Abstract
ATP is a vital molecule used by living organisms as a universal source of energy required to drive the cogwheels of intracellular biochemical reactions necessary for growth and development. Animal cells release ATP to the extracellular milieu, where it functions as the primary signaling cue at the epicenter of a diverse range of physiological processes. Although recent findings revealed that intact plant tissues release ATP as well, there is no clearly defined physiological function of extracellular ATP in plants. Here, we show that extracellular ATP is essential for maintaining plant cell viability. Its removal by the cell-impermeant traps glucose–hexokinase and apyrase triggered death in both cell cultures and whole plants. Competitive exclusion of extracellular ATP from its binding sites by treatment with β,γ-methyleneadenosine 5′-triphosphate, a nonhydrolyzable analog of ATP, also resulted in death. The death response was observed in Arabidopsis thaliana, maize (Zea mays), bean (Phaseolus vulgaris), and tobacco (Nicotiana tabacum). Significantly, we discovered that fumonisin B1 (FB1) treatment of Arabidopsis triggered the depletion of extracellular ATP that preceded cell death and that exogenous ATP rescues Arabidopsis from FB1-induced death. These observations suggest that extracellular ATP suppresses a default death pathway in plants and that some forms of pathogen-induced cell death are mediated by the depletion of extracellular ATP.
INTRODUCTION
ATP is a ubiquitous, energy-rich compound in all cells of living organisms. It is found both within organelles, such as mitochondria and chloroplasts, and in the cytoplasm of higher organisms. The energy derived from ATP is used to drive a myriad of vital biochemical reactions that are fundamental to the survival of cells and whole organisms. The presence of intracellular ATP has been recognized since its discovery in living cells in 1929 (Fiske and Subarrow, 1929). However, cells can secrete ATP to the extracellular matrix in the absence of cytolysis, an observation first reported in animal cells in 1959 (Holton, 1959) and only recently in a plant species, Arabidopsis thaliana (Thomas et al., 2000).
In animals, nonlytic release of ATP by nonsecretory cells occurs under quiescent conditions (Beigi and Dubyak, 2000; Schwiebert et al., 2002), after mechanical stimulation (Grierson and Meldolesi, 1995; Homolya et al., 2000; Yegutkin et al., 2000), or after treatment with agonists such as bradykinin, acetylcholine, and serotonin (Yang et al., 1994). Touch stimulation and osmotic stress elicit increased ATP release from Arabidopsis (Jeter et al., 2004), suggesting conservation of ATP release in response to mechanical stimulation between plants and animals. Neuronal cells and secretory cells such as mast cells and pancreatic acinar cells release ATP via regulated exocytosis (Leitner et al., 1975; Unsworth and Johnson, 1990; Sorensen and Novak, 2001). The mechanisms by which plant cells and nonsecretory animal cells release ATP are still poorly understood, but there is evidence for the involvement of ABC transporters and anion channels (Abraham et al., 1993; Roman et al., 1997; Thomas et al., 2000; Dutta et al., 2002, 2004).
In animal cells, extracellular ATP is an absolute requirement for several physiological processes, such as neurotransmission, platelet aggregation, regulation of blood vessel tone, cell growth and expansion, differentiation and function of immune cells, and muscle contraction (Gordon, 1986; Redegeld et al., 1999). In these cells, extracellular ATP funnels into signaling pathways via two mechanisms. First, it participates in extracellular phosphorylation (Redegeld et al., 1999). For example, extracellular phosphorylation is an absolute requirement in T cell–mediated immune responses. Specific abolition of ectophosphorylation, without affecting intracellular phosphorylation, by the ectoprotein kinase inhibitor K-252b is accompanied by a loss of cytolytic activity of cytotoxic T lymphocytes for target cells (Redegeld et al., 1997). Second, extracellular ATP participates in signal transmission via the activation of P2X and P2Y plasma membrane nucleotide binding receptors known as purinoceptors (Burnstock and Kennedy, 1985). P2X receptors are ligand-gated ion channels that gate extracellular cations in response to ATP, whereas P2Y receptors are G protein–coupled receptors. Some physiological processes are dependent on the activation of purinoceptors by extracellular ATP to initiate signaling cascades. For example, extracellular ATP binds to P2X7 receptors to activate astrocyte Gln release, which modulates synaptic activity and participates in brain intercellular signaling (Duan et al., 2003).
Although extracellular ATP released by intact plant tissues (Thomas et al., 2000) has no clearly defined function, two lines of evidence suggest that it may have physiological significance. The first is based on electrophysiological studies using patch–clamp techniques. Extracellular ATP depolarizes Arabidopsis root hair membranes (Lew and Dearnaley, 2000) and triggers an increase of cytosolic Ca2+ (Demidchik et al., 2003), implicating a plant homolog of animal purinoceptors; however, plant purinoceptors have yet to be identified. This Ca2+ influx mediates the activation of Arabidopsis gene expression induced by exogenous ATP (Jeter et al., 2004). The second line of evidence is based on proteomic studies of Arabidopsis extracellular matrix proteins. Identification of secreted, extracellular phosphoproteins in the Arabidopsis extracellular matrix (Chivasa et al., 2002; Ndimba et al., 2003) suggests the participation of ATP in extracellular signaling via phosphorylation.
Thus, we initiated studies to examine the role of extracellular ATP in plants via the specific removal of ATP from the extracellular matrix of cell suspension cultures and intact plants. In this article, we report the discoveries that (1) a physiological role of extracellular ATP in plants is to govern cell viability by the suppression of a default death pathway, and (2) a mechanism by which fumonisin B1 (FB1) induces death in Arabidopsis is the depletion of extracellular ATP.
RESULTS
Cell-Impermeant ATP Traps Trigger Death in Arabidopsis Cell Cultures
We confirmed that plant cells indeed secrete ATP to the extracellular matrix by showing that Arabidopsis cell cultures fed with [32P]H3PO4 synthesized and secreted radiolabeled ATP to the extracellular matrix within 1 h (Figure 1A). Six hours later, the level of radiolabeled ATP in the extracellular medium remained the same (Figure 1A, lanes 3 and 6), indicating that the cells tightly regulate extracellular ATP levels. Using Evans blue staining, we found no changes in the integrity of the plasma membrane of the suspension cells within the first hour during which the cells took up radioactive phosphate and released radioactive ATP (Figure 1C). This finding shows that the radiolabeled ATP in the growth medium was actively secreted by the cells and did not result from passive release attributable to cell death. Moreover, the fact that labeled ATP remained the same between 1 and 7 h from the addition of labeled phosphate rules out release via cell death but suggests a highly controlled dynamic balance between secretion and hydrolysis by extracellular enzymes.
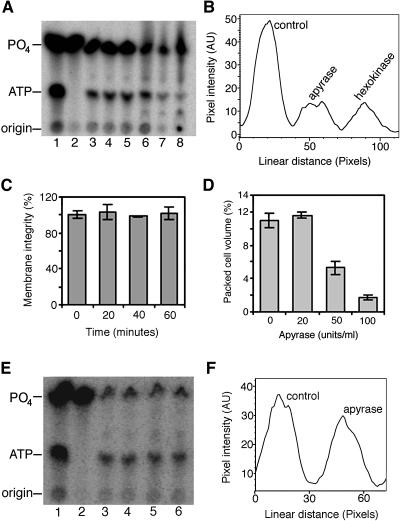
Figure 1.
Extracellular ATP and Cell Viability of Arabidopsis Cell Cultures.
(A) ATP and phosphate (PO4) standards (lane 1) and [32P]H3PO4 fed to three independent cell cultures and medium aliquots taken at time 0 (lane 2) and 1 h later (lanes 3 to 5) showing the de novo synthesis and secretion of ATP. At 1 h, solutions of ATP traps were added, and 6 h later, medium samples were taken from the control culture (lane 6) and from cultures treated with 100 units/mL apyrase (lane 7) and glucose–hexokinase (lane 8).
(B) Line graph of a pixel intensity profile showing relative levels of ATP in the 6-h samples (lanes 6 to 8) in (A).
(C) Plasma membrane integrity, measured by Evans blue staining, of cell cultures in (A) over the first 1 h.
(D) Dose response of cell viability to treatment with apyrase.
(E) ATP and phosphate standards (lane 1), [32P]H3PO4 (lane 2), and medium aliquots 1 h after adding radioactive phosphate (lanes 3 and 4) and just before adding 20 units/mL apyrase. Medium from control cells (lane 5) and cells treated with 20 units/mL apyrase (lane 6) 6 h after enzyme treatment.
(F) Line graph of a pixel intensity profile across the ATP bands in lanes 5 and 6 of (E).
Data and error bars represent means ± sd (n = 3). AU, arbitrary units.
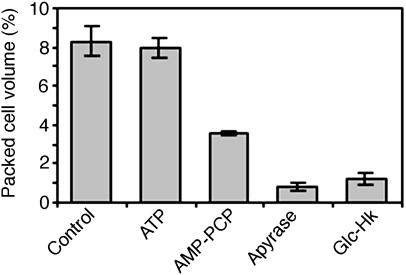
To establish its physiological function, we selectively depleted extracellular ATP from cell suspension cultures and plants using cell-impermeant ATP traps. The ATP traps used were apyrase (a hydrolase that breaks down ATP, via ADP, to AMP) and the glucose–hexokinase system. Hexokinase phosphorylates glucose in a reaction consuming a molecule of ATP. However, the plant extracellular matrix is acidic and replete with proteases, factors that could potentially inhibit the enzymatic activity of the ATP traps. Thus, we verified that both apyrase and glucose–hexokinase traps effectively reduced the extracellular ATP level in these cell cultures (Figures 1A and 1B). In initial experiments, we noted that cell death ensued within 24 to 48 h of incubating Arabidopsis cell cultures with apyrase, as confirmed by propidium iodide and fluorescein diacetate staining (data not shown). Quantitative estimation of cell death/viability was obtained by measuring the ability of the cultures to grow subsequent to the treatment. Cell cultures treated for 72 h were diluted 30-fold with fresh growth medium to diminish apyrase activity, and the cell density (packed cell volume) was determined after another 96 h of growth.
