Abstract
Resistance to infection with Toxoplasma gondii was studied in mice lacking CD4 expression. Such mice developed more brain cysts and survived for a shorter time than did wild-type controls after peroral infection with ME49 cysts. After immunization with the ts-4 strain of T. gondii, CD4-deficient mice exhibited impaired resistance to a challenge infection with virulent RH tachyzoites. Thus, deficient CD4 expression increases the susceptibility of mice to a primary peroral T. gondii infection with cysts and impairs their ability to be successfully vaccinated. CD8+ T cells from blood or spleens of Toxoplasma-infected, CD4-deficient mice expressed markers of activation at frequencies similar to those of infected wild-type mice. Production of IFN-γ in vitro was moderately depressed, and levels of Toxoplasma-specific immunoglobulin G2a in serum were substantially lower than in wild-type mice. Administration of Toxoplasma-immune serum to ts-4-vaccinated CD4-deficient mice significantly improved their resistance to RH challenge. Also, the survival of CD4-deficient mice chronically infected with ME49 was significantly prolonged by administration of immune serum. These results demonstrate that in addition to CD8+ T cells and IFN-γ, which are known to be critical for resistance, CD4+ cells also contribute significantly to protection against chronic T. gondii infections and against challenge infections with highly virulent tachyzoites in immunized mice via their role as helper cells for production of isotype-switched antibodies.
Infection with Toxoplasma gondii may cause serious disease in individuals whose immune systems are compromised from AIDS, immunoablative therapy, or developmental immaturity (19, 23, 25). Because deficiency in CD4+ T cells is a prominent feature in AIDS patients and other immunocompromised individuals, there is an obvious interest in understanding how CD4+ T cells may be involved in protection against T. gondii.
A number of studies with mice have examined whether CD4+ T cells are critically involved in resistance to T. gondii infection at the effector level. Depletion of CD4+ cells by antibody treatment exacerbated infections to an extent in chronically infected mice (9, 15, 39) and revealed a contribution, along with that of CD8+ cells, to resistance to reinfection (10, 29, 38). Taken together, these studies show that CD4+ cells may contribute to protection against T. gondii in mice with established infections or preexisting immunity. However, those studies also showed that CD8+ T cells play the predominant effector role.
Whether CD4+ T cells are needed for generation of immunity to T. gondii has also been studied. Mice treated with anti-CD4 antibody before and after infection with T. gondii cysts had more extensive mortality after several weeks than did control mice (9, 28, 39). However, mice treated with anti-CD8 antibody just before or after T. gondii infection died sooner than did mice treated with anti-CD4 (28, 34), again suggesting that CD8+ cells play a decisive effector role in adaptive immunity to T. gondii, with CD4+ cells perhaps functioning to enhance CD8+ T-cell-dependent immunity. Further evidence of CD4+ T-cell involvement in the generation of adaptive immunity to T. gondii was shown in experiments in which mice were infected with T. gondii and depleted of CD4+ cells. They developed more brain cysts than did controls and exhibited reduced blastogenic and delayed-type hypersensitivity responses to T. gondii antigens and lower T. gondii-specific serum antibody levels (3). From this and a similar study investigating mortality (2), it was concluded that CD4+ T cells are important for the generation of adaptive immunity to T. gondii.
The foregoing studies demonstrated that CD4+ cells may contribute to both the generation and expression of resistance to T. gondii, but they do not indicate the mechanism of that contribution. Candidate mechanisms include help, via interleukin-2 (IL-2) production, for the generation of CD8+ cytolytic T cells; conditioning of dendritic cells, via CD40-CD40L interaction, to activate CD8+ cells (32); production of gamma interferon (IFN-γ), a cytokine crucial to resistance to T. gondii (36, 37); control of levels of pathogen-derived ligands that drive CD8+ T-cell responses (6); and help for B cells to produce isotype-switched antibodies that may be important for resistance to T. gondii (18, 33).
In this study, we have focused on the last of these possibilities. A role for antibodies in resistance to T. gondii has been examined in several older studies (7, 16, 20, 22). For the most part, these studies have shown that immune serum, by itself, is not very protective. However, these passive-immunization studies were conducted with unimmunized mice or mice lacking T cells, which are now known to be essential for resistance. In mice lacking B cells but in which immune T cells were present, passive immunization was effective (7, 18, 33).
We have tested the hypothesis that CD4+ T cells contribute to resistance to T. gondii via their role in humoral immunity by using mice that lack conventional CD4 + T cells because they do not express the CD4 antigen but which do possess CD8+ T cells (26). After infecting such mice with a mildly virulent cyst-forming strain of T. gondii, or with an attenuated vaccine strain of the parasite, we analyzed the resulting immune responses and determined whether passive immunization with immune serum improves resistance to the parasite.
MATERIALS AND METHODS
Mice.
C57BL/6J (B6) and C57BL/6J-Cd4tm1Knw (CD4−/−) mice were used. In one experiment, CD4-deficient B10.BR mice and B10.BR CD4-sufficient controls were used. Mice were between 8 and 12 weeks of age at the start of experiments. Unless otherwise noted, male mice were used. Mice were bred in the Animal Breeding Facility of the Trudeau Institute under barrier-sustained conditions from founder stocks obtained originally from The Jackson Laboratory, Bar Harbor, Maine. Mice were given acidified water and laboratory chow ad libitum. Sera of mice bred at the Trudeau Institute are periodically screened by the University of Missouri Research Animal Diagnostic and Investigative Laboratory to verify that the mice are free of common viral pathogens of mice. The Trudeau Institute is fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
Parasites and infections.
