Abstract
Schistosoma mansoni-infected wild-type (WT) mice develop a Th2 response and chronic disease. In contrast, infected interleukin-4 double-deficient (IL-4−/−) mice develop a Th1-like response and an acute, lethal syndrome. Disease severity in these animals correlates with excessive and prolonged production of nitric oxide (NO) associated with enhanced antigen-driven gamma interferon (IFN-γ) production in the absence of IL-4. Strikingly, splenic lymphocytes from infected IL-4−/− mice failed to proliferate as well as those from infected WT mice following stimulation in vitro with antigen or anti-CD3 antibody. Contrary to antigen-driven IFN-γ responses, anti-CD3 antibody stimulation of splenocytes resulted in significantly less IFN-γ being produced by CD8 cells from infected IL-4−/− mice than by those from infected WT mice or normal mice. NO is largely responsible for the impaired T-cell functions in infected IL-4−/− mice, as inhibition of iNOS significantly enhanced proliferation and IFN-γ production.
Interleukin-4 (IL-4) plays an important role in several areas of T-cell biology, including the development of Th2 responses (25) and blocking Th1 effector mechanisms mediated by gamma interferon (IFN-γ), such as inducible nitric oxide synthase (iNOS) induction (1, 10, 28, 43, 50). Further, IL-4 can inhibit NO production by upregulating arginase expression, thereby providing an alternative pathway for metabolism of l-arginine, the precursor for NO (34).
The outcome of schistosome infection in IL-4−/− animals is different from that in wild-type (WT) mice (13, 14, 18). WT mice normally develop a strong Th2 response and survive infection (14, 18). Survival is attributed to the ability of Th2 cells to orchestrate granulomatous lesions which sequester parasite eggs away from surrounding liver tissue while at the same time secreting cytokines that are selectively anti-inflammatory (4, 13, 15, 18, 24). Infected IL-4−/− mice, in contrast, fail to develop a Th2 response and produce elevated levels of inflammatory mediators, such as IFN-γ and NO, and develop severe cachexia and die despite still being able to make adequate granulomas (13, 14, 18).
An interesting feature of schistosomiasis in infected IL-4−/− mice is the failure of these animals to develop the splenomegaly that is characteristic of infection in WT mice (39). Since NO is known to inhibit T-cell proliferation (2, 3, 8, 37, 48, 50), we hypothesized that increased NO levels in IL-4−/− animals could be contributing to the lack of splenomegaly by inhibiting the proliferation of lymphocytes during infection. Our findings presented here support this view.
MATERIALS AND METHODS
Parasites and mice.
IL-4−/− C57BL/6 (B6) mice (14) were bred at Cornell University. WT B6 mice were obtained from Taconic Farms, Germantown, N.Y. Mice were anesthetized with sodium pentobarbitone intraperitoneally (i.p.), and each was infected percutaneously with approximately 70 Schistosoma mansoni cercariae (NMRI strain). Mice were weighed using a spring balance (Forestry Suppliers, Jackson, Miss.). Experiments were terminated once infected IL-4−/− mice lost ∼20% of their body weight (14); mice were euthanatized with CO2.
Cell cultures.
Spleens were harvested, and single-cell suspensions of pooled samples were prepared using sterile 70-μm-pore-size nylon mesh (Becton Dickinson, Franklin Lakes, N.J.) and Dulbecco minimal essential medium (DMEM). Erythrocytes were lysed using ammonium chloride. Viable cells were counted using trypan blue exclusion and resuspended at 107/ml in DMEM containing 30 mM HEPES, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM glutamine, 5 × 10−5 M 2-mercaptoethanol, and 10% hiFCS (complete medium). Cells were incubated alone or were stimulated with 50 μg of soluble egg antigen (SEA)/ml (11) or with plate-bound anti-CD3 monoclonal antibody (MAb) (Pharmingen, San Diego, Calif.), 0.5 μg/well, with or without aminoguanidine (AMG) (hemisulfate salt, 1 mM), in 96-well flat-bottom plates in 5% CO2 at 37°C. Cell culture supernatants were collected at 72 h and stored at −20°C until cytokine analysis was performed.
NO and cytokine measurements.
Nitrite accumulation, used as an indicator of NO production, was measured using the Griess reaction (1% sulfanilamide and 0.1% n-1-naphthylethylene-diamine) with sodium nitrite as a standard (19). Reagents for the IL-5 enzyme-linked immunosorbent assay (ELISA) were prepared in the laboratory as previously described (51). Antibodies for the IFN-γ, IL-10, and IL-13 ELISAs were purchased from Pharmingen.
Cell proliferation.