Using this method, we observed a significant dose-dependent reduction of Arabidopsis cell culture viability in response to apyrase (Figure 1D). Effects of ATP removal have previously been confounded with the effects of buffer salts in lyophilized commercial preparations of ATP-degrading enzymes (Redegeld et al., 1991). However, we found that dialyzed and nondialyzed apyrase had indistinguishable effects on cell viability (Figure 2A), indicating that cell death was not attributable to buffer salts. Treatment of the cell cultures with BSA equivalent to the amount of apyrase used did not cause any cell death (Figure 2B), demonstrating that the death response did not result from the presence of a foreign protein in the cultures. In addition, when apyrase was denatured by boiling for 5 min before treating the cultures, there was no cell death (Figure 2A), revealing that the death response requires the active enzyme. Moreover, neither AMP nor ADP, the products of ATP hydrolysis by apyrase, caused the death of the cultures (Figure 2B). This indicates that death was in response to the depletion of ATP and not to the accumulation of ADP or AMP. The correlation of extracellular ATP depletion with cell death was further demonstrated by the observation that 20 units/mL apyrase, not enough to cause death (Figure 1D), barely reduced the level of extracellular ATP in treated cells within 6 h (Figures 1E and 1F), whereas 100 units/mL apyrase effectively reduced ATP (Figures 1A and 1B) and induced cell death (Figure 1D). Because apyrase is cell-impermeant, these results demonstrate that deprivation of extracellular ATP triggers a cell death response in Arabidopsis.
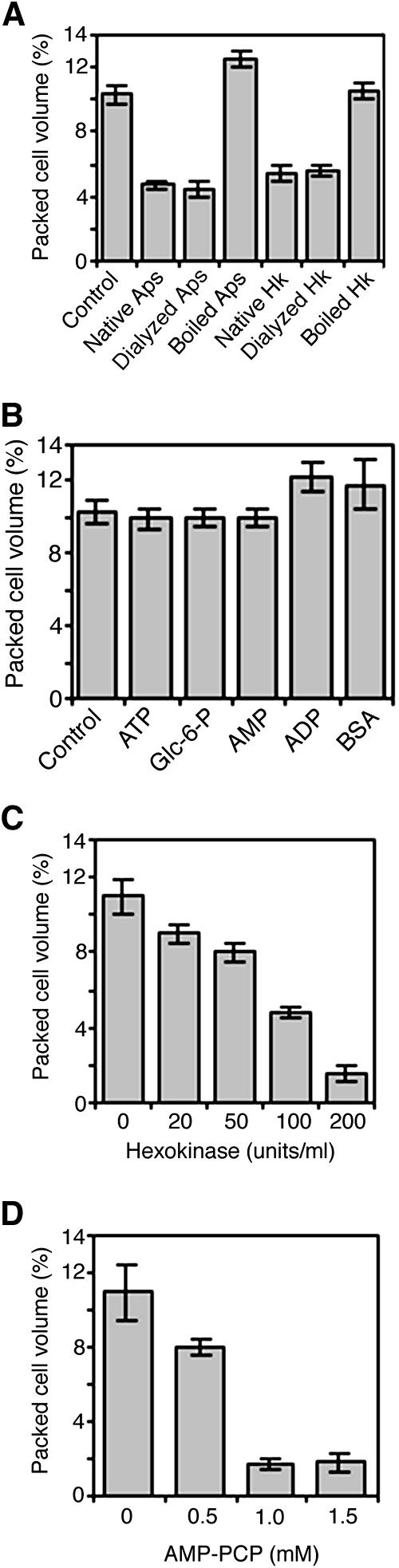
Figure 2.
Effects of Various Treatments on the Viability of Arabidopsis Cell Cultures.
(A) Effect of dialyzing or denaturing apyrase (Aps; 100 units/mL) and hexokinase (Hk; 200 units/mL) on cell viability.
(B) Effect of various solutions on cell viability. Glc-6-P, glucose-6-phosphate.
(C) Dose response of cell viability to glucose–hexokinase treatment.
(D) Dose response of cell viability to AMP-PCP treatment.
Data and error bars represent means ± sd (n = 3).
Glucose–hexokinase also functions as an ATP trap. As with apyrase, treatments caused a significant dose-dependent death response in Arabidopsis cell cultures (Figure 2C). Whereas dialyzed hexokinase evoked the cell death response, neither denatured hexokinase (Figure 2A) nor ADP and glucose-6-phosphate (Figure 2B) elicited the response. Thus, neither buffer salts nor the products of the phosphorylation of glucose by hexokinase engendered cell death. Although glucose can freely diffuse into cells, hexokinase is membrane-impermeant and remains in the external growth medium, resulting in a specific consumption of extracellular ATP. Together, these results reinforce the conclusion that removal of extracellular ATP compromises the viability of Arabidopsis cell cultures.
Treatment with a Nonhydrolyzable ATP Analog Induces Cell Death
Because ATP cannot freely diffuse across the plasma membrane (Glynn, 1968; Boldin and Burnstock, 2001), exogenous application of its nonhydrolyzable structural analogs can be used to specifically interfere with reactions requiring extracellular ATP hydrolysis (Redegeld et al., 1999). We used βγ-methyleneadenosine 5′-triphosphate (AMP-PCP), a nonhydrolyzable analog of ATP. Inundation of Arabidopsis cell cultures with exogenous AMP-PCP was attended by a concentration-dependent decrease in cell viability (Figure 2D). This finding confirms that extracellular ATP plays a role in cell viability because competitive exclusion from its binding sites by AMP-PCP, as well as its removal by hydrolyzing enzymes, result in cell death.
Extracellular ATP Is Required for the Viability of Intact Arabidopsis Plants
Extracellular ATP is also required for viability in planta. Treatment of localized areas of Arabidopsis leaves with apyrase, glucose–hexokinase, and AMP-PCP resulted in the collapse of tissue within the treated zone, leading to death between 2 and 4 d after treatment (Figure 3). Localized treatment was used to show the contrast with adjacent living tissue. When entire leaves were treated with the solutions, the whole leaf eventually died (Figure 3). Control leaves treated with BSA, ATP, AMP, ADP, glucose, or glucose-6-phosphate remained healthy (data not shown). To demonstrate that death was not the result of internalization of the ATP traps at the infiltration site caused by mechanical damage, the glucose–hexokinase system applied to the growth medium of hydroponic Arabidopsis plants caused death of the roots and some of the shoots (Figures 4 and 5). Similarly, death was triggered in Arabidopsis plants grown for 1 week on normal nutrient agar and transferred to nutrient agar supplemented with AMP-PCP (Figure 6). Control plants transferred to agar supplemented with ATP remained viable (Figure 6). Therefore, the cell death response observed in cell suspension cultures caused by extracellular ATP deprivation also occurs in whole plants.
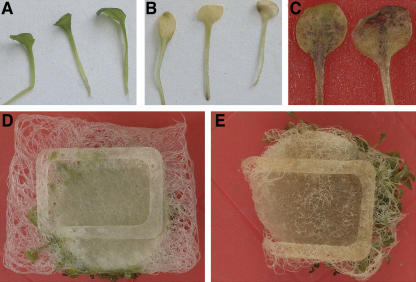
Figure 3.
Death of Intact Arabidopsis Tissues after Treatment with Extracellular ATP Traps.
Top row, leaves treated on localized areas that developed localized lesions. Bottom row, entire leaves treated and beginning to die. These leaves eventually died except for the controls. Similar results were obtained when the solutions were infiltrated with a needle or needleless syringe. Glc-Hk, glucose–hexokinase. Bar = 6 mm.
Figure 4.
Death of Hydroponic Arabidopsis Plants Caused by Glucose–Hexokinase.
(A) Healthy leaves of plants treated with glucose.
(B) Dying leaves of plants treated with glucose–hexokinase.
(C) Close-up photograph of leaves from plants treated with glucose–hexokinase showing both chlorosis and brown lesions of localized tissue death.
(D) and (E) Roots of glucose-treated plants showing normal growth (D) and retarded roots of glucose–hexokinase-treated plants (E) 7 d after treatment. Fluorescein diacetate staining and microscopy confirmed that the roots in (E) had reduced viability.
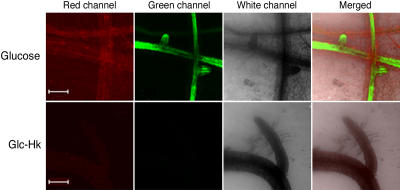
Figure 5.
Glucose–Hexokinase Causes the Death of Arabidopsis Roots.
Top row, root tissue from plants treated with glucose. Bottom row, root tissue from plants treated with glucose–hexokinase (Glc-Hk). Red channel, confocal images showing autofluorescence of the root tissues. Green channel, confocal images of fluorescein diacetate signals emitted by the same samples. White channel, images of the same samples captured using transmitted light under phase contrast. The extreme right column shows the merged images of the preceding three panels. It is clear that the glucose–hexokinase treatment caused the death of the roots, as there was no fluorescein diacetate signal in these samples. Bars = 200 μm.
Figure 6.
Death Triggered by Noninvasive Application of an ATP Analog.
Arabidopsis plants were germinated on nutrient agar and transferred to plates with agar supplemented with 1 mM ATP (A) or 1 mM AMP-PCP (B). Photographs were taken after 4 weeks. (C) and (D) show close-up photographs of representative plants from (A) and (B), respectively. It is apparent that AMP-PCP caused the death of the treated plants.