ME49 cysts, obtained originally as a gift from Jack Remington, Palo Alto Medical Foundation, were prepared from brains of chronically infected B6 females. Brain suspensions were prepared in Hanks balanced salt solution, cysts were counted, and suspensions were diluted appropriately so that 0.1 ml contained the desired number of cysts, which were administered with a 19-gauge gavage needle. Parasites of the ts-4 strain (30) were maintained in cultures of human fibroblasts (Hs68) at 33°C in a humidified 5% CO2 atmosphere in RPMI 1640 medium buffered with 10 mM HEPES and supplemented with 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U of penicillin G sodium/ml, 100 μg of streptomycin/ml, and 10% fetal bovine serum. RH strain tachyzoites were maintained essentially the same as were ts-4 tachyzoites, except that they were cultured at 37°C.
To initiate chronic infections, mice were fed brain suspensions containing 10 ME49 cysts. Mice were immunized by intraperitoneal (i.p.) injection of 2 × 104 ts-4 tachyzoites. Immunized mice were challenged by i.p. injection of 2 × 103 RH tachyzoites, a dose that is lethal for unimmunized mice.
Spleen cell cultures.
Suspensions of spleen cells from infected and uninfected control mice were cultured in the supplemented RPMI 1640 medium described above. Individual wells contained 6 × 105 spleen cells together with either concanavalin A (final concentration, 5 μg/ml), ts-4 tachyzoites (105/well), or medium only in a final volume of 200 μl. Supernatants were harvested after 72 h and stored frozen (−20°C) until assayed by enzyme-linked immunosorbent assay (ELISA).
Cytokine quantitation.
Cytokines (IFN-γ and IL-12p40) in sera and in spleen cell culture supernatants were quantitated by ELISA as previously described (17). Briefly, 96-well microtiter dishes were coated with capture antibodies (for IFN-γ, R4-6A2; for IL-12p40, C15.6), and progressive dilutions of samples to be assayed were added and incubated. Bound cytokines were then detected by addition of detection antibodies (for IFN-γ, polyclonal rabbit anti-IFN-γ [a kind gift of Edward Havell, North Carolina State University], followed by biotinylated goat-anti-rabbit immunoglobulin; for IL-12, biotinylated C17.8), followed by alkaline phosphatase-conjugated streptavidin and a chromogenic substrate. Unless otherwise noted, all antibodies were obtained from Pharmingen, San Diego, Calif. Plates were read in an automated ELISA plate reader. Sample absorbances were compared with those from standard curves for each cytokine which were generated from progressive dilutions of known amounts of a reference standard of each cytokine.
Serum IgG2a quantitation.
Titers of serum immunoglobulin G2a (IgG2a) specific for T. gondii were determined as described elsewhere (33). Briefly, ELISA plates were coated with soluble T. gondii tachyzoite lysate and dilutions of sera obtained from infected mice were added. After washing, alkaline phosphatase-conjugated goat-anti-mouse IgG2a was added, wells were washed, and a chromogenic substrate was added. To calculate the titers of individual samples, absorbances were read in an ELISA plate reader and compared with those on a standard curve generated from a laboratory reference standard serum whose titer was determined in each assay.
Flow cytometry of peripheral blood cells and spleen cells.
Peripheral blood cells (erythrocytes removed by hypotonic lysis) or spleen cells were analyzed by flow cytometry. The following antibodies were used: anti-CD4 [fluorescein isothiocyanate (FITC)-conjugated F(ab)′2 GK1.5], anti-CD8 [FITC-conjugated F(ab)′2 2.43], anti-CD45RB (phycoerythrin-conjugated 16A), anti-CD44 (Cy-chrome-conjugated IM7), anti-mouse IgG-IgM-IgA [FITC-conjugated F(ab)′2; Southern Biotechnology Associates], and anti-CD19 (phycoerythrin-conjugated 1D3). F(ab)′2 antibodies were conjugated at the Trudeau Institute. Unless stated otherwise, antibodies were purchased from Pharmingen. Cells were pretreated with Fc Block (Pharmingen) prior to incubation with specific antibodies. Cells were analyzed with a Becton-Dickinson FACScan flow cytometer and Cell Quest software.
Serotherapy.
Mice immunized with tachyzoites and mice chronically infected with cysts were given multiple injections of immune or nonimmune serum as detailed in the figure legends. Immune sera were obtained from male B6D2F1 mice immunized by oral inoculation with 20 ME49 cysts, followed by i.p. inoculation with 2 × 104 ts-4 tachyzoites 8 weeks later. Mice were bled after a further 4 weeks, and their sera were pooled and stored frozen until use. Nonimmune sera were obtained from unimmunized B6D2F1 mice. Titers of T. gondii-specific immune sera were determined by plate ELISA.
Statistics.
Survival data were compared by the Wilcoxon (Mann-Whitney) two-sample test. Cell numbers, cytokine levels, and antibody titers were compared by Student’s t test. In all figures, error bars represent standard deviations. Each experiment was performed at least twice.
