Abstract
Cell-mediated immunity during the muscular phase of Trichinella infection in humans was studied. Cell proliferation, the phenotypic changes in the T-cell population, and expression and production of cytokines were examined by using peripheral blood mononuclear cells (PBMC) collected at different times postinfection from 10 individuals who had acquired Trichinella spiralis and five individuals who had acquired Trichinella britovi in two distinct outbreaks. T. spiralis and T. britovi crude worm extracts induced proliferation of PBMC from T. spiralis- and T. britovi-infected donors. Cytokine gene expression showed a predominant type 2 pattern for the entire period of infection studied, although gamma interferon (IFN-γ) was expressed. Interleukin-2 (IL-2), IL-5, IL-10, and IFN-γ production was found in PBMC of all donors. There was a good correspondence between the cytokine expression and production patterns. Changes in PBMC composition, with a trend toward an increase in CD8+ lymphocyte counts, were observed.
The genus Trichinella is a group of nematodes which have a worldwide distribution and which cause trichinellosis in humans (15). Humans are infected by eating raw or undercooked meat or meat products (sausages, salami, etc.) from swine, horses, or game animals that are infected with larvae of these worms. In Italy, the main etiological agent of human trichinellosis is Trichinella britovi; however, some human infections have been attributed to consumption of products from imported animals infected with Trichinella spiralis (17).
From 4 to 5 days after ingestion of infected meat, the larvae develop to the adult stage in a row of columnar epithelial cells in the small intestine. Six days after ingestion, the adult worms begin to reproduce, and the newborn larvae migrate across the lamina propria of the villus into the lymph and bloodstream. However, only the newborn larvae that reach striated muscles develop to the infective stage. In laboratory mice, the intestinal phase is longer for T. spiralis than for T. britovi and the female fecundity of T. spiralis is at least twice than that of T. britovi. These differences explain the different clinical pictures obtained for humans infected with these two species (20). Intestinal symptomatology (diarrhea, abdominal pain, and vomiting) occurs more frequently and lasts longer in human T. spiralis infections than in human T. britovi infections (20).
When a larva penetrates a muscle cell, it causes the cell to become what is known as a nurse cell, and it becomes infective 15 to 16 days later (i.e., 21 to 22 days after ingestion of infected meat) (6).
After ingestion of larvae, a host develops an immunity-mediated inflammatory response at the intestinal level, and this is followed by rapid expulsion of some of the larvae from the intestine (3). Studies conducted with mouse and rat models have suggested that antibodies play the most important role in this response, which depends on type Th2 cytokines derived from CD4+ cells, and that mucosal mast cells also play an important role (11). Moreover, it appears that during the host intestinal response, the CD4+ cells initially produce type Th1 cytokines and that type Th2 cytokines are produced only later (10, 12). Altered biological parameters (muscular enzymes, eosinophilia, and leukocytosis) and specific immunoglobulin G (IgG) persist longer in people infected with T. spiralis than in people infected with T. britovi (20).
In humans, larvae in the striated muscles can remain infective for up to 30 years (8). One of the most distinctive features of Trichinella infection is its chronicity, and the resulting persistent antigenic stimulation could lead to polarization of T-cell subset populations and modification of immunoregulatory states, as observed in other parasitic infections (22). However, in humans, our understanding of the immune response is for the most part limited to the antibody response, and nothing is known about cell-mediated immunity during the course of the infection.
The objective of the present study was to characterize cell-mediated immunity during the muscular phase of Trichinella infection in humans. To do this, we studied the phenotypic changes in the T-cell populations of peripheral blood mononuclear cells (PBMC) from people infected with Trichinella and expression and the production of cytokines in these cell populations. Our results showed that CD8+ T-cell expression was increased and the cytokine pattern was predominately type 2.
MATERIALS AND METHODS
Panel of donors.
We collected blood samples from 10 people who were infected with T. spiralis, the most widespread Trichinella species, after they had ingested imported horsemeat during a 1998 outbreak in northern Italy (18). The blood samples were collected when the infected individuals returned to the hospital for checkups, 2, 9, 14, and 36 months after ingestion of the infected meat. All donors had been treated with 50 mg of prednisolone per day and 1,500 mg of mebendazole per day for 10 days; when the samples obtained 2 months postinfection (p.i.) were collected, the donors had completed treatment 3 weeks previously. We also collected blood samples 1.5, 2.5, and 12 months after ingestion of infected meat from five people who were infected with T. britovi after they ingested wild boar meat during a 1995 outbreak in central Italy (17). As described above, the sample collection times corresponded to checkup times. Two of the donors were treated with both prednisolone and mebendazole (80 mg of prednisolone per day and 1,500 mg of mebendazole per day for 10 days), whereas the other three donors were treated only with mebendazole. When the blood samples obtained 1.5 months p.i. were collected, the two donors treated with both drugs were at the eighth day of treatment. Samples from three donors who were known to have never been infected with Trichinella were used as controls in all assays. We obtained written informed consent from the donors before taking blood samples.
