Abstract
We have developed a system in which a foreign antigen is delivered and expressed on the surface of an attenuated strain of Erysipelothrix rhusiopathiae YS-1 and have examined the ability of a such recombinant E. rhusiopathiae strain to function as a mucosal vaccine vector. The C-terminal portion, including two repeat regions, R1 and R2, of the P97 adhesin of Mycoplasma hyopneumoniae strain E-1 was successfully translocated and expressed on the E. rhusiopathiae YS-1 cell surface after it was fused to SpaA.1, a cell surface protective antigen of E. rhusiopathiae. BALB/c mice subcutaneously immunized with the E. rhusiopathiae recombinant strains developed specific antibodies against SpaA.1 protein and were protected from lethal challenge with the highly virulent homologous E. rhusiopathiae Fujisawa-SmR strain, showing the efficacy of this heterologous-antigen expression system as a vaccine against E. rhusiopathiae infection. To determine whether protective immune responses are induced in target species, newborn, specific-pathogen-free piglets were immunized intranasally with a recombinant strain designated YS-19. The immunized piglets developed specific anti-SpaA.1 immunoglobulin G (IgG) antibodies in their serum and were protected from death by erysipelas, showing that mucosal vaccination of piglets with YS-19 induces systemic immune responses. Furthermore, YS-19-immunized piglets showed higher levels of P97-specific IgA antibodies in the respiratory tract than did YS-1-immunized piglets. Thus, E. rhusiopathiae YS-1 appears to be a promising vaccine vector for mucosal delivery that can induce local and systemic immune responses.
With the use of recombinant DNA technology, the potential exists to combine antigens within a single microorganism and to produce a vaccine for several different diseases simultaneously. This strategy is particularly attractive for the development of vaccines for veterinary fields in which easy-to-use and cost-effective vaccines are strongly required.
Erysipelothrix rhusiopathiae is a gram-positive, facultatively intracellular bacterial pathogen that can cause erysipelas in animals and erysipeloid in humans (21, 27). E. rhusiopathiae YS-1 is a stable acapsular mutant that was developed by a novel mechanism with transposon Tn916 (23). Although this strain is highly attenuated, it is able to induce both humoral and cell-mediated immunity in mice, suggesting that the strain may be suitable for use as a recombinant vaccine vector (23).
Recent advances in the development of vaccine vectors have demonstrated the importance of localization of the antigen to be expressed in the induction of efficient immune responses. To improve antigen presentation to the immune system, vaccine vectors should be engineered to express the foreign antigens either on the surface (9) or in secreted form (5, 20). In gram-positive bacteria, heterologous proteins have been successfully expressed on the bacterial cell surface by using the C-terminal sorting signal including a conserved LPXTG motif, followed by a segment of 15 to 20 hydrophobic amino acids that span the cytoplasmic membrane and a tail of mostly positively charged residues. The conserved C-terminal sorting signal has been shown to be responsible for attachment to the bacterial cell surface and has been found in more than 65 cell surface proteins of many gram-positive bacteria (2, 13). It has been found that heterologous proteins can be directed to the gram-positive bacterial cell surface when fused to the C-terminal sorting signal (4, 15), thus indicating that this engineering technique is applicable to most gram-positive bacteria if they express such surface proteins. A few of the E. rhusiopathiae surface proteins have been identified (21). However, in these proteins, the conserved C-terminal sorting signal has not been identified. We therefore investigated an alternative method by which to deliver and express heterologous protein on the E. rhusiopathiae YS-1 cell surface.
In this study, we explored the possible use of SpaA.1 (22), a cell surface protective antigen of E. rhusiopathiae (7, 8), for such applications. We replaced the central region of SpaA.1 with the C-terminal region of the P97 adhesin of Mycoplasma hyopneumoniae (30), the etiological agent of mycoplasmal or enzootic pneumonia in pigs (17). It has been shown that the AAKPV/E repeat sequence, referred to as the R1 repeat, in the C-terminal region of P97 is the cilium-binding epitope (6, 10). By using the system with SpaA.1, the C-terminal portion of P97 was successfully delivered and expressed on the E. rhusiopathiae YS-1 cell surface. We report herein that the E. rhusiopathiae YS-1 strain expressing a foreign antigen on the cell surface can be used as a mucosal vaccine vehicle for intranasal immunization of pigs.
MATERIALS AND METHODS
Bacterial and mycoplasmal strains.
The E. rhusiopathiae strains used were YS-1 (23) and its parent strain, Fujisawa-SmR (serovar 1a) (24), both of which were grown in a brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) containing 0.1% Tween 80, pH 7.6 (BHI-T80). M. hyopneumoniae E-1 (11, 12), originally isolated from a diseased swine, was used for chromosomal DNA preparation.
Construction of recombinant plasmids.
