlling lower initial and peak infectious loads of Giardia. Furthermore, we treated mice with a nonabsorbable oral antibiotic, neomycin, throughout the infections, which minimizes differences in G. lamblia susceptibility of different strains of mice but has no direct effects on G. lamblia, as a previous study had suggested that such susceptibility differences are largely due to differences in the intestinal microbiota (34). The antibiotic treatment protocol in our studies might have helped to reveal host defenses directed against Giardia itself rather than those that might affect Giardia indirectly by controlling the composition or density of the intestinal microbiota. In any case, the data from Singer and Nash support the presence of B-cell-independent antigiardial host defenses, which is consistent with our finding that B-cell KO mice had some, albeit limited, capacity to control G. muris infection. The relative importance of B-cell-dependent and -independent host defenses for controlling and ultimately clearing Giardia infection is likely to depend on multiple parasite factors (e.g., peak infectious burden) and host factors (e.g., intestinal microbiota), whose specific roles in antigiardial host defense remain to be defined.
Giardia spp. mostly colonize the duodenum and jejunum in humans and mice, although trophozoites can occasionally be found in the ileum, colon, stomach, and bile ducts (3, 26). The characteristic distribution of trophozoites along the orad-caudad axis of the intestine is likely to result from a complex interplay of host and parasite factors. On the parasite side, motility, the ability to attach and detach from the intestinal epithelium and mucus, proliferative capacity, the ability to differentiate into cysts, and the need for optimal nutrient access are important factors in determining their localization in the small intestine (16, 43). On the host side, physiologic functions (e.g., intestinal motility and secretory responses) and anatomic factors are likely to be important in controlling giardial numbers in different regions of the small intestine. For example, few trophozoites are present upstream of the entry point of the common bile duct into the duodenum, supporting an important role for bile and/or pancreatic secretions in giardial survival in the host (16). Our data show that specific antigiardial host defenses, i.e., antibody production, also play a significant role in determining trophozoite distribution along the orad-caudad axis of the small intestine. The mechanisms underlying this effect are not clear but could relate to direct effects (e.g., stronger B-cell defenses in the caudad portions of the small intestine) or indirect effects (e.g., a role of B cells in intestinal motility or in controlling the intestinal microbiota).
Our data and other experimental and clinical studies indicate that effective acquired immune defenses exist against Giardia which can attenuate, if not prevent, reinfections of the same host. These defenses may not be complete, since reinfections are relatively common in areas with endemic giardiasis, which may be related to differences in infecting giardial strains or antigenic variation (24). Nonetheless, antigiardial immunity elicited by infection with a specific Giardia strain or exposure to Giardia extracts can reduce severity and duration of repeated infections with the same or a different strain (25, 28), which constitutes the rationale for developing antigiardial vaccines. Successful vaccination trials have been reported in dogs and cats, validating the feasibility of antigiardial vaccination (27, 28), although the underlying immune mechanisms are not well understood. The findings reported here indicate that IgA-dependent host defenses can overcome antigenic variation as an immune evasion strategy of Giardia spp. in vivo (5, 24), possibly by targeting nonvariable antigens. Therefore, our data suggest that vaccination efforts should be targeted mostly towards stimulating antigiardial IgA.
Acknowledgments
This work was supported by NIH grant DK35108 and a Research Grant from the Crohn’s and Colitis Foundation of America.
We are grateful to Tom Blaze for invaluable help with the animal experiments and Jennifer Smith for excellent technical support.
Editor: J. M. Mansfield
REFERENCES
- 1.Adam, R. D. 1991. The biology of Giardia spp. Microbiol. Rev. 55: 706–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley, S. B., M. Zimmerman, M. Hetsko, M. E. Selsted, and F. D. Gillin. 1994. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 62: 5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belosevic, M., and G. M. Faubert. 1983. Giardia muris: correlation between oral dosage, course of infection, and trophozoite distribution in the mouse small intestine. Exp. Parasitol. 56: 93–100. [DOI] [PubMed] [Google Scholar]
- 4.Belosevic, M., G. M. Faubert, and S. Dharampaul. 1994. Antimicrobial action of antibodies against Giardia muris trophozoites. Clin. Exp. Immunol. 95: 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, M., P. Siles-Lucas, P. Wittwer, and N. Müller. 2001. vsp gene expression by Giardia lamblia clone GS/M-83-H7 during antigenic variation in vivo and in vitro. Infect. Immun. 69: 5278–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boes, M., C. Esau, M. B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160: 4776–4787. [PubMed] [Google Scholar]
- 7.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188: 2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briere, F., J. M. Bridon, D. Chevet, G. Souillet, F. Bienvenu, C. Guret, H. Martinez-Valdez, and J. Banchereau. 1994. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J. Clin. Investig. 94: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrows, P. D., and M. D. Cooper. 1997. IgA deficiency. Adv. Immunol. 65: 245–276. [PubMed] [Google Scholar]