PBMC were isolated from samples from T. spiralis-infected donors and T. britovi-infected donors by centrifugation on density gradients (Lymphoprep; Nyegaard, Oslo, Norway) and were washed twice in RPMI medium (GIBCO, Grand Island, N.Y.). The PBMC were then frozen in fetal calf serum with 10% dimethyl sulfoxide (Sigma Chemical Co.) at −80°C. After rapid thawing at 37°C in a water bath, the PBMC were washed twice and then diluted in RPMI medium supplemented with 5% pooled AB serum and antibiotics (100 IU of penicillin [GIBCO] per ml, 0.1 mg of streptomycin [GIBCO] per ml).
Parasites, antigens, and mitogens.
T. spiralis larvae (isolate code ISS597) and T. britovi larvae (ISS205) were isolated from meat from the infected horse and the infected wild boar, respectively. These two isolates were maintained under laboratory conditions by serial passages in Swiss-CD1 mice. Larvae were collected by enzymatic digestion of the infected meat, and a crude worm extract (CWE) antigen and an excretory-secretory antigen were prepared for both species by using previously described procedures (7, 9). The protein concentrations of the antigens were estimated by the method of Bradford (4). Since the two antigens induced the same proliferation in PBMC (data not shown) and the CWE antigen was easier to prepare than the excretory-secretory antigen, we used CWE in all of the experiments. For antigen selection, two doses (5.0 and 25 μg/ml) of T. spiralis CWE and T. britovi CWE were used to induce proliferative responses in PBMC from three T. spiralis-infected donors and three T. britovi-infected donors (Table 1). A partially purified mannoprotein (MP) extract of the cell wall of Candida albicans, constituting the major antigenic determinant of this microorganism, was used as an antigenic control for proliferation (1). Recombinant interleukin-2 (IL-2) (Amersham International, Buckinghamshire, United Kingdom) and purified phytohemagglutinin (PHA) (Murex Diagnostics, Dartford, United Kingdom) were used as mitogenic stimulants.
TABLE 1.
Proliferative responses to T. spiralis CWE and to a control stimulant, IL-2, on day 7 of culture of PBMC from T. spiralis-infected individuals obtained at different times p.i.
| Stimulant | [3H]thymidine incorporation (cpm, 103)a
|
|||
|---|---|---|---|---|
| 2 mo p.i. | 9 mo p.i. | 14 mo p.i. | 36 mo p.i. | |
| None | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| T. spiralis CWE | 17.8 ± 0.8 | 26.2 ± 4 | 18.7 ± 3 | 8.7 ± 3 |
| IL-2 | 27.2 ± 0.4 | 31.2 ± 1 | 30.1 ± 1 | 34.2 ± 0.9 |
The values are means ± standard deviations based on triplicate determinations for 10 donors for each group. A total of 2 × 105 PBMC/well were cultured for 7 days with 25 μg of T. spiralis CWE per ml and with 100 IU of IL-2 per ml.
PBMC proliferation and generation of activated effectors.
IL-2 (100 IU/ml) or antigens at optimal concentrations were added to PBMC from 10 T. spiralis-infected donors and five T. britovi-infected donors collected at different times p.i. PBMC proliferation was measured in 96-well flat-bottom microwell trays in triplicate. The trays were incubated in 5% CO2 at 37°C, and PBMC were harvested on the 3rd, 7th, and 10th days after 18 h of incubation in the presence of 0.5 μCi of [3H]thymidine (NEN-Du Pont, Boston, Mass.). The data are expressed below as means ± standard deviations. To isolate total RNA, PBMC were cultured at a concentration of 2 × 106 cells/ml in the presence of 25 μg of T. spiralis CWE per ml or 25 μg of T. britovi CWE per ml for 48 h under similar conditions.
RT-PCR.
Total cellular RNA was extracted from 2 × 106 cells with a QuickPrep Micro mRNA purification kit (Pharmacia). Reverse transcriptase PCRs (RT-PCR) were performed with 50-μl (total volume) mixtures by using the primer pairs specified by the manufacturer (T-primed First-Strand kit; Pharmacia). cDNAs were amplified in the presence of 5′ and 3′ primers (each at a final concentration of 0.05 μM), 2.0 mM deoxynucleotides, 1 U of Taq DNA polymerase (Promega, Madison, Wis.), and 1× PCR buffer. Each PCR was performed with a thermal cycler (Perkin-Elmer, Norwalk, Conn.) by using 10 cycles consisting of 30 s of denaturation at 94°C, 30 s of annealing at 64°C, and 30 s of extension at 72°C and then 25 cycles consisting of 30 s of denaturation at 94°C, 30 s of annealing at 62°C, and 1 min of extension at 72°C. A housekeeping gene, β-actin, was amplified in each experiment to evaluate the extraction procedure and to estimate the amount of RNA. The reaction product was visualized by electrophoresis by using 10 μl of the reaction mixture and 2 μl of loading buffer. One microgram of a 100-bp ladder (Pharmacia) was run in parallel as a molecular size marker, providing bands at 100 to 1,000 bp. Cytokine-specific primer pairs were synthesized (Applied Biosystems, Foster City, Calif.) by using previously described sequences (2).