To measure cell proliferation using [3H]thymidine incorporation, 5 × 105 cells in 200 μl of complete medium per well of a round-bottom 96-well plate (Falcon, Lincoln Park, N.J.) were incubated with or without stimulation for 4 days at 37°C in 5% CO2. Cells were pulsed with 0.5 μCi of [3H]thymidine (Amersham, Arlington Heights, Ill.) per well for the last 12 h of culture and were harvested using a 96-well plate harvester. [3H]thymidine incorporation was quantitated using liquid scintillation counting. To measure proliferation using CFSE (5- and 6-carboxyfluorescein diacetate succinimidyl ester) (Molecular Probes, Eugene, Oreg.), cells were harvested and washed in serum-free medium and suspended at 107/ml in PBS containing 1.25 μM CFSE for 8 min at room temperature. The reaction was stopped by the addition of an equal volume of hiFCS after which the cells were washed extensively, recounted, suspended at 2 × 106 to 4 × 106/ml in complete medium and plated at 200 μl per well in round-bottom 96-well plates (Falcon) with or without stimulation. Cells were cultured for 5 days at 5% CO2 and 37°C and stained for surface markers as described in the flow cytometry section below.
Flow cytometry.
Cells were surface stained with either phycoerythrin-(PE-) or CyChrome-labeled anti-CD4 and anti-CD8 antibody (Pharmingen) on ice for 30 min, washed in phosphate-buffered saline (PBS) with 1% hiFCS and 0.08% NaN3, and fixed in 1% formaldehyde in PBS. Data were acquired using a FACSCaliber flow cytometer and the Cell Quest program from Becton Dickinson.
For intracellular staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) and ionomycin (500 ng/ml) for 4 h before harvesting, and Golgi export was inhibited using Golgi-Plug (according to Pharmingen’s Cytofix/Cytoperm Plus kit instructions) for 2 h prior to harvest. Cells were surface stained as described above, fixed, and permeabilized using the Pharmingen Cytofix/Cytoperm Plus kit and stained intracellularly using PE-anti-IFN-γ (Pharmingen) according to the manufacturer’s instructions. After staining, cells were resuspended in PBS with 1% fetal calf serum (FCS) and 0.08% NaN3 and analyzed as described above.
Statistical analysis.
Differences in spleen sizes between infected WT and IL-4−/− mice were assessed using nonparametric statistical analysis by the Wilcoxon signed rank test. Cytokine and NO values were evaluated using Student’s t test. P values of <0.05 were considered significant.
RESULTS
Lack of IL-4 severely impairs splenocyte proliferation during infection.
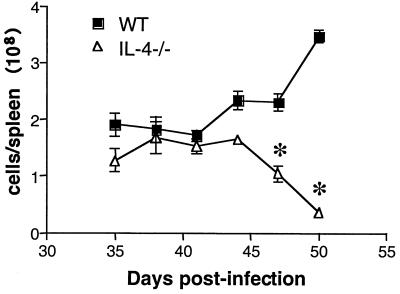
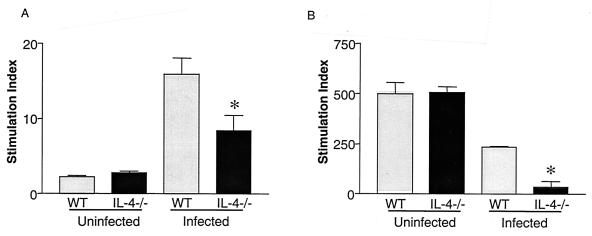
The spleens of 7-week-old infected WT mice were noticeably enlarged compared to those of uninfected controls and of infected IL-4−/− mice. To quantitate the observed differences in spleen size, spleens were weighed and the total number of nucleated cells per organ were counted. As expected, splenomegaly in WT mice was accompanied by a large increase in spleen weight (data not shown) and in splenic nucleated cell count (Fig. 1). In contrast, infected IL-4−/− mice had reduced spleen weights (data not shown) and significantly fewer nucleated cells per spleen (Fig. 1). This difference between infected WT and IL-4−/− mice was progressively more apparent as the infected IL-4−/− animals became more severely affected with time (Fig. 1). We hypothesized that this difference in spleen cell counts was due to a lymphocyte proliferation deficit in the IL-4−/− animals. Consistent with this, splenocytes from infected IL-4−/− mice incorporated significantly less [3H]thymidine following either antigen (Fig. 2A and reference 12) or anti-CD3 antibody (Fig. 2B) stimulation. To examine this issue in more detail, we labeled spleen cells with CFSE and used flow cytometry to evaluate CD4 and CD8 cell proliferation following anti-CD3 antibody-mediated T-cell receptor (TCR)-ligation. Both CD4 and CD8 cells from infected IL-4−/− mice exhibited diminished proliferative responses compared to those of WT mice (CD4 cells, 4.5% versus 11.8% [Fig. 3A]; CD8 cells, 17.25% versus 57.2% [Fig. 3B]; Table 1). This defect developed following S. mansoni infection, as spleen cells from uninfected IL-4−/− mice proliferated similarly to cells from uninfected WT mice following TCR ligation (Fig. 3).
FIG. 1.
Changes in nucleated spleen cell counts over time in infected WT and IL-4−/− mice. Mice were exposed to ∼70 cercariae, and two mice per genotype were euthanatized at the times indicated postinfection. Data represent mean ± standard error of the mean and are representative of two separate experiments. Significant differences between groups are indicated by an asterisk (P < 0.05).
FIG. 2.