RESULTS
CD4-deficient mice are susceptible to primary chronic infection and show impaired resistance to a challenge infection with virulent tachyzoites after immunization with vaccine strain tachyzoites.
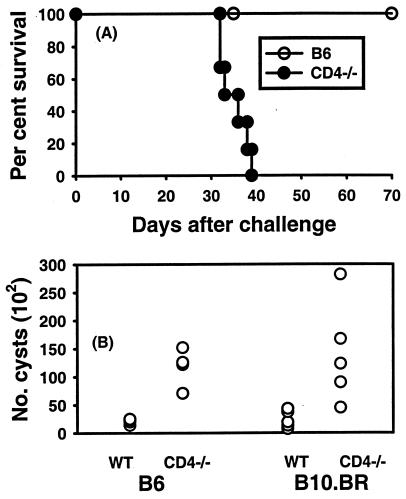
Experiments were performed to determine whether mice genetically deficient in expression of CD4 antigen were impaired in resistance to a primary peroral (p.o.) infection with T. gondii cysts and or to a secondary infection with virulent tachyzoites, similar to mice in which CD4+ cells were depleted with antibodies, as others have observed. Groups of CD4−/− mice and B6 controls were given 10 ME49 cysts p.o., and their survival was monitored. Results from a representative experiment are shown in Fig. 1A. CD4−/− mice all died within 40 days of infection, whereas B6 mice survived beyond 70 days. Brain cyst burdens were compared in p.o.-infected CD4-deficient mice and B6 controls and also in CD4-deficient B10.BR background mice and B10.BR controls 5 weeks after the mice were given ME49 cysts p.o. As shown in Fig. 1B, CD4-deficient mice had significantly more brain cysts than did CD4-sufficient controls regardless of whether the mice had the B6 genetic background (genetically susceptible to T. gondii) or the more resistant B10.BR genetic background.
FIG. 1.
(A) Survival of CD4-deficient mice and B6 mice infected p.o. with 10 ME49 cysts. Groups of six B6 and CD4-deficient mice were given 10 ME49 cysts p.o. The survival time of CD4-deficient mice was significantly shorter (P < 0.05). (B) Brain cyst burdens in infected CD4-deficient B6 and B10.BR mice. Groups of four (B6 background) or five (B10.BR background) mice were infected with 10 ME49 cysts p.o. Cyst burdens in brains were determined 5 weeks later. Significantly more cysts were found in brains of CD4-deficient mice of each strain than in brains of corresponding controls (P < 0.05). WT, wild type.
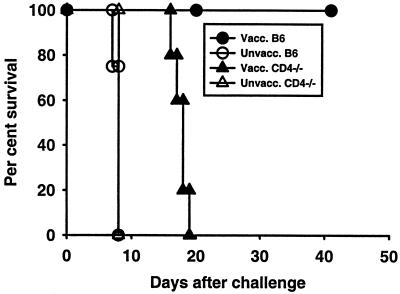
To assess acquired immunity to a challenge infection, CD4-deficient mice and CD4-sufficient B6 controls were immunized with a single injection of 2 × 104 ts-4 tachyzoites. Mice of both types survived in apparent good health for at least 100 days following such an immunization. Groups of B6 and CD4-deficient mice were also left unimmunized as controls. Eight weeks later, immunized and unimmunized mice were challenged with an i.p. injection of 2 × 103 highly virulent RH tachyzoites. Data presented in Fig. 2 show that after ts-4 immunization, B6 mice were protected whereas CD4-deficient mice were not. Essentially similar results were obtained in another experiment in which the interval between immunization and challenge was extended to 20 weeks (data not shown). ts-4-immunized, CD4-deficient mice lost body weight precipitously from day 4 after being challenged until they died or became moribund and were euthanatized (data not shown). In contrast, ts-4-immunized B6 controls exhibited only a minor, transient weight loss.
FIG. 2.
Survival of ts-4-vaccinated (Vacc.) B6 and CD4-deficient mice after a challenge with RH tachyzoites. Groups of five B6 or CD4-deficient female mice were vaccinated i.p. with 2 × 104 ts-4 tachyzoites and challenged, along with groups of five unvaccinated (Unvacc.) controls, by i.p. injection of 2 × 103 RH tachyzoites 56 days later. Vaccinated CD4-deficient mice had significantly shorter survival times than did vaccinated B6 controls (P < 0.05). Vaccinated CD4-deficient mice survived significantly longer than did unvaccinated CD4-deficient mice (P < 0.05).
Activation of CD8+ T cells and production of IL-12 are comparable in T. gondii-infected, CD4-deficient mice and B6 mice, but IFN-γ is moderately reduced.