Several Plant Species Require Extracellular ATP for Viability
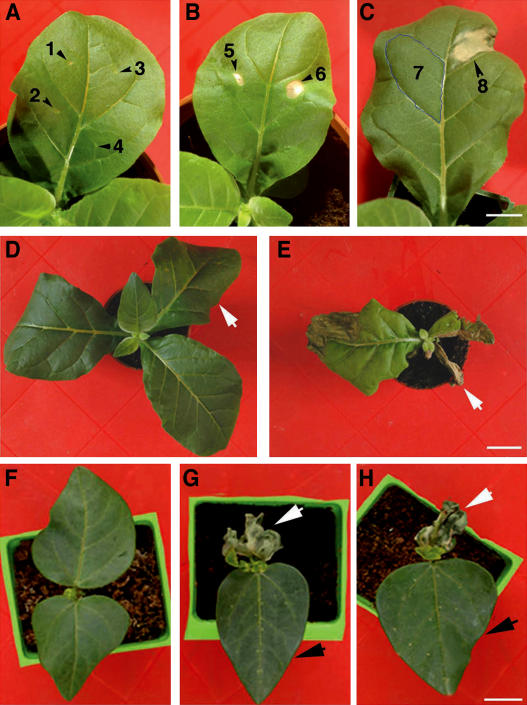
We investigated the requirement for extracellular ATP in cell viability across plant species. Tobacco (Nicotiana tabacum) plants responded to treatment in a similar manner to Arabidopsis. Local treatment with apyrase, glucose–hexokinase, and 1 mM AMP-PCP triggered the development of localized lesions (Figure 7). Treating the entire leaf caused the death of the whole leaf. However, treatment with a higher concentration (5 mM) of AMP-PCP had systemic effects: upper leaves that had not received the primary treatment died as well (Figure 7E). An equivalent concentration of ATP did not cause death (Figure 7D). Bean (Phaseolus vulgaris) plant leaves treated with apyrase or glucose–hexokinase died as well (Figure 7). Subjecting cell cultures of a monocot, maize (Zea mays), to all three treatments elicited the death response (Figure 8), indicating that the requirement of extracellular ATP for viability is not restricted to dicots. We conclude that extracellular ATP has a physiological role in plants that is evolutionarily conserved between distantly related species.
Figure 7.
Reaction of Bean and Tobacco to Extracellular ATP Removal.
(A) Control treatments with water (1), BSA (2), AMP/ADP (3), and glucose/glucose-6-phosphate/ADP (4) of tobacco.
(B) Local lesions in response to apyrase (5) or glucose–hexokinase (6) treatment of tobacco.
(C) Localized areas of a tobacco leaf treated with ATP (7) or AMP-PCP (8).
(D) Tobacco treated with ATP. The arrow indicates the treated leaf.
(E) Tobacco treated with AMP-PCP on the indicated leaf.
(F) Untreated bean plant.
(G) Water-treated (black arrow) and apyrase-treated (white arrow) bean leaves.
(H) Bean leaves treated with glucose (black arrow) or glucose–hexokinase (white arrow).
Bars = 2 cm for (A) to (C), 4 cm for (D) and (E), and 2 cm for (F) to (H).
Figure 8.
Extracellular ATP Is Required for the Viability of Maize Cell Cultures.
Viability of maize cell suspension cultures after treatment with water (control), 1 mM ATP, 1 mM AMP-PCP, 100 units/mL apyrase, or 50 mM glucose + 200 units/mL hexokinase (Glc-Hk). Data and error bars represent means ± sd (n = 3).
FB1 Triggers Extracellular ATP Depletion That Precedes Cell Death in Arabidopsis
Having established that the removal of extracellular ATP from plants or cell suspension cultures triggers death, we wondered whether some forms of plant cell death invoked during normal growth and development or during interaction with other organisms might be mediated by the ablation of extracellular ATP. We decided to investigate the pathogen-induced hypersensitive cell death, which can be simulated by treating plant tissues or cells with pathogen-derived elicitors (Levine et al., 1994). To explore the possibility that diminution of extracellular ATP mediates this response, we used FB1, a programmed cell death–eliciting mycotoxin that switches on active plant defenses in Arabidopsis (Stone et al., 2000).
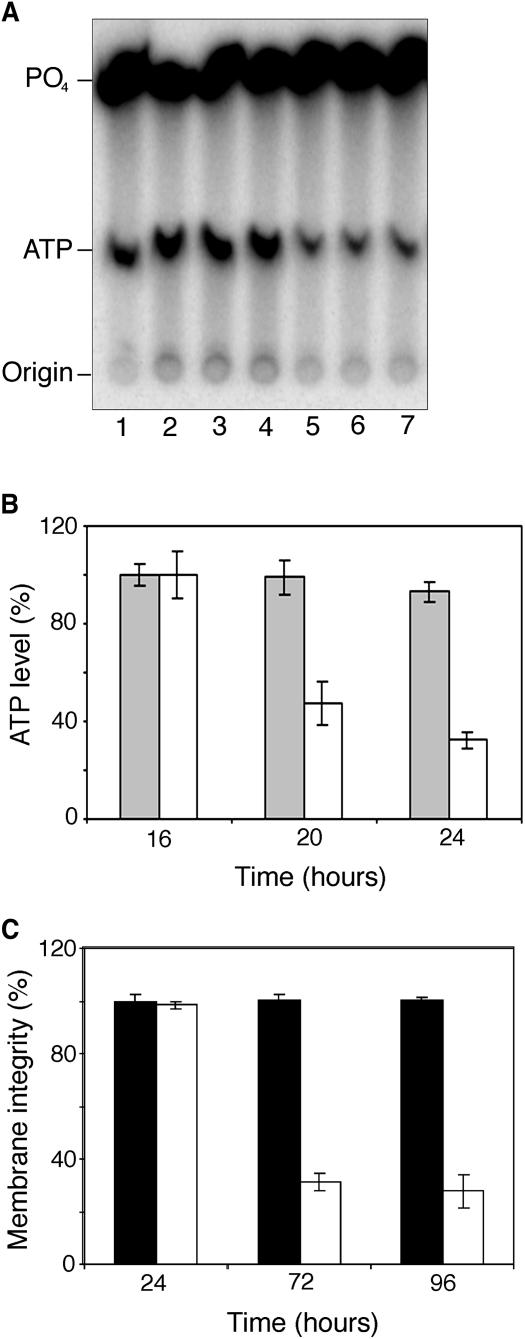
We wanted to monitor the fate of extracellular ATP in Arabidopsis tissues during FB1 treatment. Thus, we turned to cell suspension cultures, which offer a simple experimental system in which to sample the extracellular matrix without damaging cells and risking contamination with intracellular metabolites. To achieve this, we used tracer experiments in which cell cultures were spiked with [32P]ATP and treated with 1 μM FB1 or 0.014% (v/v) methanol as a control. There was a heightened increase in the hydrolysis of extracellular ATP in FB1-treated cells detectable after 16 h of treatment (Figure 9). This resulted in the rapid decrease of extracellular ATP level in FB1-treated cells to 24 h (Figure 9). The decline in extracellular ATP continued, and by 40 h, labeled ATP was undetectable in FB1-treated cultures, whereas it was still present in control cultures (data not shown). Examination of the integrity of the plasma membrane using Evans blue staining did not show any difference between control cells and FB1-treated cell cultures at 24 h (Figure 9), showing that the extracellular ATP depletion precedes the loss of plasma membrane integrity and cell death, which become obvious 72 h later (Figure 9), when the cells become chlorotic and start losing mitochondrial dehydrogenase activity (data not shown).
Figure 9.
FB1 Treatment Induces the Depletion of Extracellular ATP That Precedes the Loss of Membrane Integrity.
Light-grown Arabidopsis cell cultures were treated with methanol (control) or FB1 and spiked with [γ-32P]ATP.
(A) Growth medium aliquots from triplicate control (lanes 2 and 3) or FB1-treated (lanes 4 to 6) cultures 24 h after treatment and separated by TLC. Lane 1 contains free phosphate (PO4) and ATP standards.
(B) Level of radioactive ATP in growth medium (as a percentage of radioactivity initially added to the cell cultures) between 16 and 24 h. Gray bars, control cultures; white bars, FB1-treated cultures.
(C) Changes in the integrity of the plasma membrane over 96 h in control (black bars) and FB1-treated (white bars) cell cultures assayed by the Evans blue method.
Data and error bars represent means ± sd (n = 3).
Diminution of extracellular ATP before the loss of membrane integrity implies that the machinery for extracellular ATP removal is resident within the extracellular matrix and that this event occurs without leakage of intracellular ATP-degrading enzymes. This observation, placing extracellular ATP depletion upstream of FB1-induced death, was requisite if the hypothesis that FB1-induced death is mediated by the depletion of extracellular ATP was to be upheld.
Exogenous ATP Rescues Arabidopsis from FB1-Induced Death
Having established that FB1 treatment activates extracellular ATP depletion in Arabidopsis cell cultures, we performed experiments to establish whether this event was a mechanism that mediates FB1-induced cell death. We reasoned that if this cell death response were solely or partially mediated via this removal of extracellular ATP, then it would be interdicted or attenuated by the addition of exogenous ATP concomitant with treatment.
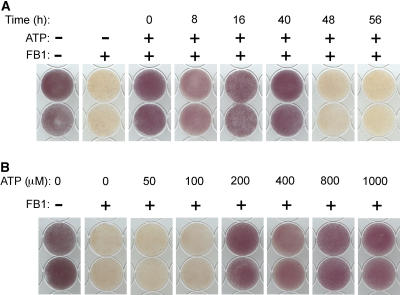
Treatment with 1 μM FB1 causes a progressive decline in the viability of Arabidopsis cell cultures, which becomes obvious at 72 h and is usually complete by 120 h. Death of the entire culture is reflected by the total loss of cellular dehydrogenase activity (Figure 10). In accordance with the prediction that extracellular ATP deficiency participates in FB1-induced death, exogenous ATP added to the cell cultures concurrently with FB1 attenuated cell death (Figure 10A) and delayed it by a minimum of 48 h. The dose response of FB1-induced death to exogenous ATP is shown in Figure 10B. We found that exogenous ATP added to the cells up to 40 h after the addition of FB1 could still rescue the cells from FB1-induced death (Figure 10A). However, when added at 48 h or later, ATP could not rescue the cells from elicitor-induced death. These results show that even though extracellular ATP depletion commences between 16 and 20 h after FB1 addition, irreversible commitment to death occurs at ∼48 h.