[3H]thymidine incorporation by lymphocytes from normal and infected WT and IL-4−/− mice. Splenocytes from mice exposed to ∼70 cercariae 48 days earlier were pooled and cultured in vitro for 96 h with either SEA or plate-bound anti-CD3 antibody. The stimulation index was calculated by dividing counts per minute following Ag or anti-CD3 antibody stimulation by the counts per minute of unstimulated cells within either genotype. Infected IL-4−/− mice had significantly less [3H]thymidine incorporation (*, P < 0.05) than infected WT mice. Data represent mean ± standard error of the mean and are representative of three separate experiments.
FIG. 3.
Cell type analysis of proliferative responses to anti-CD3 T-cell stimulation. Pooled splenocytes from infected WT and IL-4−/− mice exposed to ∼70 cercariae 53 days earlier were labeled with CFSE and cultured with plate-bound anti-CD3 MAb ± AMG for 5 days prior to staining with anti-CD4 (A) or anti-CD8 (B) MAb and analyzed on a flow cytometer. Quadrants were set based on unstimulated samples and isotype control Ab staining. Live cells were gated based on forward and side scatter, and data are expressed as the percent of total splenocytes which were proliferating (upper left quadrant) and nonproliferating (upper right quadrant) CD4 and CD8 cells. There were two or three mice per group, and data are representative of three separate experiments.
TABLE 1.
Percentage of proliferating CD4 and CD8 cellsa
| Treatment group | Stimulant | CD4
|
CD8
|
||
|---|---|---|---|---|---|
| WT | IL-4−/− | WT | IL-4−/− | ||
| Uninfected | Anti-CD3 | 6.3 | 6.5 | 64.6 | 64.0 |
| Infected | Anti-CD3 | 11.8 | 4.5 | 57.2 | 17.25 |
| Anti-CD3+AMG | 9.6 | 8.3 | 55.1 | 43.5 | |
Values are presented as percentage of all cells that are proliferating CD4 or CD8 cells as shown in Fig. 3.
NO impairs T-cell proliferative responses in infected IL-4−/− mice.
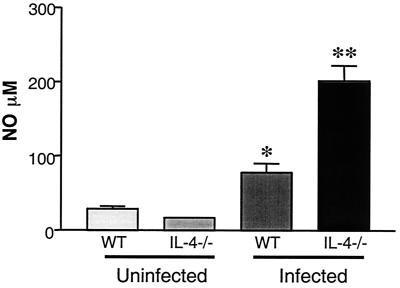
Previous studies have shown that S. mansoni infection in IL-4−/− mice is associated with elevated levels of NO in plasma and in culture supernatants from antigen- or LPS-stimulated spleen cells (12, 13, 16). This was also the case when splenic T cells were stimulated with anti-CD3 antibody where NO levels in the supernatants of the IL-4−/− cells were approximately eightfold those in WT cultures (Fig. 4). To assess the role of NO as an inhibitor of T-cell proliferation, we added AMG (a relatively selective inhibitor of iNOS) (20, 32) to spleen cell cultures and measured TCR ligation-induced proliferation (18, 22, 30). AMG inhibited NO production (data not shown) and allowed a twofold enhancement of CD4- and CD8-cell proliferation in IL-4−/− spleen cell cultures (Fig. 3 and Table 1). This demonstrates that the observed proliferation impairment in infected IL-4−/− mice was at least in part attributable to excessive NO production. The role of the Th1 response in initiating these NO-mediated effects in infected IL-4−/− mice is supported by preliminary data from our laboratory in which we found that administration of anti-IFN-γ in vivo reduced NO production and enhanced T-cell proliferation in vitro (A. C. LaFlamme, E. A. Patton, and E. J. Pearce, unpublished data).
FIG. 4.
NO production by spleen cells from infected WT and IL-4−/− mice. In vitro nitrite levels in 72-h supernatants of spleen cells cultures from mice exposed to ∼70 cercariae 47 days earlier, were measured following stimulation with plate-bound anti-CD3 MAb. IL-4−/− mice had significantly (*, P < 0.05) increased NO levels compared to WT mice. There were five mice per group. Data represent mean ± standard error of the mean and are representative of three separate experiments.
NO inhibits IFN-γ but not Th2 cytokine production by splenocytes from infected IL-4−/− mice.