Genes for the following cytokines were examined for all 10 T. spiralis-infected donors and for three of the five T. britovi-infected donors for all samples except those collected from T. spiralis-infected donors 36 months p.i and from T. britovi-infected donors 2.5 months p.i.: IL-2, IL-4, IL-5, IL-6, IL-10, and gamma interferon (IFN-γ). Each cytokine was considered expressed only if the RT-PCR signal of the T. spiralis- or T. britovi-stimulated sample was visibly more intense than the baseline response.
Cytokine production.
We determined the concentrations of IL-2, IL-5, IL-10, and IFN-γ in PBMC culture supernatants which were collected after 48 h of incubation by using a commercial enzyme-linked immunosorbent assay, as specified by the manufacturer (R&D Systems Europe).
Flow cytometric analysis.
PBMC from three T. spiralis-infected donors and three T. britovi-infected donors were cultured for 7 days at a concentration of 1 × 106cells/ml in the presence of 25 μg of T. spiralis CWE per ml, 25 μg of T. britovi CWE per ml, or 10 μg of C. albicans antigen per ml. The PBMC were then resuspended in phosphate-buffered saline and stained with phycoerythrin (PE)-conjugated or fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody; to determine vitality, cells were stained with propidium iodine.
Three-color flow cytometric analyses were performed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.). Data were acquired with CELL-Quest software (Becton Dickinson). The instrument was set up to measure the fluorescence intensities of forward-angle light scatter (FSC), side-angle light scatter, FITC (FL1), PE (FL2), and propidium iodine (FL3). The following pairs of monoclonal antibodies were used: CD3 (FITC) and CD4 (PE), CD3(FITC) and CD8 (PE), TCR-αβ (FITC) and TCR-γδ (PE), and CD45RA (FITC) and CD45RO (PE) (all obtained from Becton Dickinson). The monocyte-specific reagent CD14 (PE) (Becton Dickinson) was used to test for monocyte contamination of lymphocytes. Cells incubated with FITC- and PE-conjugated mouse IgG1/IgG2a served as an isotype control.
Statistical analysis.
The results were analyzed by one-way analysis of variance and Student’s t test. A P value of <0.05 was considered statistically significant.
RESULTS
PBMC proliferation.
Both T. spiralis CWE and T. britovi CWE induced blastogenesis in PBMC (data not shown). T. spiralis CWE induced stronger proliferation of PBMC than T. britovi CWE, independent of the infecting Trichinella species, and the greatest responses occurred at a concentration of 25 μg/ml. Thus, T. spiralis CWE was selected for the subsequent experiments. The PBMC from noninfected donors did not show any proliferative response to Trichinella antigens (data not shown).
To determine the kinetics of proliferation, PBMC from one T. spiralis-infected donor were cultured for 10 days with T. spiralis CWE (25 μg/ml) or PHA (1.5 μg/ml). The T. spiralis CWE-induced proliferation peaked on day 7 of culture, whereas the PHA-induced proliferation showed typical mitogenic kinetics, peaking on day 3 (data not shown).
The PBMC from the 10 donors infected with T. spiralis (collected 2, 9, 14, and 36 months p.i.) proliferated when they were stimulated with T. spiralis CWE, and the greatest response occurred 9 months p.i. (Table 1). The PBMC from the five T. britovi-infected donors (collected 1.5, 2.5, and 12 months p.i.) showed three different patterns of proliferative response (Table 2). For three of these donors (Table 2, donors 1 to 3), none of whom had undergone corticosteroid therapy, PBMC collected 1.5 and 2.5 months p.i. showed a high level of proliferation when they were stimulated with T. spiralis CWE and with IL-2, whereas PBMC collected 12 months p.i. showed a lower level of proliferation. For donor 4, PBMC collected 1.5 months p.i. did not respond to either T. spiralis CWE or IL-2, whereas PBMC collected 2.5 months p.i. showed a high level of proliferation with both, which decreased later (Table 2). For donor 5, PBMC collected 1.5 months p.i. did not respond to either T. spiralis CWE or IL-2. For the PBMC collected 2.5 months p.i., the level of proliferation was low with T. spiralis CWE and high with IL-2; for the PBMC collected 12 months p.i., no proliferation was observed with T. spiralis CWE, whereas proliferation was observed with IL-2, as expected (Table 2).
TABLE 2.
Proliferative responses to T. spiralis CWE and to a control stimulant, IL-2, on day 7 of culture by PBMC from five T. britovi-infected individuals obtained at different times p.i.
| Stimulant | [3H]thymidine incorporation (cpm, 103)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Donors 1 to 3 (avg)
|
Donor 4b
|
Donor 5b
|
|||||||
| 1.5 mo p.i. | 2.5 mo p.i. | 12 mo p.i. | 1.5 mo p.i. | 2.5 mo p.i. | 12 mo p.i. | 1.5 mo p.i. | 2.5 mo p.i. | 12 mo p.i. | |
| None | 0.1 ± 0 | 0.2 ± 0 | 0.4 ± 0.1 | 0.1 ± 0 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.1 ± 0 | 0.7 ± 0 | 0.3 ± 0.1 |
| T. spiralis CWE | 73.3 ± 9.1 | 69.1 ± 2 | 39.4 ± 3.2 | 0.3 ± 0.1 | 123.2 ± 91 | 23.0 ± 7.1 | 0.3 ± 0.2 | 8.8 ± 1.1 | 0.9 ± 0 |
| IL-2 | 60.0 ± 8.2 | 54.9 ± 3 | 58.9 ± 7 | 0.1 ± 0 | 82.7 ± 2 | 53.8 ± 1.7 | 0.2 ± 0.1 | 69.2 ± 2.5 | 51.2 ± 0 |
The values are means ± standard deviations based on triplicate determinations. A total of 2 × 105 PBMC/well were cultured with 25 μg of T. spiralis CWE per ml and with 100 IU of IL-2 per ml.