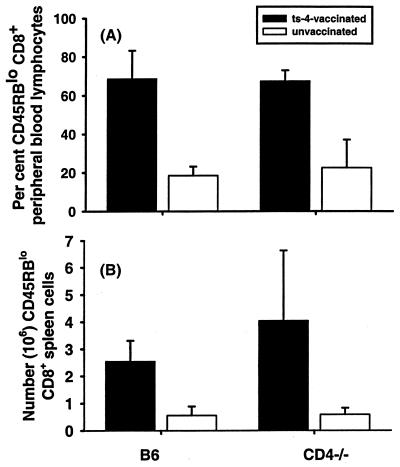
Experiments were performed to determine whether the impaired resistance to T. gondii infection in CD4-deficient mice is associated with impaired CD8+ T-cell activation or deficient production of the protective type 1 cytokines IL-12 and IFN-γ. Peripheral blood cells and spleen cells from ts-4-immunized and unimmunized CD4-deficient mice and B6 controls were analyzed by flow cytometry approximately 8 weeks after ts-4 immunization. Figure 3A shows that ts-4-vaccinated B6 and CD4-deficient mice had nearly identical percentages of peripheral blood CD8+ T cells expressing the CD45RBlo phenotype, characteristic of antigen-experienced cells, and the levels of these cells was significantly (P < 0.001) greater in immunized mice than in corresponding unimmunized controls. Quantitation of the absolute numbers of activated CD8+ peripheral blood lymphocytes revealed the same pattern of significant differences (data not shown). Similar results were found when spleen cells were analyzed (Fig. 3B). Numbers of CD8+ CD45RBlo splenic lymphocytes were not statistically significantly different in immunized B6 and CD4-deficient mice, but there were significantly more of these cells in both groups of immunized mice than in unimmunized controls.
FIG. 3.
(A) Percentage of CD45RBlo CD8+ peripheral blood lymphocytes in vaccinated and unvaccinated CD4-deficient and control B6 mice. Groups of five B6 or CD4-deficient male mice were vaccinated i.p. with 2 × 104 ts-4 tachyzoites and bled 8 weeks later. Peripheral blood leukocytes were isolated and analyzed by flow cytometry. Bars show the percentages of CD8+ lymphocytes that exhibited the CD45RBlo phenotype. These percentages were not significantly different between vaccinated CD4-deficient mice and B6 controls but were significantly greater in vaccinated mice of each strain than in corresponding unvaccinated controls (P < 0.05). (B) Numbers of CD45RBlo CD8+ splenic lymphocytes in ts-4-vaccinated and unvaccinated CD4-deficient and control B6 mice. Groups of four B6 or CD4-deficient mice were vaccinated with 2 × 104 ts-4 tachyzoites i.p., and their spleens were harvested 8 weeks later and analyzed by flow cytometry. Numbers of activated CD8+ splenic lymphocytes were not significantly different between vaccinated B6 and CD4-deficient mice, but numbers of those cells were significantly greater in spleens of vaccinated mice than in those of corresponding unvaccinated controls (P < 0.05).
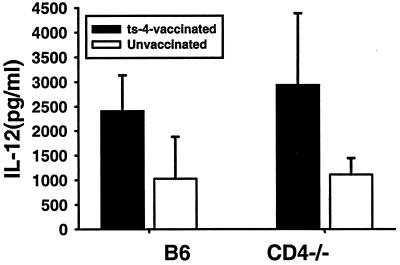
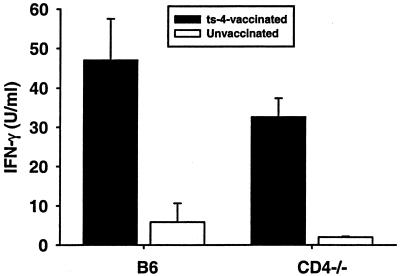
To assess type 1 cytokine levels, spleen cells from ts-4-immunized CD4-deficient mice and B6 controls, along with spleen cells from unimmunized mice of both strains, were obtained 8 weeks after mice were immunized and cells were restimulated for 3 days in vitro with ts-4 tachyzoites or medium alone. Levels of IL-12p40 and IFN-γ in culture supernatants were determined by ELISA. Levels of both of these cytokines were significantly elevated in cultures of cells from immunized mice, compared with levels in unimmunized controls (Fig. 4 and 5). The levels of each cytokine in cultures of cells from immunized B6 and CD4-deficient mice were not significantly different, although IFN-γ levels were moderately lower in the case of CD4-deficient mice. Together, these analyses revealed no gross deficiency in the ability of ts-4-immunized CD4-deficient mice to generate activated CD8+ T cells or to become primed to make IL-12 or IFN-γ upon restimulation of spleen cells with parasites. We also assayed culture supernatants for IL-10 by ELISA, but levels in all groups were beneath the limit of detection (approximately 30 pg/ml).
FIG. 4.
IL-12p40 levels in cultures of spleen cells from vaccinated CD4-deficient mice and B6 controls. Groups of four B6 or CD4-deficient mice were immunized by i.p. injection of 2 × 104 ts-4 tachyzoites. Spleen cells were harvested 8 weeks later, plated, and stimulated in vitro with ts-4 tachyzoites as described in Materials and Methods. Culture media were harvested after 72 h and analyzed for content of IL-12p40 by ELISA. IL-12 levels were not significantly different between vaccinated B6 and CD4-deficient spleen cell cultures, but levels of each were significantly higher than levels in corresponding cultures from control unvaccinated mice (P < 0.05).
FIG. 5.