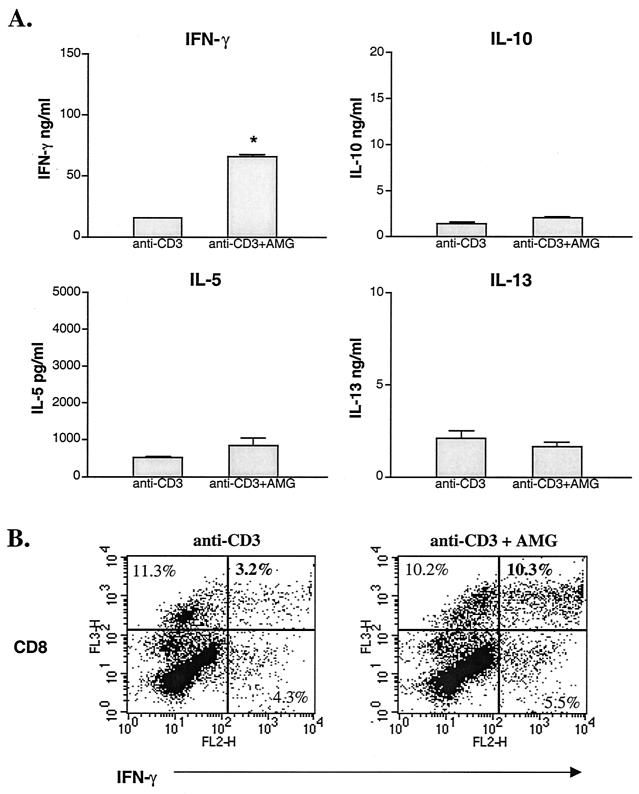
Previous studies have shown that, compared to cells from infected WT mice, splenocytes from infected IL-4−/− mice produce low levels of Th2 cytokines and more IFN-γ in response to antigen stimulation (13, 16). Paradoxically, in the absence of IL-4, T cells activated through the TCR via anti-CD3 antibody produced significantly less IFN-γ than did similarly activated WT cells (Fig. 5A). Impaired IFN-γ production was accompanied by elevated NO levels (Fig. 4). Using intracellular staining, CD8 cells from normal (WT and IL-4−/−) and infected WT mice were identified as the major producers of IFN-γ following anti-CD3 antibody stimulation (Fig. 5B). The percentage of IFN-γ-producing CD8 cells from IL-4-deficient mice, under the same stimulation conditions, was reduced by over threefold compared to cells from infected WT mice (3.2% versus 11.4%, respectively) (Fig. 5B). Overall, there was a decrease in the percentage of total CD8 cells from infected IL-4−/− mice (sum of upper quadrants in Fig. 5B). The decrease was accompanied by a decrease in the percentage of CD8 cells producing IFN-γ (Fig. 5B).
FIG. 5.
IFN-γ production by spleen cells from WT and IL-4−/− mice in response to anti-CD3 antibody stimulation. Mice were infected with ∼70 cercariae 49 days earlier. Splenocytes were pooled from two or three mice per group, and cells were cultured with plate-bound anti-CD3 MAb. (A) IFN-γ levels in 72-h culture supernatant of duplicate wells were measured by ELISA. Cytokine levels are expressed as means ± SEM. Results are representative of three separate experiments. Splenocytes from infected IL-4−/− mice produced significantly less IFN-γ than did similarly infected WT mice (*, P < 0.05). (B) IFN-γ production by CD8 cells from infected WT and IL-4−/− mice in response to anti-CD3 stimulation. Following stimulation, cells were further stimulated with PMA and ionomycin for 4 h. Intracellular cytokine production was measured using flow cytometry. Quadrants were set based on isotype control antibody staining. Live cells were gated based on forward and side scatter and data are expressed as the percent of total live cells. Results are representative of two separate experiments.
Because the inhibition of NO production by AMG treatment in vitro improved the proliferative responses of IL-4−/− T cells, we asked whether the addition of AMG would also restore the ability of these cells to produce IFN-γ. Previous studies have shown that NO can impair T-cell cytokine production in addition to proliferation (6, 34, 38, 40, 42). Inhibition of NO production in vitro enhanced IFN-γ but not Th2 cytokine production by anti-CD3 antibodies stimulated splenocytes from infected IL-4−/− mice (Fig. 6A). Most of the recovery was accounted for by an increased percentage of CD8 cells producing IFN-γ (Fig. 6B). There was no difference in IFN-γ production by CD4 cells (implied by the lack of positively stained cells in the lower right hand quadrants in Fig. 6B, and data not shown). Following anti-CD3 antibody stimulation, much higher levels of NO were accumulated in infected IL-4−/− culture supernatants than after antigen-specific stimulation (Fig. 4). At these high levels, NO may be exerting much stronger inhibitory effects on T-cell functions (proliferation and cytokine production). Therefore, NO is likely to be at least partially responsible for the low levels of IFN-γ produced following anti-CD3 antibody stimulation of spleen cells from infected mice lacking IL-4.
FIG. 6.
(A) Inhibition of NO enhanced IFN-γ but not type 2 cytokines produced by splenocytes from infected IL-4−/− mice. IL-4−/− mice were infected with ∼70 cercariae each, and spleens were harvested 50 days later and stimulated with anti-CD3 antibody ± AMG for 72 h. IFN-γ, IL-5, IL-10, and IL-13 levels were measured by ELISA in 72-h culture SN. Splenocytes from two mice were pooled and duplicate wells were analyzed. Values are expressed as means ± standard error of the mean and are representative of two separate experiments. Addition of AMG significantly increased IFN-γ production by splenocytes from infected IL-4−/− mice compared to anti-CD3 antibody stimulation alone (*, P < 0.05). (B) Effect of NO on IFN-γ production by CD8 cells from infected IL-4 mice. Splenocytes from IL-4−/− mice exposed to ∼70 cercariae 53 days earlier were stimulated with mAb anti-CD3 for 72 h and with PMA plus ionomycin for 4 h, in the presence or absence of AMG. Intracellular IFN-γ production was measured using flow cytometry. Quadrants were set based on isotype control antibody staining. Live cells were gated based on forward and side scatter, and data are expressed as the percent of total live cells. Results are representative of two separate experiments.