All cloning and analytical procedures were carried out in accordance with the standard protocol (18). A DNA fragment containing the R1 and R2 coding regions of P97 was amplified from the chromosomal DNA of M. hyopneumoniae E-1 by PCR with primers ADH1 (5′-AAGGTAAAAGAG AAGAAGTAG-3′) and ADH2 (5′-TTTTTACCTAAGTCAGGAAGG-3′). The PCR product was cloned into pCR2.1 (Invitrogen) to generate pCRTA23, and both strands of the cloned DNA were sequenced and confirmed. The cloned DNA fragment was amplified from pCRTA23 by PCR with primers containing the EcoRI sites, MHY1 (5′-CCCCGAATTCGTATATTACCTCAGCCCCCAG-3′) and MHY2 (5′-CCCCGAAT TCTTTTTACCTAAGTCAGGAAGG-3′). The PCR product was digested with EcoRI and then cloned in frame into pERc6.1 (22) to generate pERMh1.
The DNA fragment in pERMh1 was amplified by Long-Accurate PCR (Takara, Tokyo, Japan) with the ERM primer (5′-CCCCATCGATGATCTATT TATGAATCCAAT-3′), including an in-frame ClaI restriction site at the 5′ side, and the T7 primer (5′-GTAATACGACTCACTATAGGGC-3′). Because pERMh1 includes a ClaI site within the multicloning site of the vector, the PCR product containing the chimeric gene was digested with ClaI and cloned into the unique ClaI site of pGA14, an Escherichia coli-Streptococcus suis shuttle vector (26), thus generating pGA14/Mh1.
Electrotransformation of E. rhusiopathiae YS-1.
E. rhusiopathiae YS-1 was grown at 37°C in 100 ml of BHI-T80 to an optical density at 600 nm of approximately 0.6. Cells were then harvested by centrifugation and then washed twice with 50 ml of ice-cold distilled water and once with 50 ml of ice-cold 14% glycerol. The cells were resuspended in 0.5 ml of ice-cold 14% glycerol and either used directly for electrotransformation or stored at −80°C. The cells (70 μl) were mixed with 5 μg (1 μg/μl) of plasmid DNA, placed in prechilled Gene Pulser cuvettes (0.2-cm electrode gap; Bio-Rad, Tokyo, Japan), and pulsed immediately with a Bio-Rad Gene Pulser set at 1.25 kV, 600 Ω, and 25 μF. The mixture was immediately diluted with 1 ml of BHI and incubated at 37°C for 3 h. The cells were spread on BHI-T80 agar supplemented with erythromycin (1 μg/ml) and incubated at 37°C.
Southern hybridization.
Genomic DNAs from E. rhusiopathiae strains were digested with the restriction enzyme ClaI and transferred onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). An insert of pCRTA23, the DNA fragment encoding the C-terminal region of P97, was used for labeling as a probe. Subsequent hybridization and detection of the probe were performed with the digoxigenin DNA-labeling and detection kit (Boehringer Mannheim-Yamanouchi, Tokyo, Japan) in accordance with the manufacturer’s instructions.
Bacterial agglutination assay.
Expression of the R1 and R2 repeat regions of P97 on the E. rhusiopathiae bacterial cell surface was examined by bacterial agglutination reactions performed in a 96-well round-bottom Falcon microtiter plate (Becton Dickinson Labware). E. rhusiopathiae strains were grown overnight in 5 ml of BHI-T80 at 37°C, and 5-μl volumes of bacterial suspensions from the cultures were added to wells containing 50 μl of BHI-T80 supplemented with kanamycin (50 μg/ml). Anti-P97 monoclonal antibody (MAb) F1B6 (isotype immunoglobulin M [IgM]; CHEMICON International, Inc.) (30) or an irrelevant IgM MAb was added to the wells (final protein concentration of 35 μg/ml), and the plate was incubated at 37°C. After 16 h, bacterial agglutination was examined macroscopically.
Immunoblotting.
E. rhusiopathiae strains were grown in 1 ml of BHI-T80 at 37°C for 14 h. The cultures were centrifuged, and the supernatants were filtered (0.22-μm pore size; Millipore). The cells were washed once with ice-cold phosphate-buffered saline (PBS) and suspended in 100 μl of PBS. Four-microliter samples of cell suspension and supernatant fluid were loaded onto a nitrocellulose membrane (Schleicher & Schuell), and expression of the R1 region of the P97 adhesin on the surface of the recombinant E. rhusiopathiae strains was assayed by a dot blot immunoassay with anti-P97 MAb F1B6 (30). For the Western immunoblot test, the 100-μl bacterial suspensions were boltexed and centrifuged. Ten-microliter portions of the supernatants were mixed with 10 μl of sample buffer, boiled for 3 min, and used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. The proteins were blotted onto nitrocellulose membranes and developed by either rabbit antiserum raised against a recombinant protein including the R1 and R2 regions of P97 (diluted 1:100 in PBS) or rabbit antiserum raised against recombinant SpaA.1 (diluted 1:2,000 in PBS) (22).