Donors 4 and 5 were receiving corticosteroid therapy at 1.5 months p.i.
Cytokine gene expression in T. spiralis CWE-stimulated PBMC from T. spiralis- and T. britovi-infected donors.
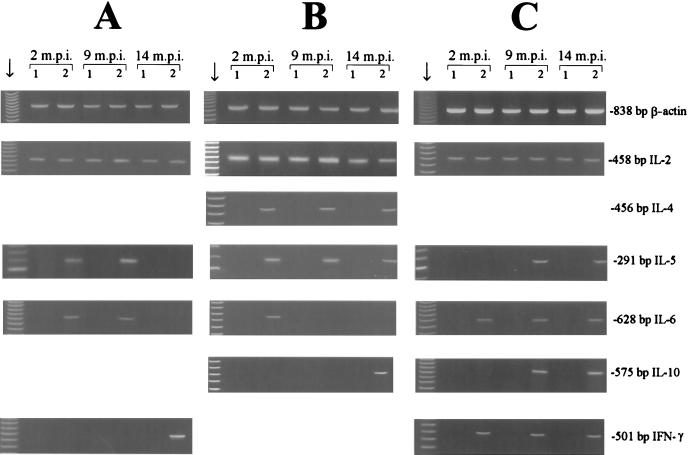
Expression of cytokine genes in T. spiralis CWE-stimulated PBMC from T. spiralis-infected donors produced three different patterns (Fig. 1). When samples collected 2 months p.i. were used, PBMC from five donors showed expression of IL-2, IL-5, and IL-6 (Fig. 1A); PBMC from three donors showed expression of IL-2, IL-4, IL-5, and IL-6 (Fig. 1B); and PBMC from two donors showed expression of IL-2, IL-6, and IFN-γ (Fig. 1C). When samples collected 9 months p.i. were used, PBMC from all donors showed expression of IL-2 and IL-5 (Fig. 1); signals were also observed for IL-6 (Fig. 1A), for IL-4 (Fig. 1B), or for IL-6, IL-10, and IFN-γ (Fig. 1C). When samples collected 14 months p.i. were used, PBMC from all donors showed expression of IL-2 (Fig. 1); signals were also observed for IFN-γ (Fig. 1A), for IL-4, IL-5, and IL-10 (Fig. 1B), or for IL-5, IL-6, IL-10, and IFN-γ (Fig. 1C).
FIG. 1.
RT-PCR analysis of cytokine gene expression in stimulated and nonstimulated PBMC cultures with representative patterns (A, B, and C) for 10 T. spiralis-infected donors at 2, 9, and 14 months p.i. (m.p.i.) for β-actin, IL-2, IL-4, IL-5, IL-6, IL-10, and IFN-γ after 48 h of culture. Lanes 1, nonstimulated PBMC; lanes 2, PBMC stimulated with T. spiralis CWE. The lanes indicated by the arrows contained molecular weight markers.
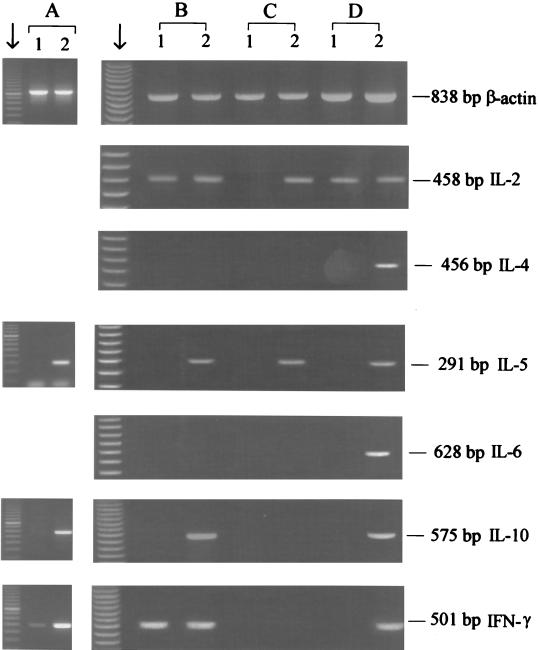
Expression of cytokine genes in T. spiralis CWE-stimulated PBMC from T. britovi-infected donors was studied for the donors who consistently showed proliferation (Table 2, donors 1 to 3). At 1.5 months p.i., PBMC from all three of these donors showed expression of IL-5, IL-10, and IFN-γ (Fig. 2A). PBMC from one donor also showed expression of IL-2, IL-4, and IL-6 (data not shown). At 12 months p.i., PBMC from all three donors showed expression of IL-2 and IL-5 (Fig. 2B, C, and D); PBMC from one donor also showed expression of IL-10 and IFN-γ (Fig. 2B); and PBMC from another donor also showed expression of IL-4, IL-6, IL-10, and IFN-γ (Fig. 2D).