DISCUSSION
IL-4−/− mice are acutely susceptible to infection with S. mansoni, dying shortly after the parasites mature and begin producing eggs (14). Severe disease in these animals is accompanied by noticeably reduced splenic enlargement compared to that observed in infected WT mice. Previous studies in our laboratory have indicated a central role for elevated iNOS expression and NO and peroxynitrite production in the disease process (13, 27). NO is known to have both inflammatory and anti-inflammatory effects (9, 46). This is further illustrated in the schistosome model, where elevated NO levels have been correlated with the severity of disease in infected IL-4−/− mice (14). However, in infected WT mice, NO serves a host protective role, as treatment with AMG induces weight loss and severe hepatic damage (13). Here we identify an underlying role for NO in the inhibition of T-cell proliferation in infected IL-4−/− mice that may in part account for the reduced splenomegaly.
This work expands upon previous work in which we demonstrated that lymphocyte proliferation is impaired in schistosome-infected IL-4−/− mice (39). In this work, we demonstrate the mechanism by which this is occurring. Specifically, we show that selective inhibition of iNOS significantly increased T-cell proliferation in vitro. NO has been implicated in the inhibition of T-cell proliferation in a variety of systems (2, 3, 8, 48, 50). In a situation that is similar to that reported here, Mycobacterium tuberculosis infection in mice induces a purified protein derivative-specific CD4-cell response in which proliferation peaks within a week of infection and then declines (35). The reduction in the proliferative response could be reversed by the addition of the iNOS inhibitor NG-monoethyl-l-arginine monoacetate to spleen cell cultures (35). How NO inhibits proliferation is not yet fully understood, but it is likely to be related in part to its ability to interact with superoxide to form peroxynitrite, a strong oxidizing (7, 40, 41) and nitrating (7, 16, 21) agent that is able to nitrate tyrosines that normally, through phosphorylation, would be involved in signaling pathways immediately downstream of TCR (12). Experimentally, peroxynitrite has been shown to inhibit anti-CD3 antibody-driven activation and tyrosine phosphorylation in purified T cells by nitrating tyrosines (12). Jak3 and STAT5 proteins are implicated as targets of peroxynitrite, and their inactivation may cause a block in the IL-2 receptor signaling cascade (8, 12). In other systems, NO-mediated T-cell proliferation is often followed by T-cell apoptosis (3, 12, 33). However, analyses of T cells from infected IL-4−/− mice have failed to reveal an increased incidence of apoptosis sufficient to account for the cell number disparity between the spleens of infected WT and IL-4−/− animals (39). The outcome (impaired proliferation or apoptosis) may be dependent upon the level of NO to which T cells are exposed (50).
Cells of the monocyte/macrophage lineage and/or GR-1-positive cells have been implicated as the source of NO and/or ONOO− that inhibits T-cell proliferation (5, 8, 12, 26, 29, 30, 48, 50). Since IL-4 inhibits but IFN-γ and tumor necrosis factor alpha (TNF-α) promote iNOS expression in macrophages (10, 17, 28, 34, 43, 49), it seems likely that macrophages will be the major source of NO in the spleens of infected IL-4−/− mice. Splenic T cells from these mice produce more IFN-γ in response to Ag stimulation than those from infected WT mice. Plasma TNF-α concentrations have been demonstrated to be elevated in infected IL-4−/− animals (14). Further, macrophage-deactivating cytokines such as IL-10 and IL-13 were produced at significantly lower levels. This situation is ideal for high-level NO production by macrophages. Additionally, the percentage of the total number of spleen cells comprised of macrophages (Mac-1+ F4/80+) increased during infection in WT and IL-4−/− mice (data not shown). Thus, the elevated levels of NO produced by splenocytes from infected compared to normal mice (both WT and IL-4−/−) could be explained by the increased number of macrophages within these cultures; however, further investigation is indicated to definitively demonstrate that it is these cells that are inhibiting proliferation.