Vaccine experiments with mice.
Nine- to 12-week-old female BALB/c mice (Japan SLC, Inc., Hamamatsu, Japan) were used throughout the vaccine experiments. Groups of four mice were immunized subcutaneously with 0.1-ml volumes of appropriate dilutions of bacterial suspensions. The exact doses were determined retrospectively by plating the serial dilutions of the bacterial suspensions used to inoculate the mice. As a negative control, mice were inoculated with BHI-T80. Fourteen days after the immunization, the mice were challenged with approximately 100 50% lethal doses of the virulent strain Fujisawa-SmR cells. The mice were observed for clinical symptoms and death for 14 days. To examine specific antibody responses to recombinant SpaA.1 and P97 proteins, groups of five mice were immunized with approximately 108 CFU of the strains. On days 14 and 21 after immunization, mice were bled and sera were collected and stored until needed for the enzyme-linked immunosorbent assay (ELISA).
Piglets.
Seven-day-old, specific-pathogen-free piglets were used. It was confirmed by a growth-agglutination test (19) that these piglets did not possess maternal antibodies against E. rhusiopathiae. It was also confirmed by ELISA (14) that the piglets did not possess maternal antibodies against M. hyopneumoniae. The piglets were housed separately in a pathogen-free environment maintained at a controlled temperature.
Pig experiment 1.
Nine piglets were divided into three groups (see Table 2), and each piglet from groups B and C received a dose of 109 cells of E. rhusiopathiae YS-19 in 1 ml of BHI-T80 via intranasal inoculation on day 0 while control piglets from group A received 1 ml of BHI-T80. On day 14, the piglets in group C were boosted intranasally with 109 cells of YS-19. On day 50, the piglets were inoculated intradermally with 107 cells of highly virulent E. rhusiopathiae strain Fujisawa-SmR. After the challenge, clinical signs of erysipelas, including increased body temperature, depression of activity, skin lesions, and death, were monitored and recorded. Serum samples were collected once a week from each piglet throughout the experimental period, and the IgG antibody response to SpaA.1 was analyzed by ELISA. At necropsy, the organs (heart, lungs, kidneys, spleen, liver, lymph nodes, and tonsils) collected from all piglets were examined for the presence of E. rhusiopathiae bacteria by culture in BHI-T80 supplemented with crystal violet (10 μg/ml).
TABLE 2.
Protection of piglets against erysipelas after intranasal immunization with E. rhusiopathiae YS-19
| Treatment group (no. of vaccinations)a and pig no. | Presence of clinical sign after challenge
|
||||
|---|---|---|---|---|---|
| Tempb | Erythemac | Depression | Death | Isolation of E. rhusiopathiae from organs | |
| A (0) | |||||
| 251 | +++ | +++ | Yes | Died (4)d | Yes |
| 252 | +++ | +++ | Yes | Died (4) | Yes |
| 253 | +++ | +++ | Yes | Died (4) | Yes |
| B(1) | |||||
| 254 | +++ | +++ | Yes | Euthanatized(5) | Yes |
| 255 | ++ | +++ | Yes | Died (3) | Yes |
| 256 | +++ | +++ | Yes | Died (5) | Yes |
| C(2) | |||||
| 257 | − | ± | No | No | |
| 258 | + | + | No | No | |
| 259 | − | ± | No | No | |
Seven-day-old SPF piglets were immunized intranasally with 109 CFU of YS-19 on day 0 and boosted on day 14 (group C only). The piglets were challenged intradermally with 107 CFU of E. rhusiopathiae Fujisawa-SmR on day 50.
Maximum measured rectal temperature. −, <40°C; +, 40 to 41°C; ++, 41 to 42°C; +++, >42°C.
±, a skin lesion at the inoculation site; +, a few localized skin lesions in addition to the lesion at the inoculation site; +++, systemic erythema.
The value in parentheses indicates the day when the piglet died or was sacrificed after the challenge.
For detection of SpaA.1-specific antibodies in serum by ELISA, the recombinant SpaA.1 protein was diluted to a 10-μg/ml concentration in carbonate buffer (pH 9.6) and used to coat the wells of polystyrene plates (100 μl/well; Nunc Immunoplates with a MaxiSorp surface). After overnight incubation at 4°C, the plates were washed four times in a buffer solution (0.85% NaCl, 0.05% Tween 20) and blocked with 1% bovine serum albumin in PBS. After 30 min of incubation at room temperature, the blocking solution was discarded and the diluted serum samples (1:400 in PBS) were added to the wells. Each sample was tested in triplicate wells. The plates were incubated for 1 h at 37°C and washed four times. In the mouse experiments, the bound antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulins (Zymed Laboratories, Inc.). In the pig experiments, horseradish peroxidase-conjugated goat anti-swine IgG (ICN Pharmaceuticals, Inc.) was used to detect the bound antibodies.