FIG. 2.
RT-PCR analysis of cytokine gene expression in cultures of stimulated and nonstimulated PBMC from three T. britovi-infected donors at 1.5 months p.i. (A) and 12 months p.i. (B, C, and D) for β-actin, IL-2, IL-4, IL-5, IL-6, IL-10, and IFN-γ after 48 h of culture. Lanes 1, nonstimulated PBMC; lanes 2, PBMC stimulated with T. spiralis CWE. The lanes indicated by the arrows contained molecular weight markers.
Cytokine production.
Production of IL-2, IL-5, IL-10, and IFN-γ was determined in supernatants of T. spiralis CWE-stimulated PBMC from T. spiralis- and T. britovi-infected donors after 48 h of culture (Table 3). In the three T. spiralis-infected donors, the IL-2 production at 2 months p.i. was similar to the IL-2 production at 9 months p.i. and to the IL-2 production in noninfected controls. At 14 months p.i., IL-2 production had slightly increased. IL-5 production was greatest at 2 months p.i. IL-10 production remained constant over time. IFN-γ production was greatest at 9 months p.i. In the three T. britovi-infected donors, IL-2, IL-5, IL-10, and IFN-γ production was also detected at 1.5 months p.i. (Table 3).
TABLE 3.
IL-2, IL-5, IL-10, and IFN-γ production in supernatants of PBMC, stimulated or not stimulated with T. spiralis CWE, from three T. spiralis-infected individuals, three T. britovi-infected individuals, and three individuals without a Trichinella infectiona
| Etiological agent | Time (mo p.i.) | Stimulant | Concn (pg/ml)b
|
|||
|---|---|---|---|---|---|---|
| IL-2 | IL-5 | IL-10 | IFN-γ | |||
| T. spiralis | 2 | None | 11.7 (3/3) | ≤3 (0/3) | ≤3.9 (0/3) | 22 (3/3) |
| T. spiralis CWE | 28 (3/3) | 190 (3/3) | 25 (3/3) | 642 (3/3) | ||
| 9 | None | 16 (3/3) | ≤3 (0/3) | ≤3.9 (0/3) | 14.9 (3/3) | |
| T. spiralis CWE | 33 (3/3) | 140 (3/3) | 23 (3/3) | 980 (3/3) | ||
| 14 | None | 17 (3/3) | ≤3 (0/3) | ≤3.9 (0/3) | 18 (3/3) | |
| T. spiralis CWE | 61 (3/3) | 40 (3/3) | 25 (3/3) | 194 (3/3) | ||
| T. britovi | 1.5 | None | 21 (3/3) | 15 (2/3) | 8 (2/3) | 60 (3/3) |
| T. spiralis CWE | 35 (3/3) | 50 (3/3) | 35 (3/3) | 586 (3/3) | ||
| None | None | 22 (3/3) | 12 (1/3) | ≤3.9 (0/3) | 41 (3/3) | |
| T. spiralis CWE | 29 (3/3) | 40 (1/3) | ≤3.9 (0/3) | 125 (3/3) | ||
Cells were cultured for 48 h and stimulated with 25 μg of T. spiralis CWE per ml.
The concentrations were determined with an enzyme-linked immunosorbent assay. The numbers in parentheses are number of samples positive/number of samples examined. The sensitivities of the cytokines were as follows: IL-2, 7.0 pg/ml; IL-5, 3.0 pg/ml; IL-10, 3.9 pg/ml; and IFN-γ, 8.0 pg/ml.
Flow cytometric analysis.
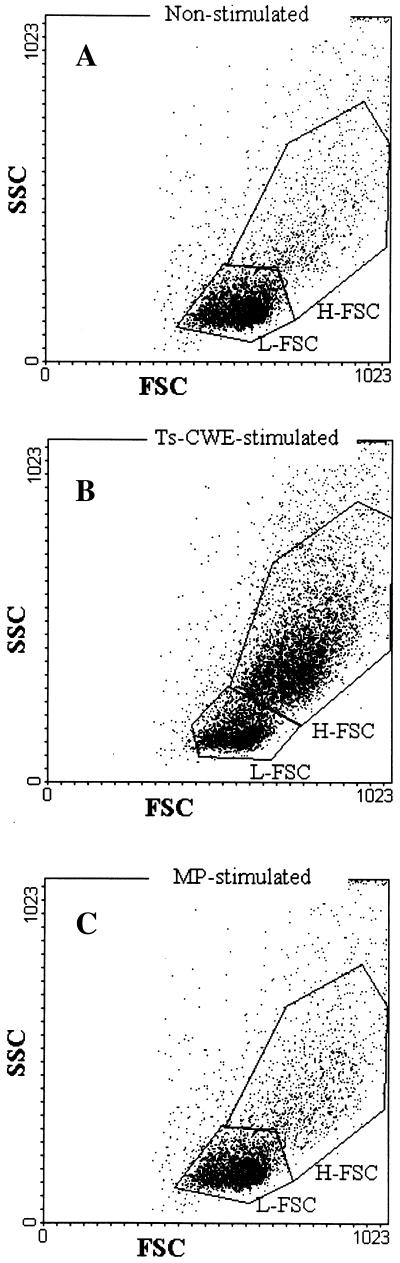
Dead cells were excluded by labeling the cells with propidium iodine. A cytometric analysis performed with T. spiralis CWE-stimulated PBMC produced similar results for all infected donors (i.e., irrespective of the etiological agent) and for all PBMC samples (i.e., irrespective of the time of blood collection). Figure 3 shows the results for 36 months p.i. for one T. spiralis-infected donor, as an example. The T. spiralis CWE stimulation induced light scatter changes in the lymphocyte population, which allowed it to be gated into two subpopulations (Fig. 3B). One subpopulation, with high forward and side scatter (H-FSC), represented 60% of the total lymphocyte population, and the other subpopulation, with low forward and side scatter (L-FSC), represented 40% of the total lymphocyte population. In the nonstimulated PBMC, the percentage of L-FSC cells was 82%, whereas the percentage of H-FSC cells was 18% (Fig. 3A); similar results were obtained when MP was used as the antigen (Fig. 3C).