Accompanying the observation that T-cell proliferation is defective in infected IL-4−/− animals, we observed that IFN-γ production in response to anti-CD3 antibody stimulation was also significantly lower than is the case for WT mice. Given that antigen-stimulated spleen cells from infected IL-4−/− but not WT mice make IFN-γ and that antigen and anti-CD3 antibody stimulation of these cells fail to result in production of high levels of Th2 cytokines but do provoke a strong NO response, we expected that IFN-γ levels in culture supernatants of spleen cells from infected IL-4−/− mice would be significantly higher than those from infected WT cultures. Detailed analysis using flow cytometry to identify IFN-γ-producing cells confirmed earlier results showing that, during infection in WT mice, CD8 cells are the major producers of IFN-γ (38). Surprisingly, this remained true for infected IL-4−/− mice, although in these animals, the numbers of CD8 cells and the number of CD8 cells making IFN-γ were significantly reduced compared to those of WT. This is likely to be due in part to the absence in vivo of help normally provided by IL-4 in this system; we have shown that this cytokine can be an excellent helper factor for IFN-γ production by CD8 cells that have been purified from the spleens of schistosome-infected mice (38). However, additional factors are clearly playing a role since addition of IL-4 to anti-CD3 antibody-stimulated isolated CD8 cells from infected IL-4−/− mice did not significantly increase the production of IFN-γ in vitro (39). Work in other experimental infectious disease and cancer models has also revealed impaired IFN-γ production in IL-4-deficient mice (31, 45). Nevertheless, the situation during schistosomiasis in the absence of IL-4 is complex since analysis of cytokine production by CD8 cells in splenocyte populations in which NO production was blocked revealed that IFN-γ production increased (Fig. 6a and b). Others have shown that in vivo suppression of NO production can enhance antitumor cytotoxic T-lymphocyte (CTL) responses by allowing increased proliferation and clonal expansion (30). Thus, reduced IFN-γ levels may be due to a combination of poor CD8-cell proliferation and thus fewer IFN-γ-producing cells in the cultures, combined with an absence of help from IL-4. Moreover, there have been multiple reports demonstrating that NO is capable of inhibiting cytokine production by T cells (6, 36, 42, 44, 47). The role of NO in downregulating type 1 responses is underscored by research utilizing iNOS−/− mice, which develop enhanced type 1 cellular and humoral responses following vaccination with attenuated S. mansoni cercariae (22).
There was little evidence from the flow cytometric data for a dominant Th1-like CD4+ population in the spleens of infected IL-4−/− mice. This result confirms previous work by some other laboratories and supports our own finding that isolated CD4 cells from schistosome-infected IL-4−/− mice make less IFN-γ than those from infected WT mice (39). However, it contrasts with the findings of Jankovic and colleagues of sharply upregulated IFN-γ production by CD4 cells in similarly infected knockout animals (23). Possibly, differences between splenic CD4 cells (our work) and mesenteric lymph node CD4 cells (23) could explain the lack of consensus. Alternatively, subtle differences in infectious dose and time postinfection at which IFN-γ production was measured could account for the differing results. Our preliminary data from studies that carefully track the appearance of IFN-γ-producing T cells in the spleens of infected IL-4−/− mice suggest a peak of Th1-like cells early following the onset of egg production by the parasites. These Th1 cells cease to be detectable as the animals become moribund, a process that happens more rapidly in heavily infected animals (La Flamme et al., submitted for publication). One possibility to account for this apparent decline in Th1 cells during infection in the absence of IL-4 is that CD4 cells succumb to NO-induced apoptosis earlier in the course of the disease.
In conclusion, the responsiveness of T cells from schistosome-infected IL-4−/− mice is suppressed in part due to the excessive NO production that occurs in the absence of a Th2 response. Suppressed T-cell-proliferative responses due to high levels of NO in vitro may reflect a similar problem in vivo, which could account for the abnormally low spleen cell count in infected IL-4−/− mice.
Acknowledgments
This work was supported by National Institutes of Health Grant AI32573 (to E.J.P.). E.J.P. is a Burroughs Wellcome Fund Molecular Parasitology Scholar award recipient. E.A.P. was supported by National Research Service Award (F32) AI10374 and A.C.L. was supported by by F32 AI10151 while performing the work. Schistosome life cycle stages for this work were supplied through NIH-NIAID contract N01-AI-55270.
We thank Andrew MacDonald for critical review of the manuscript and Dragana Jankovic for helpful discussion.