IFN-γ levels in cultures of spleen cells from vaccinated CD4-deficient mice and B6 controls. Groups of four B6 or CD4-deficient mice were immunized by i.p. injection of 2 × 104 ts-4 tachyzoites. Spleen cells were harvested 8 weeks later and stimulated in vitro with ts-4 tachyzoites as described in Materials and Methods. Culture media were harvested after 72 h and analyzed for IFN-γ content by ELISA. IFN-γ levels were not significantly different between vaccinated B6 and CD4-deficient spleen cell cultures, but levels of each were significantly higher than levels in corresponding cultures from control unvaccinated mice (P < 0.05).
Analyses were also performed on peripheral blood cells and spleen cells taken from CD4-deficient mice and B6 mice that had been infected with 10 ME49 cysts p.o. between 4 and 5 weeks earlier. This interval was chosen so that analyses could be performed just before infected CD4-deficient mice became moribund (Fig. 1A). Percentages of CD8+ CD45RBlo cells in peripheral blood were equivalently elevated in both groups of mice infected p.o., similar to what was seen in ts-4-immunized mice. Numbers of CD45RBlo CD8+ spleen cells were also approximately the same in chronically infected B6 mice (2.2 × 106 ± 1.3 × 106) and CD4-deficient mice (1.8 × 106 ± 1.6 × 106). IFN-γ levels in spleen cell cultures from both B6 and CD4-deficient mice were significantly elevated above the levels seen in unimmunized controls. However, the level of IFN-γ in CD4-deficient-cell cultures (11.1 ± 4.5 U/ml) was significantly lower than that in B6 cultures (25.5 ± 6.6 U/ml). IFN-γ levels in sera of the chronically infected B6 and CD4-deficient mice were beneath the level of detection. Levels of serum IL-12 were approximately equal (between 2.5 and 3.0 ng/ml) in both infected groups.
T. gondii-infected CD4-deficient mice have reduced titers of parasite-specific serum IgG2a.
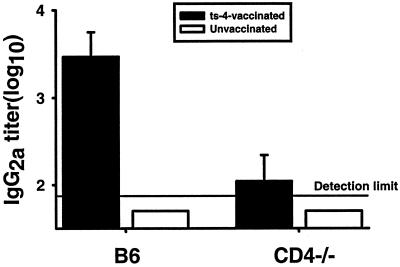
CD4+ helper T cells would be expected to be needed for generation of high titers of isotype-switched serum antibodies specific for T. gondii antigens. To investigate that requirement, we analyzed the sera of mice 8 weeks after they were inoculated with ts-4 tachyzoites. Data in Fig. 6 demonstrate that levels of T. gondii-specific serum IgG2a, the predominant isotype elicited by T. gondii infection, were barely above the limit of detection and significantly lower than that of ts-4-immunized B6 controls (P < 0.05). An almost identical pattern of results was seen in sera of immunized and unimmunized B10.BR CD4-deficient and CD4-sufficient mice that had been infected for 5 weeks with ME49 cysts (data not shown).
FIG. 6.
T. gondii-specific serum IgG2a levels in ts-4-vaccinated CD4-deficient and B6 mice. B6 and CD4-deficient mice were immunized by i.p. injection of 2 × 104 ts-4 tachyzoites and bled 8 weeks later. T. gondii-specific serum IgG2a titers were determined by ELISA. Antibody titers in immunized B6 mice were significantly (P < 0.05) higher than those in immunized CD4-deficient mice. Titers in unimmunized mice of both strains were beneath the limit of detection (log10 titer of 1.70).
Survival of ts-4-immunized, RH-challenged CD4-deficient mice, or CD4-deficient mice chronically infected with ME49 cysts is improved by administration of Toxoplasma-immune serum.
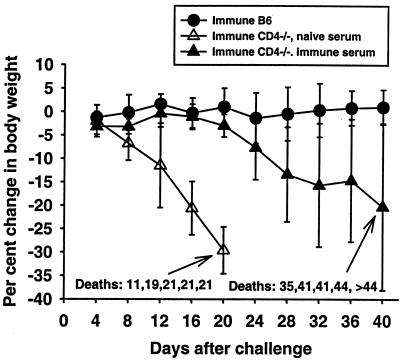
We have previously shown that the survival of ts-4-vaccinated B-cell-deficient (μMT) mice challenged with highly virulent RH tachyzoites can be significantly prolonged by administration of Toxoplasma-immune serum (33). Because CD4-deficient mice immunized with ts-4 tachyzoites generate only very low titers of T. gondii-specific serum IgG2a, we hypothesized that the survival of these mice would also be prolonged if they were given injections of Toxoplasma-immune serum when challenged with RH tachyzoites. ts-4-immunized CD4-deficient mice given a total of 6 injections (0.5 ml each) of T. gondii-immune serum (log10 IgG2a titer, 4.20) on days −1, 0, 2, 4, 7, and 10 relative to a challenge with RH tachyzoites survived significantly longer than did equivalently immunized mice treated with equal amounts of nonimmune serum (Fig. 7). However, after injections of immune serum were discontinued, the mice eventually died. To determine whether the immune sera used in these experiments contained other potentially protective constituents, we assayed immune and nonimmune sera for IFN-γ, TNF-α, and nitric oxide (as nitrite). Levels of each of these in immune sera were essentially the same as in nonimmune sera (IFN-γ and TNF-α, <125pg/ml; nitrite, approximately 35 μM) and so do not explain the differences in therapeutic effect that were seen.