Pig experiment 2.
Six piglets (group A) were vaccinated intranasally with 5 × 108 cells of E. rhusiopathiae YS-19 in 1 ml of BHI-T80 on day 0. After 2 weeks, the piglets were boosted with 4 × 108 cells of strainYS-19. As a control, three piglets (group B) were intranasally vaccinated with 2 × 108 cells of YS-1 on the same days. Two weeks after boosting, the piglets from groups A and B were sacrificed by intravenous injection of sodium pentobarbital and their lungs, including the trachea and bronchi, were collected.
To obtain IgA antibodies in the respiratory tract, 20 ml of sterile PBS was injected into the trachea and the lungs were massaged for about 1 min. The washing fluids were then aspirated. The fluids recovered (5 to 10 ml) were centrifuged, and the concentration of total IgA antibodies in the supernatant of the respiratory tract washing fluids was measured by use of a commercially available ELISA kit (Bethyl Laboratories, Inc.) in accordance with the manufacturer”s directions. To determine the concentration of P97-specific IgA antibodies in the supernatant of the respiratory tract washing fluids, the fluids were diluted with PBS to a final concentration of total IgA protein of 1.5 μg/ml and analyzed by ELISA. A recombinant protein including the R1 and R2 regions of P97 was used to coat the wells of polystyrene plates (100 μl/well; Nunc Immunoplates with a MaxiSorp surface) at a concentration of 600 ng/well. After overnight incubation at 4°C, the plates were washed three times in a buffer solution (0.005% Tween 20, PBS) (T-PBS) and blocked with T-PBS containing 5% bovine serum. After 1 h of incubation at 37°C, the blocking solution was discarded and the plate was washed three times with T-PBS. One hundred microliters of the PBS-diluted fluid was added to the wells, and the plate was incubated for 1 h at 37°C. Each sample was tested in triplicate wells. After three washes with T-PBS, the bound antibodies were detected by horseradish peroxidase-conjugated goat anti-swine IgA alpha chain antibody (Bethyl Laboratories, Inc.) diluted with T-PBS containing 5% bovine serum (1:500). The results were expressed as the mean optical density at 450 nm ± the standard error of the mean (SEM).
Statistical analyses.
Statistical significance was determined by Student’s unpaired t test, except in the case of the animal immunization and challenge experiments, in which significance was assessed by Fisher’s exact test.
RESULTS
Construction of recombinant E. rhusiopathiae strains expressing the R1 and R2 regions on the cell surface.
We have previously cloned and characterized the spaA.1 gene that encodes a protective antigen of E. rhusiopathiae (22). To deliver and express the cilium-binding epitope of P97 adhesin on the cell surface of E. rhusiopathiae YS-1, we utilized the SpaA.1 molecule as a carrier of the heterologous protein to the bacterial surface.
The central region of the spaA.1 gene was deleted and replaced in frame with the DNA fragment encoding the cilium-binding domain of P97 adhesin. The resulting chimeric gene, which generates a hybrid protein containing the R1 and R2 repeat regions flanked by SpaA.1 domains, which constitute the protection-eliciting domain on the N terminus (22) and the cell surface-anchoring domain on the C terminus (8), was cloned in pGA14, an E. coli-S. suis shuttle vector (26). pGA14 is a pWV01-based plasmid and has been shown to replicate in a considerable number of gram-positive bacteria (26). We found that pGA14 can also replicate within E. rhusiopathiae bacteria but that it is unstable in the absence of erythromycin. We took advantage of its instability for the isolation of E. rhusiopathiae strains in which the chimeric gene is integrated into the chromosome by a single-crossover recombination event.