Editor: J. M. Mansfield
REFERENCES
- 1.al-Ramadi, B. K., J. J. Meissler, D. Huang, and T. K. Eisenstein. 1992. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur. J. Immunol. 22: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 2.Albina, J. E., J. A. Abate, and W. L. Henry. 1991. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J. Immunol. 147: 144–148. [PubMed] [Google Scholar]
- 3.Allione, A., P. Bernabei, M. Bosticardo, S. Ariotti, G. Forni, and F. Novelli. 1999. Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. J. Immunol. 163: 4182–4191. [PubMed] [Google Scholar]
- 4.Amiri, P., R. M. Locksley, T. G. Parslow, M. Sadick, E. Rector, D. Ritter, and J. H. McKerrow. 1992. Tumor necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356: 604–607. [DOI] [PubMed] [Google Scholar]
- 5.Angulo, I., F. G. de las Heras, J. F. Garcia-Bustos, D. Gargallo, M. A. Munoz-Fernandez, and M. Fresno. 2000. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood 95: 212–220. [PubMed] [Google Scholar]
- 6.Bauer, H., T. Jung, D. Tsikas, D. O. Stichtenoth, J. C. Frolich, and C. Neumann. 1997. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology 90: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman, J. S., and W. H. Koppenol. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271: C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 8.Bingisser, R. M., P. A. Tilbrook, P. G. Holt, and U. R. Kees. 1998. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 160: 5729–5734. [PubMed] [Google Scholar]
- 9.Bogdan, C. 1998. The multiplex function of nitric oxide in (auto)immunity. J. Exp. Med. 187: 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdan, C., Y. Vodovotz, J. Paik, Q. W. Xie, and C. Nathan. 1994. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J. Leukoc. Biol. 55: 227–233. [DOI] [PubMed] [Google Scholar]
- 11.Boros, D. L., and K. S. Warren. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J. Exp. Med. 132: 488–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brito, C., M. Naviliat, A. C. Tiscornia, F. Vuillier, G. Gualco, G. Dighiero, R. Radi, and A. M. Cayota. 1999. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J. Immunol. 162: 3356–3366. [PubMed] [Google Scholar]
- 13.Brunet, L. R., M. Beall, D. W. Dunne, and E. J. Pearce. 1999. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J. Immunol. 163: 4976–4984. [PubMed] [Google Scholar]
- 14.Brunet, L. R., F. D. Finkelman, A. W. Cheever, M. A. Kopf, and E. J. Pearce. 1997. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159: 777–785. [PubMed] [Google Scholar]
- 15.Byram, J. E., M. J. Doenhoff, R. Musallam, L. H. Brink, and F. von Lichtenberg. 1979. Schistosoma mansoni infections in T-cell deprived mice, and the ameliorating effect of administering homologous chronic infection serum. II. Pathology. Am. J. Trop. Med. Hyg. 28: 274–285. [DOI] [PubMed] [Google Scholar]
- 16.Crow, J. P., and H. Ischiropoulos. 1996. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 269: 185–194. [DOI] [PubMed] [Google Scholar]
- 17.Drapier, J. C., J. Wietzerbin, and J. B. Hibbs, Jr. 1988. Interferon-gamma and tumor necrosis factor induce the l-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur. J. Immunol. 18: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 18.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164: 2585–2591. [DOI] [PubMed] [Google Scholar]
- 19.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126: 131–138. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, M. J., M. Messent, R. J. MacAllister, and T. W. Evans. 1993. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 110: 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ischiropoulos, H., L. Zhu, J. Chen, M. Tsai, J. C. Martin, C. D. Smith, and J. S. Beckman. 1992. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 298: 431–437. [DOI] [PubMed] [Google Scholar]
- 22.James, S. L., A. W. Cheever, P. Caspar, and T. A. Wynn. 1998. Inducible nitric oxide synthase-deficient mice develop enhanced type 1 cytokine-associated cellular and humoral immune responses after vaccination with attenuated Schistosoma mansoni cercariae but display partially reduced resistance. Infect. Immun. 66: 3510–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankovic, D., M. C. Kullberg, N. Noben-Trauth, P. Caspar, J. M. Ward, A. W. Cheever, W. E. Paul, and A. Sher. 1999. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J. Immunol. 163: 337–342. [PubMed] [Google Scholar]
- 24.Kaplan, M. H., J. R. Whitfield, D. L. Boros, and M. J. Grusby. 1998. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160: 1850–1856. [PubMed] [Google Scholar]
- 25.Kopf, M., G. Le Gros, M. Bachmann, M. C. Lamers, H. Bluethmann, and G. Kohler. 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362: 245–248. [DOI] [PubMed] [Google Scholar]
- 26.Kusmartsev, S. A., Y. Li, and S. H. Chen. 2000. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 165: 779–785. [DOI] [PubMed] [Google Scholar]
- 27.La Flamme, A. C., E. A. Patton, B. Bauman, and E. J. Pearce. 2001. Il-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 166: 1903–1911. [DOI] [PubMed] [Google Scholar]
- 28.Liew, F. Y., Y. Li, A. Severn, S. Millott, J. Schmidt, M. Salter, and S. Moncada. 1991. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur. J. Immunol. 21: 2489–2494. [DOI] [PubMed] [Google Scholar]