FIG. 3.
Dot plots of cytometric analysis (SSC and FSC) of nonstimulated (A), T. spiralis CWE-stimulated (B), and C. albicans MP-stimulated (C) PBMC collected 36 months p.i. from one T. spiralis-infected donor, showing the L-FSC and H-FSC regions. (A) L-FSC accounted for 82% and H-FSC accounted for 18% of the total cell population. (B) L-FSC accounted for 40% and H-FCS accounted for 60% of the total cell population. (C) L-FSC accounted for 80% and H-FSC accounted for 20% of the total cell population.
A phenotypic analysis was carried out with the H-FSC and L-FSC subpopulations of T. spiralis CWE-stimulated PBMC, MP-stimulated PBMC, and nonstimulated PBMC from three T. spiralis-infected donors and three T. britovi-infected donors. In the L-FSC subpopulation, no phenotypic variation was observed over time, nor were any variations observed among the six donors or for the specific antigens (data not shown).
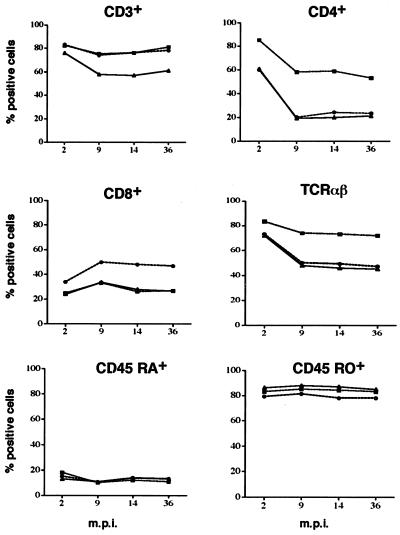
In the H-FSC subpopulation from T. spiralis-infected donors, the percentages of CD3+ and CD4+ cells and of cells bearing αβ T-cell receptor (TCR) decreased from 2 to 9 months p.i.; thereafter, no variation was observed. The percentage of CD8+ cells increased slightly from 2 to 9 months p.i. and did not change thereafter. The percentages of CD45RO+ and CD45RA+ cells and of cells bearing γδ TCR (data not shown) remained almost constant from 2 to 36 months p.i. (Fig. 4).
FIG. 4.
Kinetics and percentages of H-FSC PBMC stimulated with T. spiralis CWE expressing CD3+, CD4+, CD8+, TCR αβ, CD45RA+, and CD45RO+ markers from three T. spiralis-infected donors. m.p.i., months postinfection.
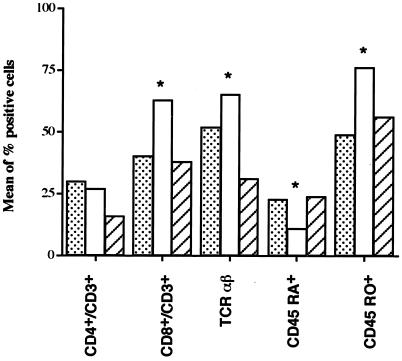
In the H-FSC subpopulations from T. britovi-infected donors, at 12 months p.i. the T. spiralis CWE-stimulated PBMC consisted of 27% ± 7% CD4+ CD3+ cells and 62% ± 9% CD8+ CD3+ cells with 11% ± 6% CD45RA+, 76% ± 13% CD45RO+, and 56% ± 9% αβ TCR (Fig. 5). At the same time, the nonstimulated PBMC consisted of 30% ± 8% CD4+ CD3+ cells and 40% ± 13% CD8+ CD3+ cells with 23% ± 6% CD45RA+, 56% ± 12% CD45RO+, and 59% ± 9% αβ TCR (Fig. 5). When interpreting the difference in the percentages of CD8+ CD3+ cells in the stimulated and nonstimulated PBMC (62% ± 9% and 40% ± 13%, respectively), we had to take into account the finding that in the nonstimulated PBMC the H-FSC subpopulation represented only 18% of the total (Fig. 3A), whereas in the T. spiralis CWE-stimulated PBMC the H-FSC subpopulation represented 60% (Fig. 3B).
FIG. 5.