FIG. 7.
Changes in body weight and survival of ts-4-immune CD4-deficient mice given immune or nonimmune serum and challenged with RH tachyzoites. Two groups of five CD4-deficient mice and one group of five B6 mice were immunized by i.p. injection of 2 × 104 ts-4 tachyzoites. Six weeks later, CD4-deficient mice were given injections of 0.5 ml of immune (log10 T. gondii-specific IgG2a titer, 4.20) or nonimmune serum on days −1, 0, 2, 4, 7, and 10 relative to an i.p. challenge with 2 × 103 RH tachyzoites. CD4-deficient mice given nonimmune serum survived for a significantly shorter time than did CD4-deficient mice given immune serum (P < 0.05).
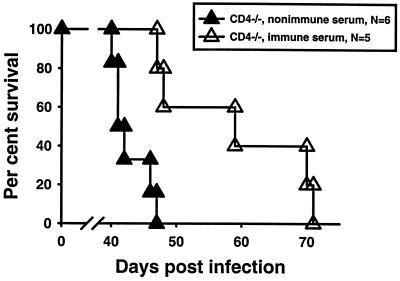
Experiments similar in concept were performed to determine whether serotherapy would prolong the survival of CD4-deficient mice chronically infected with T. gondii cysts. Groups of CD4-deficient mice and B6 mice were infected with 10 ME49 cyst p.o. After 29 days, cyst burdens in brains were checked in two mice per group to determine that heavier burdens were found in CD4-deficient mice (average of 2,400 cysts per brain) than in B6 mice (average of 750 cysts per brain). Injections of 0.5 ml of immune or nonimmune serum were initiated on day 32 of infection in the remaining CD4-deficient mice and repeated on days 36, 40, 42, 44, 46, 50, and 54 in surviving mice. As evidenced in Fig. 8, all recipients of nonimmune serum were dead by day 47 whereas recipients of immune serum survived significantly longer (P < 0.05), eventually dying between days 47 and 71. All of the infected but otherwise untreated B6 mice survived beyond 70 days in this experiment.
FIG. 8.
Survival of chronically infected CD4-deficient mice given serotherapy. Groups of CD4-deficient mice were infected with 10 ME49 cysts p.o. Mice were given i.p. injections of 0.5 ml immune or nonimmune serum on days 32, 36, 40, 42, 44, 46, 50, and 54. Mice given immune serum survived significantly longer than did mice given nonimmune serum (P < 0.05).
DISCUSSION
We have shown in this study that CD4-deficient mice have impaired resistance to both chronic primary T. gondii infection and to challenge infections after being vaccinated with ts-4 tachyzoites. Although CD4-deficient mice lack cells expressing the CD4 antigen, they may possess compensatory populations of major histocompatibility complex (MHC) class II-restricted CD8+ cells (13, 24) and significant numbers of CD4− CD8− CD3+ T cells (14). However, it is unlikely that either of these populations of cells plays a major protective role during T. gondii infection, in view of the impaired resistance of CD4−/− mice to chronic primary infection or to challenge infections with virulent tachyzoites.
A number of possible explanations can be considered for the lesser resistance to T. gondii in CD4-deficient mice. CD4+ cells may be needed as helper cells for the generation CD8+ cells, which are known to play a significant protective role against a challenge infection with virulent RH tachyzoites (10). This help might be provided by CD4+ cell-dependent “conditioning” of dendritic cells that present antigens on MHC class II molecules so that the dendritic cells become capable of activating CD8+ T cells, encountered later, that recognize antigens presented in association with MHC class I on the dendritic cells (32). Our finding that CD8+ T cells from infected CD4-deficient mice express an activated (antigen-experienced) phenotype argues against this explanation.
Despite displaying an antigen-experienced phenotype, CD8+ T cells from infected CD4-deficient mice may be unable to perform cytolysis of infected targets. Whether the cytolytic function of CD8+ cells is an important component of their protective function is not clear. ts-4-vaccinated perforin gene knockout mice were completely resistant to a challenge infection with virulent parasites but did die sooner than wild-type controls and developed heavier cyst burdens when chronically infected with ME49 cysts (5). Although we have not examined whether CD4-deficient mice immunized with ts-4 tachyzoites generate T. gondii-specific cytotoxic T lymphocytes (CTL), we infer from the cited studies that it is unlikely that the impaired resistance to RH challenge in CD4-deficient mice immunized with ts-4 tachyzoites results from an inability to generate CD4-dependent CTL activity against T. gondii-infected cells. Whether chronically infected CD4-deficient mice have impaired CTL activity against T. gondii-infected targets remains to be determined. It seems unlikely, however, that passive immunization with immune serum would overcome the susceptibility of chronically infected CD4-deficient mice, as we have seen, if it were caused by a deficiency in CTL. We also note that there are many reports of pathogen-specific CTL being generated in the absence of CD4+ cells (4, 27, 31, 32), and perhaps this is true for T. gondii.