- 29.Mabuchi, A., M. Kitajima-Shimizu, K. Kikuchi, Y. Nakagawa, H. Takahashi, T. Kakiuchi, and K. Yokomuro. 1999. Cultured murine parenchymal liver cells induce differentiation of bone marrow cells to macrophage-like cells which present antigen to Th1 clones but inhibit their proliferation by nitric oxide and prostaglandins. Cell Immunol. 196: 14–22. [DOI] [PubMed] [Google Scholar]
- 30.Medot-Pirenne, M., M. J. Heilman, M. Saxena, P. E. McDermott, and C. D. Mills. 1999. Augmentation of an antitumor CTL response In vivo by inhibition of suppressor macrophage nitric oxide. J. Immunol. 163: 5877–5882. [PubMed] [Google Scholar]
- 31.Mencacci, A., G. Del Sero, E. Cenci, C. F. d’Ostiani, A. Bacci, C. Montagnoli, M. Kopf, and L. Romani. 1998. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J. Exp. Med. 187: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misko, T. P., W. M. Moore, T. P. Kasten, G. A. Nickols, J. A. Corbett, R. G. Tilton, M. L. McDaniel, J. R. Williamson, and M. G. Currie. 1993. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 233: 119–125. [DOI] [PubMed] [Google Scholar]
- 33.Mossalayi, M. D., P. A. Becherel, and P. Debre. 1999. Critical role of nitric oxide during the apoptosis of peripheral blood leukocytes from patients with AIDS. Mol. Med. 5: 812–819. [PMC free article] [PubMed] [Google Scholar]
- 34.Munder, M., K. Eichmann, J. M. Moran, F. Centeno, G. Soler, and M. Modolell. 1999. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 163: 3771–3777. [PubMed] [Google Scholar]
- 35.Nabeshima, S., M. Nomoto, G. Matsuzaki, K. Kishihara, H. Taniguchi, S. Yoshida, and K. Nomoto. 1999. T-cell hyporesponsiveness induced by activated macrophages through nitric oxide production in mice infected with Mycobacterium tuberculosis. Infect. Immun. 67: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nukaya, I., K. Takagi, T. Kawabe, and Y. Suketa. 1995. Suppression of cytokine production in T helper type 2 cells by nitric oxide in comparison with T helper type 1 cells. Microbiol. Immunol. 39: 709–714. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor, R. A., J. S. Jenson, and E. Devaney. 2000. NO contributes to proliferative suppression in a murine model of filariasis. Infect. Immun. 68: 6101–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedras-Vasconcelos, J. A., and E. J. Pearce. 1996. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J. Immunol. 157: 3046–3053. [PubMed] [Google Scholar]
- 39.Pedras-Vasconcelos, J. A., L. Rosa Brunet, and E. J. Pearce. 2001. Profound effect of the absence of IL-4 on T cell responses during infection with Schistosoma mansoni. J. Leukoc. Biol. 70: 737–744. [PubMed] [Google Scholar]
- 40.Radi, R., J. S. Beckman, K. M. Bush, and B. A. Freeman. 1991. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266: 4244–4250. [PubMed] [Google Scholar]
- 41.Radi, R., J. S. Beckman, K. M. Bush, and B. A. Freeman. 1991. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 288: 481–487. [DOI] [PubMed] [Google Scholar]
- 42.Roozendaal, R., E. Vellenga, D. S. Postma, J. G. De Monchy, and H. F. Kauffman. 1999. Nitric oxide selectively decreases interferon-gamma expression by activated human T lymphocytes via a cGMP-independent mechanism. Immunology 98: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sands, W. A., V. Bulut, A. Severn, D. Xu, and F. Y. Liew. 1994. Inhibition of nitric oxide synthesis by interleukin-4 may involve inhibiting the activation of protein kinase C epsilon. Eur. J. Immunol. 24: 2345–2350. [DOI] [PubMed] [Google Scholar]
- 44.Stefani, M. M., I. Muller, and J. A. Louis. 1994. Leishmania major-specific CD8+ T cells are inducers and targets of nitric oxide produced by parasitized macrophages. Eur. J. Immunol. 24: 746–752. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, Y., Q. Yang, S. Yang, N. Nguyen, S. Lim, O. Liesenfeld, T. Kojima, and J. S. Remington. 1996. IL-4 is protective against development of toxoplasmic encephalitis. J. Immunol. 157: 2564–2569. [PubMed] [Google Scholar]
- 46.Taylor, B. S., L. H. Alarcon, and T. R. Billiar. 1998. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry 63: 766–781. [PubMed] [Google Scholar]
- 47.Taylor-Robinson, A. W., F. Y. Liew, A. Severn, D. Xu, S. J. McSorley, P. Garside, J. Padron, and R. S. Phillips. 1994. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur. J. Immunol. 24: 980–984. [DOI] [PubMed] [Google Scholar]
- 48.van der Veen, R. C., T. A. Dietlin, J. Dixon Gray, and W. Gilmore. 2000. Macrophage-derived nitric oxide inhibits the proliferation of activated T helper cells and is induced during antigenic stimulation of resting T cells. Cell Immunol. 199: 43–49. [DOI] [PubMed] [Google Scholar]
- 49.van der Veen, R. C., T. A. Dietlin, F. M. Hofman, L. Pen, B. H. Segal, and S. M. Holland. 2000. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J. Immunol. 164: 5177–5183. [DOI] [PubMed] [Google Scholar]
- 50.van der Veen, R. C., T. A. Dietlin, L. Pen, and J. D. Gray. 1999. Nitric oxide inhibits the proliferation of T-helper 1 and 2 lymphocytes without reduction in cytokine secretion. Cell Immunol. 193: 194–201. [DOI] [PubMed] [Google Scholar]
- 51.Vella, A. T., M. D. Hulsebosch, and E. J. Pearce. 1992. Schistosoma mansoni eggs induce antigen-responsive CD44-hi T helper 2 cells and IL-4-secreting CD44-lo cells. Potential for T helper 2 subset differentiation is evident at the precursor level. J. Immunol. 149: 1714–1722. [PubMed] [Google Scholar]