Phenotypic analysis of the high-scatter population of PBMC from one representative T. britovi-infected donor that were not stimulated (dotted bars) or were stimulated with T. spiralis CWE (open bars) or with C. albicans MP (striped bars). Asterisks indicate significant differences (P ≤ 0.05).
DISCUSSION
Our results show that T. spiralis CWE can induce proliferation for at least 36 months in PBMC from T. spiralis-infected individuals and for at least 12 months in PBMC from T. britovi-infected individuals. A trend toward an increase in CD8+ CD3+ lymphocytes and a type 2 pattern of cytokines were also observed during the muscular phase of Trichinella infection. Muscle biopsy samples collected 36 months p.i. from T. spiralis-infected donors were still positive for the presence of viable and mouse-infecting muscle larvae (data not shown) in spite of the mebendazole treatment (19).
Although both T. spiralis CWE and T. britovi CWE were able to induce proliferation of PBMC from Trichinella-infected donors, acting as T-cell recall antigens (data not shown), the proliferation induced by T. spiralis CWE was stronger than that induced by T. britovi CWE, independent of the etiological agent of infection. It is known that in T. spiralis-infected mice, T. spiralis CWE induces shock and death more often than T. britovi CWE (5). Furthermore, in humans, T. spiralis induces a stronger humoral immune response than T. britovi (20). Our results also suggest that the immunogenicity of T. spiralis CWE and the immunogenicity of T. spiralis larvae are greater than the immunogenicity of T. britovi CWE and the immunogenicity of T. britovi larvae, respectively, as observed in other studies (5).
In the T. spiralis-infected donors, blastogenesis peaked at 9 months p.i. (Table 1), whereas in the T. britovi-infected donors it peaked at 1.5 months p.i. (Table 2). In the two T. britovi-infected donors who were treated with prednisolone, at 1.5 months p.i. there was a lack of PBMC proliferation with both T. spiralis CWE and IL-2; proliferation with both was restored at 2.5 months p.i. in one donor (Table 2, donor 4) and was only partially restored (IL-2) at 12 months p.i. in the other donor (donor 5). For donor 5, the low level of proliferation at 2.5 months p.i. and the lack of proliferation at 12 months p.i. with T. spiralis CWE could have been due to successful mebendazole treatment in a person who had acquired a low-level infection.
The pattern of cytokine gene expression varied among donors. Independent of the time after infection, most of the T. spiralis CWE-stimulated PBMC from T. spiralis-infected donors showed expression of IL-5 and IL-6, and some of these PBMC also showed expression of IL-4, IL-10, and IFN-γ (Fig. 1). Expression of IL-2 was not considered because it also occurred in nonstimulated PBMC. Τhe results suggest that type 2 cytokines were predominant in the first 9 months p.i., and although 14 months p.i. a type 2 pattern was still predominant, the presence of IFN-γ expression suggested an intermediate type 0 pattern, even if a non-T-cell origin of IFN-γ could be postulated. T. spiralis CWE-stimulated PMBC from T. britovi-infected donors showed a pattern similar to that of T. spiralis-infected donors but with earlier expression of IL-10 and IFN-γ (Fig. 2).
Production of cytokines by the T. spiralis CWE-stimulated PBMC from the T. spiralis-infected donors showed different trends; IL-2 production increased slightly over time, IL-10 production was constant, IL-5 production reached the highest level 2 months p.i., and IFN-γ production increased up to 9 months p.i. and decreased thereafter. There was a good correspondence between the cytokine expression and production patterns and the antibody response because infected individuals still had high antibody levels (i.e., IgG titers of ≥1/1,600) at 36 months p.i. (data not shown). The finding that the T. spiralis CWE-stimulated PBMC from the T. britovi-infected donors exhibited lower IL-5 and IFN-γ cytokine production than the T. spiralis CWE-stimulated PBMC from the T. spiralis-infected donors is further evidence that T. spiralis larvae in humans and antigen in vitro both have greater immunogenicity than T. britovi larvae and antigen (5, 20). A good correspondence between expression and production of cytokines by PBMC from all infected individuals was observed, supporting the conclusion that the cytokine response is predominantly a type 2 response during the first 9 months p.i.
The phenotypic analysis of the H-FSC subpopulation showed that there was a trend toward a decrease in CD4+ cells and a trend toward an increase in CD8+ cells in all Trichinella-infected individuals. Phenotypic changes over time were observed in T. spiralis CWE-stimulated PBMC from both T. spiralis- and T. britovi-infected donors compared with nonactivated PBMC and MP-activated PBMC.
The increase in the number of CD8+ cells and the decrease in the number of CD4+ cells were accompanied by a reduction in the number of naïve cells and an increase in the number of memory cells. Changes in PBMC composition, with an increase in the number of CD8+ cells and a decrease in the number of CD4+ cells, have been observed in individuals with general immune activation caused by persistent helminthic infection (13, 16).
Although PBMC cultures provide no information concerning the nature of the cells that express and produce cytokines, it has been shown that not only CD4+ cells but also CD8+ cells can be placed in different subsets on the basis of their cytokine production profiles (14, 21). In conclusion, our study of cellular immunity during the muscular phase of Trichinella infection in humans showed that CD8+ cells were involved and that the cytokine pattern was predominantly a type 2 pattern.