IFN-γ is critical for protection of chronically infected mice and against RH challenge in ts-4-immunized mice, and CD4-deficient mice exhibit a modest deficiency in IFN-γ production. Therefore, it is possible that this deficiency might underlie the susceptibility to T. gondii in these mice, at least to some extent. In fact, IFN-γ responses in vivo may be even more depressed than those observed in vitro. IFN-γ is detectable for only a relatively short period in the serum of T. gondii-infected mice. For the first few days after a primary infection, IFN-γ is made independently of CD4+ or CD8+ T cells (17) but these cells produce IFN-γ a few days later (34). We compared IFN-γ levels in sera of B6 and CD4-deficient mice 8 days after feeding them cysts or injecting them with ts-4 tachyzoites i.p. We saw no significant difference in IFN-γ levels in mice fed cysts but saw significantly lower levels in CD4-deficient mice injected with ts-4 tachyzoites (data not shown). We speculate that this difference is attributable to the lack of production by CD4+ cells, which are believed to be significant producers of IFN-γ at that stage of infection (8), and in vaccinated and rechallenged mice (10). Deficient levels of IFN-γ could also result from an indirect effect on B cells in CD4-deficient mice. B cells are capable of IFN-γ production in response to T. gondii (11), and the B-cell responses to an infection may be severely affected in the absence of CD4+ cells (12). However, whatever the cause, this decrease in IFN-γ did not cause deaths of mice, as we and many others have observed in mice completely lacking IFN-γ and given ts-4, so we are uncertain of its significance.
It has been a repeatable finding in these studies that ts-4-vaccinated CD4-deficient mice survive slightly longer than unvaccinated mice when challenged with RH tachyzoites. We have not determined the basis for this effect. However, we have observed similar resistance in ts-4-vaccinated B-cell-deficient μMT mice, which appears to depend on IFN-γ. We speculate that IFN-γ, and probably CD8+ T cells too, will be found to contribute to the similar modest resistance seen in vaccinated CD4-deficient mice after an RH challenge.
The most significant finding of this study is that the deficiency in resistance to a primary p.o. infection in CD4-deficient mice can be temporarily overcome by administration of immune serum, as can the defective resistance to a challenge with virulent tachyzoites in ts-4-vaccinated CD4-deficient mice, so long as the CD4-deficient mice have been vaccinated with ts-4 tachyzoites or are chronically infected with cysts. This suggests that one of the protective functions of conventional CD4+ T cells in these models is to provide help for antibody responses to T. gondii. We have previously shown that resistance to a challenge infection with virulent RH tachyzoites can be markedly improved in ts-4-immunized B-cell-deficient mice by administration of serum from donors immunized with T. gondii (33), and others have shown that repeated injections of immune serum begun early in infection can diminish the severity of a primary infection in the chronic stage in B-cell-deficient mice (18). The present study thus lends further support to the idea that an inability to produce isotype-switched antibodies leads to impaired resistance to T. gondii. We stress, however, that antibody alone is insufficient to protect T. gondii-infected mice. It is clear from studies cited above that T cells, especially CD8+ cells, and IFN-γ are essential components of protective immunity.
We have not determined the mechanism by which antibodies, along with immune T cells, may function protectively in these experiments. However, in a previously published study, we observed that serum from T. gondii-immunized mice was able to severely limit the ability of tachyzoites to infect cells that were readily infected in the presence of nonimmune serum (33). Thus, one can speculate that antibody may limit the infection of parenchymal cells of the host, thus leaving fewer infected cells to be dealt with by cell-mediated immune mechanisms. Alternatively, antibody-coated parasites may be more readily destroyed by host effector cells (1) that have been activated by IFN-γ and TNF-α, as suggested by in vitro studies (21, 35).
Acknowledgments
We thank Paula Lanthier for excellent technical assistance.
This work was supported by U.S. Public Health Service grant AI-46571 and by funds provided by the Trudeau Institute.
Editor: J. M. Mansfield
REFERENCES
- 1.Anderson, S. E., Jr., S. C. Bautista, and J. S. Remington. 1976. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin. Exp. Immunol. 26: 375–380. [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo, F. G. 1992. Depletion of CD4+ T cells but not inhibition of the protective activity of IFN-gamma prevents cure of toxoplasmosis mediated by drug therapy in mice. J. Immunol. 149: 3003–3007. [PubMed] [Google Scholar]
- 3.Araujo, F. G. 1991. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect. Immun. 59: 1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller, R. M. L., K. L. Holmes, A. Hugin, T. N. Fredrickson, and H. C. Morse III. 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328: 77–79. [DOI] [PubMed] [Google Scholar]
- 5.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Hieny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159: 1903–1908. [PubMed] [Google Scholar]
- 6.Flano, E., D. L. Woodland, and M. A. Blackman. 1999. Requirement for CD4+ T cells in Vβ4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J. Immunol. 163: 3403–3408. [PubMed] [Google Scholar]