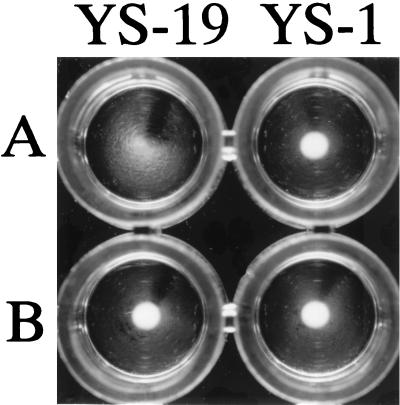
The chimeric gene in pGA14/Mh1 was introduced into E. rhusiopathiae YS-1 by electroporation, and transformants were selected for erythromycin resistance. Unexpectedly, in contrast to the transformation efficiency obtained with pGA14, the number of transformants obtained with pGA14/Mh1 decreased drastically and only one erythromycin-resistant strain, designated YS-5, was obtained. YS-5 was passaged in BHI-T80 for overnight cultivation for a week in the absence of erythromycin, and then 108 cells of YS-5 were plated for selection of erythromycin-resistant strains. Sixty-one clones were selected from the colonies that appeared in the selective plating and were examined for expression of the R1 region on the cell surface by bacterial dot blotting with F1B6 (30), a MAb specific to the R1 region of the P97 adhesin. The results showed that two clones, designated YS-19 and YS-24, were positive in the dot blotting assay with MAb F1B6 (data not shown), suggesting that in the positive strains, the hybrid protein was expressed on the cell surface. The expression of the R1 region on the cell surface of YS-19 was confirmed in a bacterial agglutination assay with MAb F1B6 (Fig. 1).
FIG. 1.
Agglutination of E. rhusiopathiae YS-19 expressing the C-terminal portion of the P97 adhesin. Strain YS-19 and parental strain YS-1 were grown in BHI-T80 in the presence of anti-P97 MAb F1B6 (A) or an irrelevant IgM MAb (B).
To further confirm the expression of the hybrid protein in these strains, a Western blot analysis of cell surface extracts was performed with anti-P97 or anti-SpaA.1 rabbit serum. Doublet bands of native SpaA.1 protein were detected at 69 kDa. In addition, doublet bands at 67 kDa, corresponding to the hybrid protein consisting of P97 fused to SpaA.1, were also detected by anti-SpaA.1 rabbit serum (Fig. 2). It was found that the lower band in the doublet disappeared when samples from the bacteria were used that had been grown for a shorter duration (data not shown). These findings suggest that the lower band was the degraded or normally processed form of the fusion protein.
FIG. 2.
Expression of the R1 and R2 regions of P97 in E. rhusiopathiae YS-19. Western immunoblots were developed with either anti-P97 rabbit serum (diluted 1:100) or anti-SpaA.1 rabbit serum (diluted 1:2,000) (27). Molecular size markers (in kilodaltons) are shown on the left. Data are not shown for YS-24.
Southern hybridization of the genomic DNAs from YS-19 and YS-24 using the P97 gene as a probe revealed that the chimeric gene was integrated into the chromosome of the strains (Fig. 3). Taken together, these results suggest that in these strains, the chimeric gene is integrated into the chromosome via a single-crossover recombination event, resulting in the original SpaA.1-positive phenotype. The YS-19 strain was passaged without selection, and expression of the hybrid protein on the strain was confirmed by bacterial dot blotting. Our results show that expression of the hybrid protein was not lost after growth without selection for more than 50 generations, suggesting that these recombinants are genetically stable.
FIG. 3.
Detection of the p97 gene in E. rhusiopathiae strains by Southern hybridization. The amplified DNA fragment encoding the C-terminal regions of R1 and R2 of the p97 gene was labeled and used as a probe. Genomic DNAs of E. rhusiopathiae strains were digested with ClaI and transferred onto a nitrocellulose membrane. Lanes: M, DNA molecular size marker VII (Roche Diagnostics Corporation); 1, YS-1; 2, YS-19. A marker size is shown on the left. Data are not shown for YS-24.
Virulence and vaccine efficacy of recombinant E. rhusiopathiae strains in mice.
To examine the protective capabilities of recombinant YS-19 and YS-24 strains against infection, BALB/c mice were immunized subcutaneously with appropriate dilutions and then challenged with the virulent Fujisawa-SmR strain. It was found that during the vaccination period, all of the vaccinated mice remained healthy and there was no difference in the level of protection between the mice vaccinated with the recombinant strains and those vaccinated with strain YS-1 (Table 1).
TABLE 1.
Protection of mice immunized with E. rhusiopathiae strains against lethal challenge with a homologous virulent straina
| Immunization | Immunizing dose (CFU) | No. of survivors/total |
|---|---|---|
| YS-1 | 108 | 4/4b |
| 106 | 4/4b | |
| 104 | 3/4 | |
| YS-19 | 108 | 4/4b |
| 106 | 4/4b | |
| 104 | 4/4b | |
| YS-24 | 108 | 4/4b |
| 106 | 4/4b | |
| 104 | 3/4 | |
| Control | 0/4 |
Groups of four BALB/c mice were immunized subcutaneously with 0.1-ml volumes of appropriate dilutions of the bacterial suspensions. After immunization, mice were challenged subcutaneously with approximately 100 50% lethal doses of the Fujisawa-SmR strain and observed for clinical symptoms and death for 14 days.
Significant difference (P < 0.05) compared to the control determined by Fisher’s exact test.