Figure 10.
Extracellular ATP Attenuates FB1-Induced Cell Death in Arabidopsis Cell Cultures.
(A) Light-grown cell cultures at a density of 5% (w/v) were treated with 1 μM FB1 at time 0 and spiked with 1 mM ATP at the given times. Cells were harvested at 120 h and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoium bromide (MTT) solution.
(B) Cell cultures were treated with 1 μM FB1 at time 0, and increasing concentrations of ATP were added 40 h later. Cells were harvested at 120 h and processed as in (A).
The formation of the purple formazan is an indication of viability; unstained cells are dead; + or – indicates addition or omission of the indicated compound, respectively.
Effect of Phosphate-Containing Compounds on FB1-Induced Death
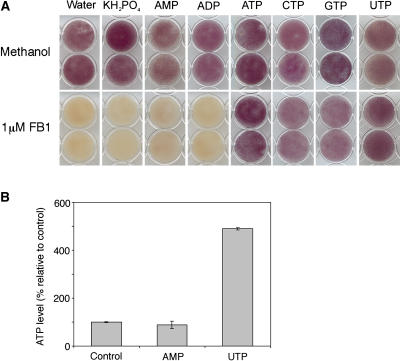
We were interested to determine whether or not the abrogation of FB1-induced death was unique to ATP and not an effect of the degradation products of ATP. It is clear from Figure 11A that exogenous phosphate, AMP, and ADP were unable to rescue the cells from FB1-induced death. This finding indicates that intact ATP, and not ADP, AMP, or phosphate released by nonspecific phosphatases (Bozzo et al., 2002; Olczak et al., 2003), was responsible for the rescue.
Figure 11.
Nucleoside Triphosphates Rescue Arabidopsis Cells from FB1-Induced Death.
(A) Light-grown cell cultures adjusted to a density of 5% (w/v) were treated with either 0.014% methanol (control) or 1 μM FB1 mixed with 1 mM of the indicated compounds. Cells were harvested 120 h later, and viability was determined via the MTT assay.
(B) Cell cultures were treated with water (control), 1 mM AMP, or 1 mM UTP and ATP in the growth medium assayed by the luciferin–luciferase method. Data and error bars represent means ± sd (n = 3).
Next, we decided to investigate the effects of other nucleoside triphosphates on FB1-induced death. CTP, GTP, and UTP had similar effects on FB1-induced death as ATP had (Figure 11A). There are reports in the literature suggesting that the addition of other nucleoside triphosphates to cell cultures leads to the accumulation of endogenous extracellular ATP via competitive inhibition of ATP hydrolysis by nonspecific ectonucleotide-degrading enzymes (Ostrom et al., 2000; Lazarowski et al., 2003). Thus, we tested the possibility that the other nucleotides may exert their effects on FB1-induced death indirectly, via the protection of endogenous extracellular ATP from hydrolysis. We treated cell cultures with AMP or UTP for 4 h and measured the level of ATP in the medium using the luciferin–luciferase method. The stock solutions of these compounds were tested for ATP contamination before addition to the cell cultures, and we found that they were not contaminated. We discovered that the ATP in the medium remained the same in AMP-treated cells but accumulated >4.5-fold in UTP-treated cell cultures (Figure 11B). This confirmed the possibility that the nucleotides may have interfered with FB1-induced death by acting as decoy substrates for the ATP-degrading enzymes and allowing ATP accumulation to protect the cells. This possibility is supported by the correlative evidence that AMP, a compound that fails to rescue cells, does not cause the accumulation of endogenous extracellular ATP, whereas UTP, a nucleotide that rescues elicitor-treated cells, does.
ATP Rescues Intact Plant Tissues from FB1-Induced Death
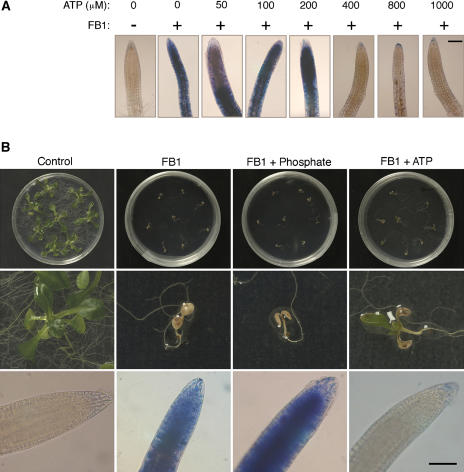
FB1 kills the roots of submerged hydroponic Arabidopsis plants, which become stainable with Evans blue 72 h after treatment. In conformity with a requirement for extracellular ATP depletion in FB1-induced death, exogenous ATP added to the plants concurrently with FB1 rescued the roots from death (Figure 12). Dose–response experiments revealed that a minimum of ∼400 μM ATP is required to rescue the roots of hydroponic Arabidopsis plants (Figure 12A). In other experiments, Arabidopsis plants were sown on agar plates and transferred 5 d later to plates supplemented with FB1 or FB1 mixed with ATP. At this growth stage, the plants had fully expanded cotyledons. The cotyledons of plants in both treatments died within 1 week, and growth of true leaves proceeded in the plants with FB1 + ATP but not with FB1 only (Figure 12B). This finding shows that FB1 treatment led to the death of these plants, whereas ATP rescued the plants despite the death of the cotyledons.
Figure 12.
ATP, but Not Phosphate, Rescues Arabidopsis Plants from FB1-Induced Death.
(A) Hydroponic plants were treated with methanol (control) or 1 μM FB1 mixed with the indicated concentrations of ATP. Roots were stained 72 h later with Evans blue to determine viability. Bar = 1 μm; + or – implies addition or omission of the indicated compound, respectively.
(B) Arabidopsis plants were germinated on nutrient agar and transferred after 5 d to plates with agar supplemented with 0.014% methanol (control), 1 μM FB1, or 1 μM FB1 mixed with either 1 mM ATP or 1 mM KH2PO4. Top row, retardation of growth in all treatments containing FB1. Middle row, close-up photographs of representative plants from corresponding plates in the top row. Bottom row, root tips from hydroponic plants subjected to the same treatments for 72 h and stained with Evans blue to reveal cell death. It is clear from the middle and bottom rows that only ATP was able to rescue the plants from FB1-induced death. Bar = 1 μm for the bottom row.
One interpretation of this is that the plants initially absorbed FB1 into the cotyledons and the lack of uptake of the membrane-impermeant ATP precluded the protection of the cotyledons by extracellular ATP. Because ATP was restricted to the rhizosphere, it rescued the roots from death. After FB1 degradation or depletion from the vicinity of the roots, the roots were able to supply the apical meristem with nutrients and growth resumed. However, ATP did not rescue the plants from FB1-induced growth retardation (Figure 12), demonstrating that ATP does not indiscriminately abolish FB1-induced effects but specifically targets the death response. Exogenous phosphate could not rescue the plants from FB1-induced death (Figure 12), indicating that phosphate cleaved from ATP by nonspecific acid phosphatases, ATPases, or apyrases does not account for the observed ATP effects. Overall, these results suggest that removal of extracellular ATP is a critical event mediating FB1-induced death in Arabidopsis.
Extracellular ATP Depletion Alters the Abundance of Cellular Protein
We wanted to investigate whether there were any changes in the abundance of cellular proteins associated with the removal of extracellular ATP. Total soluble proteins from cells treated with glucose (control) or glucose–hexokinase were analyzed by two-dimensional difference gel electrophoresis and the BVA module of the DeCyder software (GE Healthcare). Protein spots whose abundance reproducibly increased or decreased significantly (t test; P < 0.05) by a factor of ≥20% across five independent biological replicates in response to extracellular ATP removal were identified by mass spectrometry and database searches. A total of 34 protein spots responsive to treatment were identified via mass spectrometry, and 13 of these decreased while the rest increased in abundance (Table 1).
Table 1.