Acknowledgments
We are grateful to F. Bruschi for his insightful criticism and helpful suggestions. We are also grateful to M. J. Gomez Miguel for providing the C. albicans MP.
This work was supported by a research project on emerging and reemerging infectious diseases (Istituto Superiore di Sanità no. 502/12, Ministry of Health of Italy).
Editor: T. R. Kozel
REFERENCES
- 1.Ausiello, C. M., G. C. Spagnoli, and M. Boccanera. 1986. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J. Med. Microbiol. 22:195–202. [DOI] [PubMed] [Google Scholar]
- 2.Ausiello, C. M., F. Urbani, S. Gessani, G. Spagnoli, M. J. Gomez, and A. Cassone. 1996. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect. Immun. 61:4105–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, R. G. 1998. The generation and expression of immunity to Trichinella spiralis in laboratory rodents. Adv. Parasitol. 41:149–217. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. [DOI] [PubMed] [Google Scholar]
- 5.Bruschi, F., E. Pozio, W. Watanabe, M. A. Gomez Morales, M. Ito, Y. Huang, and R. Binaghi. 1999. Anaphylactic response to parasite antigens: IgE and IgG1 independently induce death in Trichinella-infected mice. Int. Arch. Allergy Immunol. 119:291–296. [DOI] [PubMed] [Google Scholar]
- 6.Despommier, D. D. 1998. How does Trichinella spiralis make itself at home? Parasitol. Today 14:318–323. [DOI] [PubMed] [Google Scholar]
- 7.Engvall, E., and I. Ljungstrom. 1975. Detection of human antibodies to Trichinella spiralis by enzyme-linked immunosorbent assay (E.L.I.S.A.). Acta Pathol. Microbiol. Scand. 83:231–237. [Google Scholar]
- 8.Fröscher, W., F. Gullotta, M. Saathoff, and W. Tackmann. 1988. Chronic trichinosis. Clinical, bioptic, serological and electromyographic observations. Eur. Neurol. 28:221–226. [DOI] [PubMed] [Google Scholar]
- 9.Gamble, H. R., W. R. Anderson, C. E. Graham, and K. D. Murrell. 1983. Diagnosis of swine trichinosis by enzyme-linked immunosorbent assay using an excretory-secretory antigen. Vet. Parasitol. 13:349–361. [DOI] [PubMed] [Google Scholar]
- 10.Goyal, P. K., J. Hermanek, and D. Wakelin. 1994. Lymphocyte proliferation and cytokine production in mice infected with different geographical isolates of Trichinella spiralis. Parasitol. Immunol. 16:105–110. [DOI] [PubMed] [Google Scholar]
- 11.Grencis, R. K., L. Hultner, and K. J. Else. 1991. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology 74:329–332. [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa, N., P. K. Goyal, Y. R. Mahida, K. Fli, and D. Wakelin. 1998. Early cytokine responses during intestinal parasitic infections. Immunology 93:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinkovich, A., Z. Weisman, Z. Greenberg, J. Nahmias, S. Eitan, M. Stein, and Z. Bentwich. 1998. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin. Exp. Immunol. 114:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosman, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 15.Murrell, K. D., and E. Pozio. 2000. Trichinellosis: the zoonosis that won’t go quietly. Int. J. Parasitol. 30:1339–1349. [DOI] [PubMed] [Google Scholar]
- 16.Pollack, S., B. Fuad, and A. Etzioni. 1993. CD4 T-lymphocytopenia without opportunistic infections in HIV-seronegative Ethiopian immigrants to Israel. Lancet 342:50–51. [DOI] [PubMed] [Google Scholar]
- 17.Pozio, E., G. La Rosa, and M. A. Gomez Morales. 2001. Epidemiology of human and animal trichinellosis in Italy since its discovery in 1887. Parasite 8:S106–S108. [DOI] [PubMed] [Google Scholar]
- 18.Pozio, E., D. Sacchini, P. Boni, A. Tamburrini, F. Alberici, and F. Paterlini. 1998. Human outbreak of trichinellosis associated with the consumption of horsemeat in Italy. Eurosurveillance 3:85–86. [DOI] [PubMed] [Google Scholar]
- 19.Pozio, E., D. Sacchini, L. Sacchi, A. Tamburrini, and F. Alberici. 2001. Failure of mebendazole in treating Trichinella spiralis infection in humans at the stage of encapsulating larvae. Clin. Infect. Dis. 32:638–642. [DOI] [PubMed] [Google Scholar]
- 20.Pozio, E., P. Varese, M. A. Gomez Morales, G. P. Croppo, D. Pelliccia, and F. Bruschi. 1993. Comparison of human trichinellosis caused by Trichinella spiralis and by Trichinella britovi. Am. J. Trop. Med. Hyg. 48:568–575. [DOI] [PubMed] [Google Scholar]
- 21.Sad, S., R. Marcotte, and T. R. Mossman. 1995. Cytokine induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2:271–279. [DOI] [PubMed] [Google Scholar]
- 22.Sher, A., and R. L. Coffman. 1993. Regulation of immunity to parasites by T cells and T-cell derived cytokines. Annu. Rev. Immunol. 10:385–409. [DOI] [PubMed] [Google Scholar]