Sera were taken from individual mice at 2 and 3 weeks after immunization with either recombinant E. rhusiopathiae YS-19 or YS-24 and tested by ELISA for specific antibodies to SpaA.1 and regions R1 and R2 of the P97 protein. As shown in Fig. 4A, mice immunized with strains YS-19 and YS-24 developed the same levels of SpaA.1-specific antibodies at 2 and 3 weeks after immunization as did those immunized with strain YS-1. The levels of antibody to the C-terminal portion of the P97 protein were slightly increased at 3 weeks after inoculation. However, the differences between YS-1 and YS-19 or YS-24 were not found to be significant (Fig. 4B). As we could not detect any differences between the phenotypes of the two recombinant strains, the YS-19 strain was used for the following experiments.
FIG. 4.
ELISA detection of SpaA.1-specific (A) and P97-specific (B) antibodies in the sera of mice vaccinated with E. rhusiopathiae strains. Results are expressed as the mean ± the standard error of the mean of five mice. Asterisks indicate significant differences (P < 0.01) compared to the control determined by using Student”s unpaired t test. OD 450, optical density at 450 nm.
Pig experiment 1: virulence and vaccine efficacy of YS-19 against erysipelas.
Seven-day-old newborn piglets were intranasally vaccinated once or twice with YS-19, and the virulence of the strain was then examined. It was found that all of the piglets vaccinated with the strain displayed neither an elevated body temperature nor loss of appetite, demonstrating that the vaccine strain was safe even when very young piglets received the highest dose. The YS-19-immunized piglets were challenged with the virulent E. rhusiopathiae Fujisawa-SmR strain, and its vaccine potential was then evaluated. As shown in Table 2, both unvaccinated control pigs (group A) and piglets receiving a single dose of the vaccine (group B) developed severe clinical signs, with five piglets dying of septicemia within 5 days after challenge, and one piglet was euthanatized. Piglets that received two doses of the vaccine (group C) did not develop erysipelas. Only one piglet (no. 258) had an elevated temperature on day 3 after the challenge and showed localized erythema near the site of inoculation until 4 days after the challenge. However, the erythema in the latter piglet disappeared on day 5. These piglets remained healthy for 14 days after the challenge, and all of them survived. Thus, the piglets that had received two doses of the vaccine did not show any clinical symptoms. However, no statistically significant differences in mortality were observed due to the small sample size. Bacterial examination revealed that the challenge E. rhusiopathiae strain Fujisawa-SmR was isolated from the organs of all of the unprotected piglets but not from those of the piglets in group C. No vaccine strain was recovered from any of the organs examined in any of the piglets. ELISA revealed significant IgG responses to SpaA.1 in the serum of the protected piglets (groups C) but not in that of the unprotected piglets in groups A and B (Fig. 5).
FIG. 5.
ELISA detection of SpaA.1-specific IgG antibodies in the sera of piglets vaccinated intranasally with E. rhusiopathiae YS-19. Results are expressed as the mean ± the standard error of the mean of three piglets. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) compared to the controls determined by using Student’s unpaired t test. OD 450, optical density at 450 nm.
Pig experiment 2: antigen-specific IgA response in the respiratory tract.
We examined whether intranasal vaccination of piglets with YS-19 induces a mucosal immune response that may result in protection against mycoplasmal infection. Piglets were intranasally immunized with either YS-19 or YS-1, and the concentration of P97-specific IgA antibodies in the respiratory tract washing fluids was determined. The result showed that the concentration of P97-specific IgA antibodies in the respiratory tract washing fluid from the YS-19-immunized group was 1.12 ± 0.09 versus 0.80 ± 0.11 in that of the control group. However, no statistically significant difference was observed, which may be due to the small sizes of the groups examined.
DISCUSSION
Because most infections begin at mucosal sites, immunization via mucosal routes is an attractive strategy for blocking of pathogens at the entry point. Mucosal immunization of whole bacteria, especially intranasal immunization, has been shown to elicit strong local and systemic immune responses (16). Recently, we developed an acapsular, attenuated strain, E. rhusiopathiae YS-1, for live erysipelas vaccines (23). In contrast to the virulent parental Fujisawa-SmR strain, acapsular E. rhusiopathiae mutant strains fail to resist phagocytosis (24) and cannot survive within macrophages (25); such observations show why acapsular strains of this sort are highly attenuated. The acapsular YS-1 strain has been shown to induce strong humoral and cell-mediated immune responses in mice when delivered subcutaneously (23), suggesting that the strong immunogenicity of the strain may make it suitable for use as a recombinant vaccine vector. This finding led us to propose that E. rhusiopathiae YS-1, like other enteric pathogens, such as Salmonella spp., may have the ability to induce strong local and systemic immune responses when administered via a mucosal route.