Proteins Altered in Abundance by Treatment with Glucose–Hexokinase
| Spot No.a | Protein Name | Accessionb | Ratioc |
|---|---|---|---|
| Protein folding | |||
| 632 | Heat shock protein 81-2 | At5g56030 | −1.50 |
| 633 | Heat shock protein 81-2 | At5g56030 | −1.50 |
| 636 | Heat shock protein 81-2 | At5g56030 | −1.49 |
| 729 | Putative heat shock protein 70 | At4g24280 | 1.20 |
| 1063 | Putative protein disulfide isomerase | At1g21750 | 1.21 |
| Mitochondrial proteins | |||
| 440 | Putative aminopeptidase | At1g63770 | 1.22 |
| 1238 | Aldehyde dehydrogenase, mitochondrial | At3g48000 | 1.29 |
| 1242 | Aldehyde dehydrogenase, mitochondrial | At3g48000 | 1.25 |
| 1959 | Malate dehydrogenase (NAD), mitochondrial | At1g53240 | 1.27 |
| Amino acid metabolism | |||
| 482 | Nodulin/Gln-ammonia ligase–like | At3g53180 | 1.42 |
| 1135 | Putative Ala aminotransferase | At1g17290 | 1.24 |
| 1187 | Putative Ala aminotransferase | At1g17290 | 1.30 |
| 1098 | Ketol-acid reductoisomerase | At3g58610 | 1.23 |
| 1516 | S-Adenosylmethionine synthetase | At3g17390 | −1.37 |
| 1525 | S-Adenosylmethionine synthetase | At3g17390 | −1.37 |
| 1570 | S-Adenosylmethionine synthetase | At3g17390 | −1.54 |
| 1616 | Aminoacylase | At4g38220 | 1.29 |
| 1718 | Gln synthetase–related protein | At3g17820 | −1.38 |
| Carbohydrate metabolism | |||
| 1497 | Putative phosphoglycerate kinase | At1g79550 | −1.41 |
| 1731 | Fructose-bisphosphate aldolase | At3g52930 | 1.23 |
| 1817 | Glyceraldehyde-3-phosphate dehydrogenase | At3g04120 | 1.21 |
| 1943 | Malate dehydrogenase (EC 1.1.1.3) | At3g47520 | 1.24 |
| 2200 | Phosphoglycerate mutase | At2g17280 | −1.26 |
| Antioxidative proteins | |||
| 2562 | Putative GST | At2g30870 | −1.29 |
| 2575 | Putative GST | At1g02930 | 1.96 |
| 2591 | Putative GST | At1g02930 | 3.01 |
| 2602 | Putative GST | At1g02930 | 2.98 |
| 2605 | Putative quinone reductase | At5g54500 | 1.69 |
| Unclassified | |||
| 396 | Putative aconitase | At4g26970 | 1.20 |
| 848 | Putative ferredoxin–nitrite reductase | At2g15620 | 1.21 |
| 906 | Vacuolar ATPase, A subunit | At1g78900 | 1.21 |
| 1200 | Tubulin α-3/α-5 chain | At5g19770 | −1.66 |
| 2282 | Expressed protein | At3g22850 | −1.29 |
| 2370 | ATP-dependent Clp protease, proteolytic subunit | At1g09130 | −1.71 |
Spot number on the master gel.
Munich Information Center for Protein Sequences database accession number.
Paired average ratio: treatment:control for upregulation, control:treatment for downregulation. A minus sign denotes a decrease in abundance.
These results reveal that deprivation of extracellular ATP triggers a change in cellular metabolism by engendering significant alterations in the abundance of cellular proteins. The list of responsive proteins includes mitochondrial, cytosolic, chloroplast, and endoplasmic reticulum proteins (Table 1), suggesting a requirement for extracellular ATP in the maintenance of metabolic homeostasis across cellular organelles. Of note was the massive response of several spots of the stress-responsive glutathione S-transferase (GST) proteins. Three spots identified as a single GST (At1g02930) were upregulated, whereas another isoform (At2g30870) found in a single protein spot was downregulated. This finding confirms at the protein level that the presence of extracellular ATP is so vital that its withdrawal elicits a stress response that results in death, as described above.
DISCUSSION
ATP Release
The existence of extracellular ATP in plants has been reported previously (Thomas et al., 2000; Jeter et al., 2004). This study has gone further and demonstrated, using radiolabeling, that ATP is rapidly synthesized and secreted in plant cell cultures and that its level is tightly regulated, presumably by a dynamic balance between active release and hydrolysis or utilization by enzymes in the extracellular matrix. This is supported by the observation that within 1 h of feeding cells with radioactive phosphate, the cells had synthesized and released radioactive ATP and its level remained unchanged 6 h later. In animal cell systems, extracellular ATP homeostasis is governed by a complex interplay of dephosphorylating and transphosphorylating ectoenzyme activities in concert with regulated release from cells. A balance between ectonucleotide pyrophosphatase and nucleoside diphosphokinase has been reported to play a role in regulating extracellular ATP in human and rat cell lines (Lazarowski et al., 2000). Extracellular ATP level in human blood is regulated by a balance of extracellular hydrolytic activity of nucleotide pyrophosphatase and 5′-nucleotidase and the opposite extracellular ATP-generating pathway requiring ectoadenylate kinase and ectonucleoside diphosphokinase (Yegutkin et al., 2003). Although ATP hydrolytic enzymes have been identified in the plant extracellular matrix, no extracellular adenylate kinases or nucleoside diphosphokinases have been reported; therefore, a role for extracellular ATP-generating reactions in plant extracellular ATP homeostasis remains to be determined.
Extracellular ATP and Cell Viability
To find the physiological function of ATP in plants, we used a strategy of depleting the level of endogenous extracellular ATP and examining the plant tissues for any attendant responses. Our results have revealed a new role for extracellular ATP in plants, namely, the regulation of plant cell viability. Enzymatic removal of extracellular ATP was consistently followed by death, both in cell cultures and in intact plants of several species. This suggests that extracellular ATP is a crucial factor required to suppress a default cell death pathway in plants. Our results reveal a fundamental difference between the effects of extracellular ATP in plants and animals; although extracellular ATP is a crucial requirement for plant cell viability, it is a cytotoxic factor in particular animal cells expressing P2X7 purinoceptors (Ferrari et al., 1997; von Albertini et al., 1998).
The observation that ATP in the pericellular space is vital for sustaining cell viability is consistent with several reports demonstrating that the extracellular matrix is indispensable for cell viability in both plants and animals. For example, perturbation of Arabidopsis extracellular arabinogalactans using Yariv reagents induces programmed cell death (Gao and Showalter, 1999), implying that arabinogalactans are part of an extracellular matrix machinery that sustains viability during normal growth. In animals, default cell death is switched on by ablation of adrenocorticotropic hormone, a hormone that binds to cell surface receptors and normally suppresses the death of adrenocortical cells (Wyllie et al., 1973; Negoescu et al., 1995).
The presence of such a default death pathway regulated by extracellular signal molecules secreted by cells has been demonstrated before in plants. This default death pathway is switched on when plant cells are plated below a critical cell density, but cell-free growth medium from high-density cultures rescues these cells from death (McCabe et al., 1997). The nature of these secreted signals is still unclear, but our study has identified extracellular ATP as a hitherto unknown extracellular metabolite that regulates cell death and is indispensable for viability.
However, in addition to its role in modulating cell death/viability, we predict that extracellular ATP in plants will turn out to participate in numerous physiological processes depending on cell type and developmental stage, similar to the situation in animal cells (Gordon, 1986; Redegeld et al., 1999). The clues obtained from the observed effects of exogenous ATP on Arabidopsis will eventually lead to the discovery of more physiological functions of endogenous extracellular ATP in plants. The effects of exogenous ATP currently known include the inhibition of root gravitropism (Tang et al., 2003) and the activation of mitogen-activated protein kinases and the ethylene biosynthetic pathway (Jeter et al., 2004) in Arabidopsis.
How Does Extracellular ATP Sustain Cell Viability?
The mechanism by which extracellular ATP sustains the viability of plant cells is unclear, but we discuss the potential mechanisms below. Extracellular ATP might directly or indirectly interact with an extracellular protein whose activity is vital for viability and is dependent on ATP. The interaction may serve to trigger a cascade of events that results in the inactivation of a prodeath protein by its binding to a death inhibitor protein. Removal of extracellular ATP from its extracellular interacting protein leads to the disassociation of such a complex, and the death activator triggers death. This model requires cells to perceive the presence of extracellular ATP and activate intracellular biochemical signaling events. There is already evidence that, in Arabidopsis, extracellular ATP activates Ca2+ influx (Demidchik et al., 2003; Jeter et al., 2004), a universal progenitor of intracellular signaling cascades.
ATP could possibly exert its protective effects by interacting with its extracellular target in one of three ways or a combination of them. First, it could provide its γ-phosphate for phosphorylating a target protein. This notion is supported by the observation that overriding the binding of extracellular ATP to targets by a nonhydrolyzable analog induced death. A simplistic prediction would be that extracellular ATP is required for phosphorylating some extracellular or membrane-bound pro-death substrate that becomes inactivated when phosphorylated. An example of this type of regulation is the phosphorylation-dependent posttranslational modulation of plant nitrate reductase activity. Inhibitory 14-3-3 proteins bind to and inactivate only phosphorylated nitrate reductase but neither bind nor inhibit nonphosphorylated forms of the same protein (Kaiser and Huber, 2000; Lillo et al., 2004). Although the presence of extracellular phosphoproteins has been reported (Chivasa et al., 2002; Ndimba et al., 2003), plant ectoprotein kinases responsible for the phosphorylation have yet to be identified. Second, extracellular ATP could act as a ligand, binding directly to the extracellular domain of a transmembrane receptor similar to animal P2X and P2Y receptors. There is biochemical and electrophysiological evidence for the presence of P2X-type receptors in plants, but none has been identified to date. Third, the full ATP molecule may mediate the binding of two extracellular proteins. For example, the neuronal apoptosis inhibitor protein (NAIP) undergoes a conformational change that enables it to bind to caspase-9 only when there is an ATP molecule in its nucleotide binding domain (Davoodi et al., 2004). Binding of NAIP to caspases prevents default cell death in neurons (Maier et al., 2002).
Results obtained with the nonhydrolyzable ATP analog indicate that ATP cleavage is required for cell viability. The potential role of P2X- or P2Y-type receptor activation and the possibility that ATP functions as an adaptor for binding cognate protein partners during the initiation of signaling cascades to sustain cell viability will require further investigation. The primary target protein/molecule for ATP in the extracellular milieu is not known, and its discovery will lead to the identification of components of this pathway. There is precedence from animal cells for individual extracellular proteins to sustain viability by inhibiting default cell death. For example, neutrophin-3, a secretory protein that binds to cell surface receptors of neuronal cells, is required to sustain their viability, and its gene deletion or blockade by specific antiserum triggers a default programmed cell death response (Zhou and Rush, 1995). Unraveling the mechanism by which extracellular ATP sustains plant cell viability is now the subject of further experimental investigations by our group.