- 7.Frenkel, J. K., and D. W. Taylor. 1982. Toxoplasmosis in immunoglobulin M-suppressed mice. Infect. Immun. 38: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavrilescu, L. C., and E. Y. Denkers. 2001. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 167: 902–909. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149: 175–180. [PubMed] [Google Scholar]
- 10.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146: 286–292. [PubMed] [Google Scholar]
- 11.Harris, D. P., L. Haynes, P. C. Sayles, D. K. Duso, S. M. Eaton, N. M. Lepak, L. L. Johnson, S. L. Swain, and F. E. Lund. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1: 475–482. [DOI] [PubMed] [Google Scholar]
- 12.Harris, D. P., S. Kock, L. M. Mullen, and S. L. Swain. 2001. B cell immunodeficiency fails to develop in CD4-deficient mice infected with BM5: murine AIDS as a multistep disease. J. Immunol. 166: 6041–6049. [DOI] [PubMed] [Google Scholar]
- 13.Heemskerk, M. H., M. W. Schilam, H. M. Schoemaker, G. Spierenburg, W. J. Spaan, and C. J. Boog. 1995. Activation of virus-specific major histocompatibility complex class II-restricted CD8+ cytotoxic T cells in CD4-deficient mice. Eur. J. Immunol. 25: 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornquist, C. E., L. Ekman, K. D. Grdic, K. Schon, and N. Y. Lycke. 1995. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J. Immunol. 155: 2877–2887. [PubMed] [Google Scholar]
- 15.Israelski, D. M., F. G. Araujo, F. K. Conley, Y. Suzuki, S. Sharma, and J. S. Remington. 1989. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J. Immunol. 142: 954–958. [PubMed] [Google Scholar]
- 16.Johnson, A. M., P. J. McDonald, and S. H. Neoh. 1983. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J. Protozool. 30: 351–356. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, L. L., F. P. VanderVegt, and E. A. Havell. 1993. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect. Immun. 61: 5174–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α, and inducible nitric oxide synthase. J. Immunol. 164: 2629–2634. [DOI] [PubMed] [Google Scholar]
- 19.Kasper, L. H., and J. C. Boothroyd. 1993. Toxoplasma gondii and toxoplasmosis, p. 269–301. In K. Warren (ed.), Immunology and molecular biology of parasitic infections, 3d ed. Blackwell Scientific Publications, Boston, Mass.
- 20.Krahenbuhl, J. L., J. Ruskin, and J. S. Remington. 1972. The use of killed vaccines in immunization against an intracellular parasite: Toxoplasma gondii. J. Immunol. 108: 425–431. [PubMed] [Google Scholar]
- 21.Langermans, J. A., M. E. Van der Hulst, P. H. Nibbering, P. S. Hiemstra, L. Fransen, and R. Van Furth. 1992. IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J. Immunol. 148: 568–574. [PubMed] [Google Scholar]
- 22.Lindberg, R. E., and J. K. Frenkel. 1977. Toxoplasmosis in nude mice. J. Parasitol. 63: 219–221. [PubMed] [Google Scholar]
- 23.Luft, B. J., R. Hafner, A. H. Korzun, et al. 1993. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 329: 995–1000. [DOI] [PubMed] [Google Scholar]
- 24.Matechak, E. O., N. Killeen, S. M. Hedrick, and B. J. Fowlkes. 1996. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity 4: 337–347. [DOI] [PubMed] [Google Scholar]
- 25.McCabe, R. E., and J. S. Remington. 1990. Toxoplasma gondii, p. 2090–2103. In G. L. Mandell, J. Douglas, R. G., and J. E. Bennett (ed.), Principles and practices of infectious diseases, 3rd ed. Churchill Livingstone, Inc., New York, N.Y.
- 26.McCarrick, J. W., III, J. R. Parnes, R. H. Seong, D. Solter, and B. B. Knowles. 1993. Positive-negative selection gene targeting with the diphtheria toxin A-gene in mouse embryonic stem cells. Transgenic Res. 2: 183–190. [DOI] [PubMed] [Google Scholar]
- 27.Moretto, M., L. Casciotti, B. Durell, and I. A. Khan. 2000. Lack of CD4+ T cells does not affect induction of CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect. Immun. 68: 6223–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasawa, H., T. Manabe, Y. Maekawa, M. Oka, and K. Himeno. 1991. Role of L3T4+ and Lyt-2+ T cell subsets in protective immune responses of mice against infection with a low or high virulent strain of Toxoplasma gondii. Microbiol. Immunol. 35: 215–222. [DOI] [PubMed] [Google Scholar]
- 29.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1976. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 39: 365–376. [DOI] [PubMed] [Google Scholar]
- 31.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kundig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, R. M. Zinkernagel, R. G. Miller, and T. W. Mak. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353: 180–184. [DOI] [PubMed] [Google Scholar]
- 32.Ridge, J. P., F. DiRosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper cell and a T-killer cell. Nature 393: 474–478. [DOI] [PubMed] [Google Scholar]
- 33.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirahata, T., T. Yamashita, C. Ohta, H. Goto, and A. Nakane. 1994. CD8+ T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondii infection in mice. Microbiol. Immunol. 38: 789–796. [DOI] [PubMed] [Google Scholar]
- 35.Sibley, L. D., L. B. Adams, Y. Fukutomi, and J. L. Krahenbuhl. 1991. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J. Immunol. 147: 2340–2345. [PubMed] [Google Scholar]
- 36.Suzuki, Y., F. K. Conley, and J. S. Remington. 1989. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143: 2045–2050. [PubMed] [Google Scholar]
- 37.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240: 516–518. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140: 3943–3946. [PubMed] [Google Scholar]
- 39.Vollmer, T. L., M. K. Waldor, L. Steinman, and F. K. Conley. 1987. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J. Immunol. 138: 3737–3741. [PubMed] [Google Scholar]