To test this hypothesis, we chose M. hyopneumoniae P97 adhesin for delivery to the mucosal surface by use of E. rhusiopathiae YS-1 as a vaccine vector. In our system, SpaA.1, a cell surface protective antigen of E. rhusiopathiae, was used to carry the P97 adhesin to the bacterial cell surface. As has been shown in Streptococcus pneumoniae PspA (29) and LytA (3), the tandem repeats, starting with the dipeptide GW, which has also been found in the C-terminal repeating region of the SpaA.1 protein, have been suggested to be involved in cell wall association (1). On the basis of this cell surface display system, we deleted and replaced the DNA segment encoding the central, surface-exposed domain, but not that involved in protection of the SpaA.1 molecule, with the DNA fragment encoding the C-terminal region of the P97 adhesin. The chimeric gene integrated into the chromosome was found to be properly translocated and expressed on the YS-1 bacterial cell surface, suggesting that our engineering technique may also be applicable to other gram-positive bacteria. In the recombinant E. rhusiopathiae strains, designated YS-19 and YS-24, expression of the native spaA.1 gene was found not to be hampered by the presence of the chimeric gene, demonstrating that the recombinant strains express an additional protection-eliciting domain of the SpaA.1 molecule. Thus, our genetic system allowed recombinant strains to be stable in the absence of selective pressure, which may have important consequences in the induction of protective immunity against erysipelas.
Protection studies done with mice have demonstrated that the recombinant E. rhusiopathiae strains expressing the mycoplasmal antigen on the cell surface are safe and effective as a vaccine against erysipelas, suggesting that fusion of the foreign protein to SpaA.1 alters neither the attenuation characteristic of the YS-1 strain nor its ability to protect against infection. Furthermore, the virulence and vaccine potential of recombinant strain YS-19 was confirmed in a natural host by intranasal vaccination. These data support our hypothesis that intranasal vaccination of the YS-19 strain indeed induces a systemic immune response. This observation is very important for E. rhusiopathiae-based vaccine vectors because serum IgG antibodies play an important role as an opsonin in the prevention of infection (21, 28).
We also examined whether intranasal vaccination of piglets with YS-19 induces a local immune response that may result in protection against mycoplasmal infection. Although no significant difference was observed, intranasal vaccination with YS-19 induced antigen-specific IgA antibodies in the respiratory tract. We found that P97-specific IgA antibodies in serum from YS-19-immunized piglets were below the detection limit of the assay (data not shown). Taken together, these results suggest that the IgA antibodies detected in the respiratory tract were locally produced. Thus, it was found that the P97 antigen expressed on the cell surface is accessible to the immune system and that it is able to stimulate a local immune response. Our findings suggest that the present delivery system may be broadly applicable to a variety of antigens of interest.
In this study, we could not isolate the YS-19 strain from pig organs. In spite of such low infectivity, the strain was found to induce local and systemic immune responses in the experimental piglets. We cannot account for the immunogenicity mechanisms of the strain. However, in our preliminary experiment with YS-1, we were able to isolate the bacteria from the tonsils of one of the three pigs used 2 weeks after intranasal vaccination (data not shown). This finding suggests that the YS-19 strain may have been able to colonize the tonsils to such a degree that it stimulated the immune system but was cleared from the organs at the time of necropsy. It remains unclear whether the YS-19 strain can colonize the tonsils or other organs.
Finally, we constructed recombinant E. rhusiopathiae strains expressing a hybrid protein consisting of M. hyopneumoniae P97 adhesin fused to SpaA.1 on the bacterial cell surface. The animal experiments demonstrated that recombinant strain YS-19 is safe, even in newborn, specific-pathogen-free piglets, and that it induces local and systemic immune responses in these animals when administered intranasally. We are currently investigating the efficacy of YS-19 as a vaccine against enzootic pneumonia.
Acknowledgments
We thank H. E. Smith (Institute for Animal Science and Health, Lelystad, The Netherlands) for donating plasmid pGA14.