Changes in Protein Abundance in Response to Extracellular ATP Removal
To elucidate the molecular basis for the ability of ATP to sustain plant cell viability, we used the glucose–hexokinase system to deplete extracellular ATP and analyzed attendant changes in the proteome of Arabidopsis. We found that removal of ATP in the pericellular space perturbed the steady state levels of a diverse range of cellular proteins in various organelles and numerous biochemical pathways. These changes in intracellular protein abundance in response to extracellular ATP removal suggest that signals initiated by extracellular ATP perception funnel into numerous intracellular metabolic pathways spanning several cellular compartments. This reinforces, at the molecular level, the finding that cells are dependent on extracellular ATP for viability.
Previous studies of plant programmed cell death responses have identified changes in the expression of genes involved in primary metabolism, molecular chaperones, and other enzymes regulating protein folding, mitochondrial proteins, and antioxidative stress proteins (Desikan et al., 1998; Swidzinski et al., 2002, 2004). Arabidopsis microarray data available via the Genevestigator database (http://www.genevestigator.ethz.ch) and web browser data-mining interface (Zimmermann et al., 2004) reveal that most of the genes for the proteins identified in this study are differentially expressed in response to a diverse range of death-inducing treatments such as herbicides, toxins, and attack by bacterial and fungal pathogens. This finding suggests that cell death induced by extracellular ATP depletion possibly funnels into a shared core death pathway at some point downstream from the position where ATP deprivation is crucial. The concept of a shared core death machinery into which disparate signaling pathways activated by various pro-death stimuli feed and converge has been proposed before (McCabe and Leaver, 2000). This is similar to the sensory and signaling cascades in the cyanobacterial stress response, in which various stresses have unique components close to the initial stimulus sensing but share common components of the signaling cascades (Marin et al., 2003).
The strongest response was observed in GSTs, a family of stress-responsive proteins whose gene expression can be induced by pathogen attack (Lieberherr et al., 2003) or treatment with pathogen-derived elicitors (Desikan et al., 1998). Wagner et al. (2002) reported that salicylic acid suppresses gene expression of the GST (At2g30870) whose abundance at the protein level decreased in response to extracellular ATP deprivation in this study. Gene expression of the GST protein (At1g02930) upregulated by ATP removal in our study is also activated by endogenous signaling molecules such as H2O2, salicylic acid, ethylene, and jasmonic acid (Wagner et al., 2002). This suggests that the cell death activated by the removal of extracellular ATP may potentially be mediated by some of these signaling molecules. Indeed, it is already known that FB1, which triggers death via extracellular ATP removal, induces the accumulation of reactive oxygen species in Arabidopsis and that FB1-induced death in the same species requires ethylene, salicylic acid, and jasmonic acid signaling (Asai et al., 2000).
Depletion of Extracellular ATP Mediates Elicitor-Induced Cell Death
Cell death is an essential part of plant development that is regulated by a programmed genetic template and affects single cells, particular cell layers, or entire organs (Buchanan-Wollaston, 1997; Fukuda, 1997; Groover et al., 1997) and is invoked in the hypersensitive response to pathogen attack (Lam et al., 2001). Having established that extracellular ATP is vital to sustain cell viability and that artificially removing it triggers cell death, we discovered that depletion of extracellular ATP mediates an elicitor-induced cell death response. FB1, a mycotoxin that acts as an elicitor in Arabidopsis and triggers defense responses and cell death, induced a heightened hydrolysis of extracellular ATP preceding the breach in plasma membrane integrity and the onset of death. Crucially, exogenous ATP was able to rescue plants treated with the elicitor from death. Elicitor-induced cell death requires an influx of apoplastic Ca2+ into the cell, and its chelation can block cell death (Levine et al., 1996). However, the effect of exogenous ATP on FB1-induced death is unlikely the result of chelation of divalent cations because 1 mM solutions of AMP and ADP, which have a higher chelation capacity than 200 μM ATP, failed to abolish cell death when 200 μM ATP blocked it. In cell cultures, prevention of ATP depletion by adding exogenous ATP concurrently with FB1 or correcting the deficit by the addition of exogenous ATP several hours later, up to 40 h, was able to prevent death. When added 48 h after FB1 or later, exogenous ATP was unable to rescue cells from death, implying that commitment to death occurs ∼48 h from FB1 addition.
In cell cultures, it is clear that FB1 upregulates an extracellular ATP hydrolytic activity after a lag of ∼16 h. This activity does not discriminate between different nucleoside triphosphates and appears not to act on nucleoside monophosphates and diphosphates, as shown by the failure of AMP and ADP to block FB1-induced death, which was abolished by all of the tested nucleoside triphosphates. The observation that a pathogen elicitor stimulates extracellular nucleoside triphosphatase activity is not unprecedented because elicitors have been reported to activate membrane-bound and cell wall–bound ATPase activity (Kiba et al., 1995, 1999). The exact mechanism by which FB1 activates extracellular ATP hydrolysis is not yet defined. However, plant extracellular ATP hydrolysis is performed by ectoapyrases (Komoszynski and Wojtczak, 1996), cell wall–bound ATPases (Kivilaan et al., 1961; Shiraishi et al., 1991), and extracellular purple acid phosphatases (Bozzo et al., 2002; Olczak et al., 2003). Perhaps FB1 stimulates the activity of a similar extracellular enzyme, as has been reported for other elicitors. For example, chitin, a pathogen-derived elicitor known to trigger cell death (Tada et al., 2001), has been reported to specifically bind and stimulate the activity of an extracellular apyrase (Etzler et al., 1999). Recently, a fungal pathogen elicitor was reported to stimulate a recombinant plant apyrase in vitro (Kawahara et al., 2003). However, the delay between FB1 addition and the activation of extracellular ATP hydrolysis might be an indication that FB1 does not directly activate the enzymes but requires de novo synthesis of secondary messengers or, alternatively, that synthesis and secretion of the hydrolytic enzymes occur during the lag period.
Our results allow a rationalization of extracellular ATP, cell death, and the response of plant cells to some pathogen elicitors. Elicitors clearly affect cell viability (Levine et al., 1994) and have been reported to activate enzymes that modulate the level of extracellular ATP (Kiba et al., 1995; Etzler et al., 1999; Kawahara et al., 2003). Further studies on a wide range of elicitors and incompatible host–pathogen combinations are now required to investigate whether or not hypersensitive cell death in general is mediated via extracellular ATP depletion.
METHODS
Plant Material and Growth Conditions
Cell suspension cultures of Arabidopsis thaliana var erecta were maintained as described previously (May and Leaver, 1993). Maize (Zea mays) cell cultures were maintained by weekly 10-fold dilution in fresh Murashige and Skoog (MS) growth medium (Murashige and Skoog, 1962) with 2% (w/v) sucrose and 2 mg/L 2,4-D. Suspension cell cultures of both species were grown either in continuous darkness or with a 16-h photoperiod at 25°C and were used for treatment 3 to 5 d after transfer to fresh medium. Dark-grown cell cultures were used in all experiments except for those involving FB1 treatments, in which light-grown cell cultures were used because FB1-induced cell death in plants is dependent on light (Asai et al., 2000; Stone et al., 2000). Glucose–hexokinase treatments were performed with both light- and dark-grown cell cultures. Tobacco (Nicotiana tabacum), Landsberg erecta and Columbia ecotypes of Arabidopsis, and bean (Phaseolus vulgaris) plants for injecting with solutions were grown in soil in a growth cabinet with a 16-h photoperiod at 20°C and 8 h of darkness at 15°C. RH was maintained at 60%, and the photon flux density was 250 μmol·m−2·s−1. Plants were used for treatment 5 to 8 weeks after sowing.
Treatments
Enzymes were dissolved in water and filter-sterilized (0.2 μm). Dialysis was performed against water using membranes with a molecular mass cutoff of 8 kD. AMP, ADP, ATP, and AMP-PCP stock solutions in water were neutralized to pH 7.0 using KOH and filter-sterilized. Whole plants were treated by infiltration of the apoplast of a small area or entire leaf on the abaxial surface using a syringe with or without a hypodermic needle. Concentrations used to treat intact plant tissues were 6 mg/mL BSA, 1 mM AMP, 1 mM ADP, 5 mM ATP, 5 mM AMP-PCP, 50 mM glucose, 1 mM glucose-6-phosphate, 0.5 units/μL apyrase, and 1.85 units/μL hexokinase. In cell culture experiments, glucose was used at a concentration of 100 mM. Cell cultures were incubated with the appropriate solutions in a final volume of 1.5 mL and a cell density of 5% (w/v). After 72 h, the cultures were diluted to 50 mL in fresh growth medium. Ninety-six hours later, 1-mL aliquots were withdrawn and the packed cell volume was determined as a percentage of the culture volume. A 7 mM stock solution of FB1 was prepared in absolute methanol, and a working solution of 50 μM (diluted in water) was used for treatments at a final concentration of 1 μM, with an equivalent volume of 0.71% methanol being used to treat controls.
Hydroponic Experiments
Floating Plants
Seeds of Arabidopsis ecotype Columbia were surface-sterilized as described previously (Topping and Lindsey, 1991) and sown on 7-cm-diameter, 1-cm-thick polyurethane foam discs soaked in MS medium (0.22% [w/v] MS salts, 1% [w/v] sucrose, adjusted to pH 5.7 with KOH/HCl). The discs were placed in sterile phytatrays (Sigma-Aldrich) and incubated under a 16-h-light/8-h-dark cycle at 22°C. One week later, when the seeds had germinated and the roots had penetrated the foam, 30 mL of MS medium was added to the phytatray, which caused the foam discs to float. The trays were transferred to a shaking platform (25 rpm) under the same environmental conditions. During the second week, the roots of the plants had emerged through the other side of the foam and were submerged in the nutrient-rich medium. Exactly 2 weeks after sowing, the plants were treated with water (control), 45 mM glucose, 45 mM glucose + 1 mM glucose-6-phosphate, and 45 mM glucose + 100 units/mL hexokinase.