- 10.Byrd, L. G., J. T. Conrad, and T. E. Nash. 1994. Giardia lamblia infections in adult mice. Infect. Immun. 62: 3583–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels, C. W., and M. Belosevic. 1994. Serum antibody responses by male and female C57BL/6 mice infected with Giardia muris. Clin. Exp. Immunol. 97: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson, B. L., K. Badizadegan, Z. Wu, J. C. Ahouse, X. Zhu, N. E. Simister, R. S. Blumberg, and W. I. Lencer. 1999. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J. Clin. Investig. 104: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckmann, L., F. Laurent, T. D. Langford, M. L. Hetsko, J. R. Smith, M. F. Kagnoff, and F. D. Gillin. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164: 1478–1487. [DOI] [PubMed] [Google Scholar]
- 14.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13: 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan, P. A. 1992. Giardia–diagnosis, clinical course and epidemiology: a review. Epidemiol. Infect. 109: 1–22. [PMC free article] [PubMed] [Google Scholar]
- 16.Gillin, F. D., D. S. Reiner, and J. M. McCaffery. 1996. Cell biology of the primitive eukaryote Giardia lamblia. Annu. Rev. Microbiol. 50: 679–705. [DOI] [PubMed] [Google Scholar]
- 17.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162: 2521–2529. [PubMed] [Google Scholar]
- 18.Heyworth, M. F. 1986. Antibody response to Giardia muris trophozoites in mouse intestine. Infect. Immun. 52: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyworth, M. F. 1992. Relative susceptibility of Giardia muris trophozoites to killing by mouse antibodies of different isotypes. J. Parasitol. 78: 73–76. [PubMed] [Google Scholar]
- 20.Heyworth, M. F., and J. A. Vergara. 1994. Giardia muris trophozoite antigenic targets for mouse intestinal IgA antibody. J. Infect. Dis. 169: 395–398. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B-cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350: 423–426. [DOI] [PubMed] [Google Scholar]
- 22.Lai, P. S., and L. Mayer. 1997. Gastrointestinal manifestations of primary immunodeficiency disorders. Semin. Gastrointest. Dis. 8: 22–32. [PubMed] [Google Scholar]
- 23.Marconi, M., A. Plebani, M. A. Avanzini, R. Maccario, A. Pistorio, M. Duse, M. Stringa, and V. Monafo. 1998. IL-10 and IL-4 cooperate to normalize in vitro IgA production in IgA-deficient (IgAD) patients. Clin. Exp. Immunol. 112: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller, N., and B. Gottstein. 1998. Antigenic variation and the murine immune response to Giardia lamblia. Int. J. Parasitol. 28: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 25.Nash, T. E., D. A. Herrington, G. A. Losonsky, and M. M. Levine. 1987. Experimental human infections with Giardia lamblia. J. Infect. Dis. 156: 974–984. [DOI] [PubMed] [Google Scholar]
- 26.Oberhuber, G., N. Kastner, and M. Stolte. 1997. Giardiasis: a histologic analysis of 567 cases. Scand. J. Gastroenterol. 32: 48–51. [DOI] [PubMed] [Google Scholar]
- 27.Olson, M. E., H. Ceri, and D. W. Morck. 2000. Giardia vaccination. Parasitol. Today 16: 213–217. [DOI] [PubMed] [Google Scholar]
- 28.Olson, M. E., D. W. Morck, and H. Ceri. 1996. The efficacy of a Giardia lamblia vaccine in kittens. Can. J. Vet. Res. 60: 249–256. [PMC free article] [PubMed] [Google Scholar]
- 29.Prigent-Delecourt, L., B. Coffin, J. F. Colombel, J. P. Dehennin, J. P. Vaerman, and J. C. Rambaud. 1995. Secretion of immunoglobulins and plasma proteins from the colonic mucosa: an in vivo study in man. Clin. Exp. Immunol. 99: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince, H. E., G. L. Norman, and W. L. Binder. 2000. Immunoglobulin A (IgA) deficiency and alternative celiac disease-associated antibodies in sera submitted to a reference laboratory for endomysial IgA testing. Clin. Diagn. Lab. Immunol. 7: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan, C. P., E. Ruffet, K. Arihiro, R. Pires, and J. P. Bouvet. 1996. High affinity serum-derived Fab fragments as another source of antibodies in the gut lumen of both neonates and adults. Scand. J. Immunol. 44: 108–114. [DOI] [PubMed] [Google Scholar]
- 32.Roberts-Thomson, I. C., D. P. Stevens, A. A. Mahmoud, and K. S. Warren. 1976. Giardiasis in the mouse: an animal model. Gastroenterology 71: 57–61. [PubMed] [Google Scholar]
- 33.Singer, S. M., and T. E. Nash. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect. Immun. 68: 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer, S. M., and T. E. Nash. 2000. The role of normal flora in Giardia lamblia infections in mice. J. Infect. Dis. 181: 1510–1512. [DOI] [PubMed] [Google Scholar]
- 35.Skea, D. L., and B. J. Underdown. 1991. Acquired resistance to Giardia muris in X-linked immunodeficient mice. Infect. Immun. 59: 1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snider, D. P., J. Gordon, M. R. McDermott, and B. J. Underdown. 1985. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J. Immunol. 134: 4153–4162. [PubMed] [Google Scholar]
- 37.Snider, D. P., D. Skea, and B. J. Underdown. 1988. Chronic giardiasis in B-cell-deficient mice expressing the xid gene. Infect. Immun. 56: 2838–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snider, D. P., and B. J. Underdown. 1986. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect. Immun. 52: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stäger, S., B. Gottstein, H. Sager, T. W. Jungi, and N. Müller. 1998. Influence of antibodies in mother’s milk on antigenic variation of Giardia lamblia in the murine mother-offspring model of infection. Infect. Immun. 66: 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stäger, S., R. Felleisen, B. Gottstein, and N. Müller. 1997. Giardia lamblia variant surface protein H7 stimulates a heterogeneous repertoire of antibodies displaying differential cytological effects on the parasite. Mol. Biochem. Parasitol. 85: 113–124. [DOI] [PubMed] [Google Scholar]
- 41.Stäger, S., and N. Müller. 1997. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect. Immun. 65: 3944–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strober, W., and M. C. Sneller. 1991. IgA deficiency. Ann. Allergy 66: 363–375. [PubMed] [Google Scholar]
- 43.Upcroft, J., and P. Upcroft. 1998. My favorite cell: Giardia. Bioessays 20: 256–263. [DOI] [PubMed] [Google Scholar]