This work was supported in part by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Cossart, P., and R. Jonquieres. 2000. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 97: 5013–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischetti, V. A. 2000. Surface proteins on gram-positive bacteria, p. 11–24. In V. A. Fischetti et al. (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 3.Garcia, P., J. L. Garcia, E. Garcia, and R. Lopez. 1986. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene 43:265–272. [DOI] [PubMed] [Google Scholar]
- 4.Hansson, M., S. Stahl, T. N. Nguyen, T. Bachi, A. Robert, H. Binz, A. Sjolander, and M. Uhlen. 1992. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. J. Bacteriol. 174:4239–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess, J., I. Gentschev, D. Miko, M. Welzel, C. Ladel, W. Goebel, and S. H. Kaufmann. 1996. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc. Natl. Acad. Sci. USA 93:1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu, T., S. Artiushin, and F. C. Minion. 1997. Cloning and functional analysis of the P97 swine cilium adhesin gene of Mycoplasma hyopneumoniae. J. Bacteriol. 179:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitajima, T., E. Oishi, K. Amimoto, U. Satoshi, H. Nakamura, K. Oda, S. Katayama, A. Izumida, and Y. Shimizu. 2000. Quantitative diversity of 67 kDa protective antigen among serovar 2 strains of Erysipelothrix rhusiopathiae and its implication in protective immune response. J. Vet. Med. Sci. 62:1073–1077. [DOI] [PubMed] [Google Scholar]
- 8.Makino, S., K. Yamamoto, S. Murakami, T. Shirahata, K. Uemura, T. Sawada, H. Wakamoto, and Y. Morita. 1998. Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb. Pathog. 25:101–109. [DOI] [PubMed] [Google Scholar]
- 9.Medaglini, D., G. Pozzi, T. P. King, and V. A. Fischetti. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. USA 92:6868–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minion, F. C., C. Adams, and T. Hsu. 2000. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 68:3056–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori, Y., Y. Yoshida, C. Kuniyasu, and K. Hashimoto. 1983. Improvement of complement fixation test antigen for the diagnosis of Mycoplasma hyopneumoniae infection. Natl. Inst. Anim. Health Q. 23:111–116. [PubMed] [Google Scholar]
- 12.Mori, Y., T. Hamaoka, S. Sato, and S. Takeuchi. 1988. Immunoblotting analysis of antibody response in swine experimentally inoculated with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 19:239–250. [DOI] [PubMed] [Google Scholar]
- 13.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolet, J., P. Paroz, and S. Bruggmann. 1980. Tween 20 soluble proteins of Mycoplasma hyopneumoniae as antigen for an enzyme linked immunosorbent assay. Res. Vet. Sci. 29:305–309. [PubMed] [Google Scholar]
- 15.Pozzi, G., M. Contorni, M. R. Oggioni, R. Manganelli, M. Tommasino, F. Cavalieri, and V. A. Fischetti. 1992. Delivery and expression of a heterologous antigen on the surface of streptococci. Infect. Immun. 60:1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renauld-Mongenie, G., N. Mielcarek, J. Cornette, A. M. Schacht, A. Capron, G. Riveau, and C. Locht. 1996. Induction of mucosal immune responses against a heterologous antigen fused to filamentous hemagglutinin after intranasal immunization with recombinant Bordetella pertussis. Proc. Natl. Acad. Sci. USA 93:7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross, R. F. 1992. Mycoplasmal diseases, p.535–549. In A. D. Leman et al. (ed.), Diseases of swine. Iowa State University Press, Ames, Iowa.
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Seto, K., K. Ando, Y. Nishimura, M. Fujiki, and T. Matsuo. 1965. Studies on the swine erysipelas. Evaluation of growth agglutination and killed cell agglutination. Annu. Rep. Vet. Assay Lab. 4:151–154. [Google Scholar]
- 20.Shen, H., J. F. Miller, X. Fan, D. Kolwyck, R. Ahmed, and J. T. Harty. 1998. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92:535–545. [DOI] [PubMed] [Google Scholar]
- 21.Shimoji, Y. 2000. Pathogenicity of Erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microbes Infect. 2:965–972. [DOI] [PubMed] [Google Scholar]
- 22.Shimoji, Y., Y. Mori, and V. A. Fischetti. 1999. Immunological characterization of a protective antigen of Erysipelothrix rhusiopathiae: identification of the region responsible for protective immunity. Infect. Immun. 67:1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoji, Y., Y. Mori, T. Sekizaki, T. Shibahara, and Y. Yokomizo. 1998. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: use of excision of Tn916 to inactivate a target gene. Infect. Immun. 66:3250–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoji, Y., Y. Yokomizo, T. Sekizaki, Y. Mori, and M. Kubo. 1994. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect. Immun. 62:2806–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimoji, Y., Y. Yokomizo, and Y. Mori. 1996. Intracellular survival and replication of Erysipelothrix rhusiopathiae within murine macrophages: failure of induction of the oxidative burst of macrophages. Infect. Immun. 64:1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, H. E., H. J. Wisselink, U. Vecht, A. L. Gielkens, and M. A. Smits. 1995. High-efficiency transformation and gene inactivation in Streptococcus suis type 2. Microbiology 141:181–188. [DOI] [PubMed] [Google Scholar]
- 27.Wood, R. L. 1992. Erysipelas, p. 475–486. In A. D. Leman et al. (ed.), Diseases of swine. Iowa State University Press, Ames.
- 28.Yokomizo, Y., and Y. Isayama. 1972. Antibody activity of IgM and IgG fractions from rabbit anti-Erysipelothrix rhusiopathiae sera. Res. Vet. Sci. 13:294–296. [PubMed] [Google Scholar]
- 29.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]