Submerged Plants
Surface-sterilized Arabidopsis seeds were incubated in 5 mL of MS medium in 25-mL conical glass flasks on an orbital shaker (120 rpm) at 22°C in a 16-h photoperiod regime. Five days later, the plants were treated with 1 μM FB1 and 1 μM FB1 mixed with either 1 mM ATP or 1 mM KH2PO4. At 72 h after treatment, the plants were stained with Evans blue dye as described below.
Agar Plate Assays
Surface-sterilized Arabidopsis seedlings were plated on 0.4% (w/v) agar-containing MS medium. Five days later, the plants were transferred to agar plates supplemented with 1 μM FB1 or 1 μM FB1 + 1 mM ATP. The plants were photographed 4 weeks later. This experiment was performed using both the Landsberg erecta and Columbia ecotypes of Arabidopsis.
Microscopy
Roots of Arabidopsis treated with glucose or glucose–hexokinase were stained with fluorescein diacetate and examined using a LSM 510 Meta microscope (Carl Zeiss). Autofluorescence was acquired at an excitation wavelength of 543 nm and an emission long pass of 560 nm. The fluorescence of fluorescein diacetate was detected at an excitation wavelength of 488 nm and an emission band pass between 505 and 530 nm. The same tissues were also imaged using transmitted light phase contrast, and all three images were merged.
Cell Death Assay
Determination of cell viability by the MTT assay was performed as described previously (Watts et al., 1989) with minor modifications. An aliquot of 400 μL of 5 mg/mL MTT solution in water was incubated with 5 mL of cell culture for 4 h. A lawn of cells was placed in wells of a microtiter plate and scanned to show the color development.
Evans blue assay for the death (and plasma membrane integrity) of cell cultures was performed by incubating triplicate 100-μL cell aliquots with a final 0.0125% (w/v) Evans blue solution for 20 min. Cell death levels in the treatments were obtained by spectrophotometrically (600 nm) determining the amount of dye taken up by cells and assuming that control cells were 100% viable and cells boiled for 5 min before staining were 0% viable. For hydroponic plants, five plants in 5 mL of 0.04% (w/v) Evans blue solution were vacuum-infiltrated by holding the vacuum for 30 min and then washed three times with water for 4 h each wash.
ATP Measurements
De novo synthesis and secretion of ATP were performed by feeding 100 μCi of [32P]H3PO4 to 5 mL of 3-d-old light-grown cell cultures adjusted to a density of 1% (w/v). Cell-free 170 μL of medium was withdrawn 1 h later and acidified with 0.6% trichloroacetic acid. Aliquots of 2 μL were mixed with an equal volume of 50 mM citrate buffer, pH 5.5, and separated by thin layer chromatography (TLC) on silica gel plates with dioxane:ammonium hydroxide:water (6:1:6, v/v/v). To demonstrate the activity of apyrase and hexokinase, cell cultures labeled with a similar amount of [32P]H3PO4 for 1 h were treated with 100 units/mL apyrase or 100 mM glucose + 200 units/mL hexokinase for a further 6 h, and medium samples were analyzed by TLC. Apyrase at 20 units/mL was also used in parallel experiments.
To monitor extracellular ATP during FB1 treatment, 5 mL of light-grown cell cultures was adjusted to 1% (w/v) density, treated with 0.014% (v/v) methanol as a control or 1 μM FB1, and spiked with 4.5 μCi [γ-32P]ATP 16 h later. Cell-free medium aliquots were withdrawn periodically and analyzed by TLC as described above.
In other experiments, medium ATP was measured by the nonradioactive luciferin–luciferase method using the Enlighten ATP assay kit (Promega). Cell-free 100-μL medium aliquots were mixed with 2 μL of 50% (w/v) trichloroacetic acid containing 0.0005% (w/v) acid blue 147 and stored at −20°C. For ATP assay, 5 μL was mixed with 95 μL of 100 mM Tris-acetate buffer, pH 7.8, and the reaction was started by the addition of 30 μL of luciferin–luciferase followed by a reading delay of 0.3 s and an integration time of 10 s using the Anthos Lucy 1 luminometer (Labtech International).
Proteomic Analyses
Aliquots of 5 mL each of 3-d-old, dark-grown Arabidopsis cell cultures were treated with 100 mM glucose (to serve as controls) or a combination of 100 mM glucose plus 200 units/mL hexokinase. The experiment was performed using five independent biological replicates. Cells were harvested 48 h later, and protein was extracted and analyzed by two-dimensional difference gel electrophoresis as described previously (Ndimba et al., 2005). Gel images were scanned using the Typhoon 9400 scanner (GE Healthcare), and image analysis was performed using the DIA and BVA modules of the DeCyder software (GE Healthcare) as described before (Ndimba et al., 2005). Proteins whose abundance changed (up or down) by a minimum of 20% were excised from silver-stained gels, with a total protein loading of 400 μg, and identified via mass spectrometry and database (National Center for Biotechnology Information nonredundant database) searches using the methods described by Chivasa et al. (2002).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Antoni R. Slabas (a.r.slabas@durham.ac.uk).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.036806.
References
- Abraham, E., Prat, A., Gerweck, L., Seneveratne, T., Arceci, R., Kramer, R., Guidotti, G., and Cantiello, H. (1993). The multidrug resistance (MDR1) gene product functions as an ATP channel. Proc. Natl. Acad. Sci. USA 90, 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, T., Stone, J.M., Heard, J.E., Kovtun, Y., Yorgey, P., Sheen, J., and Ausubel, F.M. (2000). Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signalling pathways. Plant Cell 12, 1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi, R.D., and Dubyak, G.R. (2000). Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J. Immunol. 165, 7189–7198. [DOI] [PubMed] [Google Scholar]
- Boldin, P., and Burnstock, G. (2001). Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969. [DOI] [PubMed] [Google Scholar]
- Bozzo, G.G., Raghothama, K.G., and Plaxton, W.C. (2002). Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures. Eur. J. Biochem. (Tokyo) 269, 6278–6286. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston, V. (1997). The molecular biology of leaf senescence. J. Exp. Bot. 48, 181–199. [Google Scholar]
- Burnstock, G., and Kennedy, C. (1985). Is there a basis for distinguishing two types of P2-purinoceptors? Gen. Pharmacol. 16, 433–440. [DOI] [PubMed] [Google Scholar]
- Chivasa, S., Ndimba, B.K., Simon, W.J., Robertson, D., Yu, X.-Y., Knox, J.P., Bolwell, P., and Slabas, A.R. (2002). Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis 23, 1754–1765. [DOI] [PubMed] [Google Scholar]
- Davoodi, J., Lin, L., Kelly, J., Liston, P., and MacKenzie, A.E. (2004). Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase-9. J. Biol. Chem. 279, 40622–40628. [DOI] [PubMed] [Google Scholar]
- Demidchik, V., Nichols, C., Oliynyk, M., Dark, A., Glover, B.J., and Davies, J.M. (2003). Is ATP a signalling agent in plants? Plant Physiol. 133, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R., Reynolds, A., Hancock, J.T., and Neill, S.J. (1998). Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 330, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, S., Anderson, C.M., Keung, E.C., Chen, Y., Chen, Y., and Swanson, R.A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, A.K., Okada, Y., and Sabirov, R.Z. (2002). Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J. Physiol. 542, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, A.K., Sabirov, R.Z., Uramoto, H., and Okada, Y. (2004). Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J. Physiol. 559, 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzler, M.E., Kalsi, G., Ewing, N.N., Roberts, N.J., Day, R.B., and Murphy, J.B. (1999). A nod factor binding lectin with apyrase activity from legume roots. Proc. Natl. Acad. Sci. USA 96, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, D., Wesselborg, S., Bauer, M.K., and Schulze-Osthoff, K. (1997). Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoceptor by selectively targeting NF-kappaB p65 (RE1A). J. Cell Biol. 139, 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske, C.H., and Subarrow, Y. (1929). Phosphorous compounds of muscle and liver. Science 70, 381–382. [DOI] [PubMed] [Google Scholar]
- Fukuda, H. (1997). Tracheary element differentiation. Plant Cell 9, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M., and Showalter, A.M. (1999). Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant Cell 19, 321–331. [DOI] [PubMed] [Google Scholar]
- Glynn, I.M. (1968). Membrane adenosine triphosphatase and cation transport. Br. Med. Bull. 24, 165–169. [DOI] [PubMed] [Google Scholar]
- Gordon, J.L. (1986). Extracellular ATP: Effects, sources and fate. Biochem. J. 233, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson, J.P., and Meldolesi, J. (1995). Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J. Biol. Chem. 270, 4451–4456. [DOI] [PubMed] [Google Scholar]
- Groover, A., Dewitt, N., Heidel, A., and Jones, A. (1997). Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma 196, 197–211. [Google Scholar]
- Holton, P. (1959). The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. (Lond.) 145, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya, L., Steinberg, A.D., and Boucher, R.C. (2000). Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarised epithelia. J. Cell Biol. 150, 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter, C.R., Tang, W., Henaff, E., Butterfield, T., and Roux, S.J. (2004). Evidence of a novel cell signalling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16, 2652–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, W.M., and Huber, S.C. (2000). Post-translational regulation of nitrate reductase: Mechanism, physiological relevance and environmental triggers. J. Exp. Bot. 52, 1981–1989. [DOI] [PubMed] [Google Scholar]
- Kawahara, T., Toyoda, K., Kiba, A., Miura, A., Ohgawara, T., Yamamoto, M., Inagaki, Y., Ichinose, Y., and Shiraishi, T. (2003). Cloning and characterization of pea apyrases: Involvement of PsAPY1 in response to signal molecules from the pea pathogen Mycosphaerella pinodes. J. Gen. Plant Pathol. 69, 33–38. [Google Scholar]
- Kiba, A., Takeda, T., Kanemitsu, T., Toyoda, K., Ichinose, Y., Yamada, T., and Shiraishi, T. (1999). Induction of defence responses by synthetic glycopeptides that have a partial structure of the elicitor in the spore germination fluid of Mycosphaerella pinodes. Plant Cell Physiol. 40, 978–985. [DOI] [PubMed] [Google Scholar]
- Kiba, A., Toyoda, K., Ichinose, Y., Yamada, T., and Shiraishi, T. (1995). Specific inhibition of cell wall-bound ATPases by fungal suppresser from Mycosphaerella pinodes. Plant Cell Physiol. 36, 809–817. [Google Scholar]
- Kivilaan, A., Beaman, T.C., and Bandurski, R.S. (1961). Enzymatic activities associated with cell wall preparations from corn coleoptiles. Plant Physiol. 36, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoszynski, M., and Wojtczak, A. (1996). Apyrases (ATP diphosphohydrolases, EC 3.6.1.5): Function and relationship to ATPases. Biochim. Biophys. Acta 1310, 233–241. [DOI] [PubMed] [Google Scholar]
- Lam, E., Kato, N., and Lawton, M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 848–853. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., Boucher, R.C., and Harden, T.K. (2000). Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 275, 31061–31068. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., Boucher, R.C., and Harden, T.K. (2003). Mechanisms of release of nucleotides and integration of their actions as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795. [DOI] [PubMed] [Google Scholar]
- Leitner, J.W., Sussman, K.E., Vatter, A.E., and Schneider, F.H. (1975). Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology 96, 662–677. [DOI] [PubMed] [Google Scholar]
- Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R., and Lamb, C. (1996). Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6, 427–437. [DOI] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Lew, R.R., and Dearnaley, J.D.W. (2000). Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci. 153, 1–6. [Google Scholar]
- Lieberherr, D., Wagner, U., Dubuis, P.-H., Metraux, J.-P., and Mauch, F. (2003). The rapid induction of glutathione S-transferases AtGSTF2 and AtGSTF6 by avirulent Pseudomonas syringae is the result of combined salicylic acid and ethylene signalling. Plant Cell Physiol. 44, 750–757. [DOI] [PubMed] [Google Scholar]
- Lillo, C., Meyer, C., Lea, U.S., Provan, F., and Oltedal, S. (2004). Mechanism and importance of post-translational regulation of nitrate reductase. J. Exp. Bot. 55, 1275–1282. [DOI] [PubMed] [Google Scholar]
- Maier, J.K., Lahoua, Z., Gendron, N.H., Fetni, R., Johnston, A., Davoodi, J., Rasper, D., Roy, S., Slack, R.S., Nicholson, D.W., and MacKenzie, A.E. (2002). The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J. Neurosci. 22, 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, K., Suzuki, I., Yamaguchi, K., Ribbeck, K., Yamamoto, H., Kanesaki, Y., Hagemann, M., and Murata, N. (2003). Identification of histidine kinases that act as sensors in the perception of salt stress in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 100, 9061–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, M.J., and Leaver, C.J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe, P.F., and Leaver, C.J. (2000). Programmed cell death in cell cultures. Plant Mol. Biol. 44, 359–368. [DOI] [PubMed] [Google Scholar]
- McCabe, P.F., Levine, A., Meijer, P., Tapon, N.A., and Pennell, R.I. (1997). A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 12, 267–280. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15, 473–497. [Google Scholar]
- Ndimba, B.K., Chivasa, S., Hamilton, J.M., Simon, W.J., and Slabas, A.R. (2003). Proteomic analysis of changes in the extracellular matrix of Arabidopsis cell suspension cultures induced by fungal elicitors. Proteomics 3, 1047–1059. [DOI] [PubMed] [Google Scholar]
- Ndimba, B.K., Chivasa, S., Simon, W.J., and Slabas, A.R. (2005). Identification of Arabidopsis salt and osmotic stress responsive proteins using two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics 5, in press. [DOI] [PubMed]
- Negoescu, A., Labat-Moleur, F., Defaye, G., Mezin, P., Drouet, C., Brambilla, E., Chambaz, E.M., and Feige, J.J. (1995). Contribution of apoptosis to the phenotypic changes of adrenocortical cells in primary culture. Mol. Cell. Endocrinol. 110, 175–184. [DOI] [PubMed] [Google Scholar]
- Olczak, M., Morawiecka, B., and Watorek, W. (2003). Plant purple acid phosphatases—Genes, structures and biological function. Acta Biochim. Pol. 50, 1245–1256. [PubMed] [Google Scholar]
- Ostrom, R.S., Gregorian, C., and Insel, P.A. (2000). Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J. Biol. Chem. 275, 11735–11739. [DOI] [PubMed] [Google Scholar]
- Redegeld, F., Filippini, A., and Sitkovsky, M. (1991). Comparative studies of the cytotoxic T-lymphocyte-mediated cytotoxicity and extracellular ATP-induced cell lysis: Different requirements in extracellular Mg2+ and pH. J. Immunol. 147, 3638–3645. [PubMed] [Google Scholar]
- Redegeld, F.A., Caldwell, C.C., and Sitkovsky, M.V. (1999). Ecto-protein kinases: Ecto-domain phosphorylation as a novel target for pharmacological manipulation? Trends Pharmacol. Sci. 20, 453–459. [DOI] [PubMed] [Google Scholar]
- Redegeld, F.A., Smith, P., Apasov, S., and Sitkovsky, M.V. (1997). Phosphorylation of T-lymphocyte plasma membrane-associated proteins by ectoprotein kinases: Implications for a possible role for ectophosphorylation in T-cell effector functions. Biochim. Biophys. Acta 1328, 151–165. [DOI] [PubMed] [Google Scholar]
- Roman, R., Wang, Y., Lidofsky, S., Feranchak, A., Lomri, N., Scharschmidt, B., and Fitz, J. (1997). Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J. Biol. Chem. 272, 21970–21976. [DOI] [PubMed] [Google Scholar]
- Schwiebert, L.M., Rice, W.C., Kudlow, B.A., Taylor, A.L., and Schwiebert, E.M. (2002). Extracellular ATP signalling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am. J. Physiol. 282, C289–C301. [DOI] [PubMed] [Google Scholar]
- Shiraishi, T., Araki, M., Yoshioka, H., Kobayashi, I., Yamada, T., Ichinose, Y., Kunoh, H., and Oku, H. (1991). Inhibition of ATPase activity in pea plasma membranes in situ by a suppresser from a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol. 32, 1067–1075. [Google Scholar]
- Sorensen, C.E., and Novak, I. (2001). Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. J. Biol. Chem. 276, 32925–32932. [DOI] [PubMed] [Google Scholar]
- Stone, J.M., Heard, J.E., Asai, T., and Ausubel, F.M. (2000). Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1–resistant (fbr) Arabidopsis mutants. Plant Cell 12, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidzinski, J.A., Leaver, C.J., and Sweetlove, L.J. (2004). A proteomic analysis of plant programmed cell death. Phytochemistry 65, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Swidzinski, J.A., Sweetlove, L.J., and Leaver, C.J. (2002). A custom microarray analysis of gene expression during programmed cell death in Arabidopsis thaliana. Plant J. 30, 431–446. [DOI] [PubMed] [Google Scholar]
- Tada, Y., Hata, S., Takata, Y., Nakayashiki, H., Tosa, Y., and Mayama, S. (2001). Induction and signalling of an apoptotic response typified by DNA laddering in the defense response of oats to infection and elicitors. Mol. Plant Microbe Interact. 14, 477–486. [DOI] [PubMed] [Google Scholar]
- Tang, W., Brady, S.R., Sun, Y., Muday, G.K., and Roux, S.J. (2003). Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol. 131, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, C., Rajagopal, A., Windsor, B., Dudler, R., Lloyd, A., and Roux, S.J. (2000). A role for ectophosphatase in xenobiotic resistance. Plant Cell 12, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping, J.F., and Lindsey, K. (1991). Shoot cultures and root cultures of tobacco. In Plant Tissue Culture Manual, K. Lindsey, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. A4: 1–13.
- Unsworth, C.D., and Johnson, R.G. (1990). Acetylcholine and ATP are coreleased from the electromotor nerve terminals of Narcine brasiliensis by an exocytotic mechanism. Proc. Natl. Acad. Sci. USA 87, 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Albertini, M., Palmetshofer, A., Kaczmarek, E., Koziak, K., Stroka, D., Grey, S.T., Stuhlmeier, K.M., and Robson, S.C. (1998). Extracellular ATP and ADP activate transcription factor NF-kappa B and induce endothelial cell apoptosis. Biochem. Biophys. Res. Commun. 248, 822–829. [DOI] [PubMed] [Google Scholar]
- Wagner, U., Edwards, R., Dixon, D.P., and Mauch, P. (2002). Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 49, 515–532. [DOI] [PubMed] [Google Scholar]
- Watts, M.E., Roberts, I.J., and Woodcock, M.A. (1989). Comparison of colorimetric and clonogenic assays for hypoxic-specific toxins with hamster and human cells. Int. J. Radiat. Oncol. Biol. Phys. 16, 939–942. [DOI] [PubMed] [Google Scholar]
- Wyllie, A.H., Kerr, J.F.R., Macaskill, I.A.M., and Currie, A.R. (1973). Adrenocortical cell deletion: The role of ACTH. J. Pathol. 111, 85–94. [DOI] [PubMed] [Google Scholar]
- Yang, S., Cheek, D.J., Westfall, D.P., and Buxton, I.L. (1994). Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ. Res. 74, 401–407. [DOI] [PubMed] [Google Scholar]
- Yegutkin, G., Bodin, P., and Burnstock, G. (2000). Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 129, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin, G.G., Samburski, S.S., and Jalkanen, S. (2003). Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 17, 1328–1330. [DOI] [PubMed] [Google Scholar]
- Zhou, X.F., and Rush, R.A. (1995). Sympathetic neurons in neonatal rats require endogenous neutrophin-3 for survival. J. Neurosci. 15, 6521–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). Genevestigator. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136, 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]