Abstract
Scrapie in small ruminants belongs to transmissible spongiform encephalopathies (TSEs), or prion diseases, a family of fatal neurodegenerative disorders that affect humans and animals and can transmit within and between species by ingestion or inoculation. Conversion of the host-encoded prion protein (PrP), normal cellular PrP (PrPc), into a misfolded form, abnormal PrP (PrPSc), plays a key role in TSE transmission and pathogenesis. The intensified surveillance of scrapie in the European Union, together with the improvement of PrPSc detection techniques, has led to the discovery of a growing number of so-called atypical scrapie cases. These include clinical Nor98 cases first identified in Norwegian sheep on the basis of unusual pathological and PrPSc molecular features and “cases” that produced discordant responses in the rapid tests currently applied to the large-scale random screening of slaughtered or fallen animals. Worryingly, a substantial proportion of such cases involved sheep with PrP genotypes known until now to confer natural resistance to conventional scrapie. Here we report that both Nor98 and discordant cases, including three sheep homozygous for the resistant PrPARR allele (A136R154R171), efficiently transmitted the disease to transgenic mice expressing ovine PrP, and that they shared unique biological and biochemical features upon propagation in mice. These observations support the view that a truly infectious TSE agent, unrecognized until recently, infects sheep and goat flocks and may have important implications in terms of scrapie control and public health.
Keywords: sheep prion, transgenic mice
Scrapie in sheep and goats is the longest known and most widely spread of the transmissible spongiform encephalopathies (TSEs), or prion diseases, that also include Creutzfeldt-Jakob disease in humans and bovine spongiform encephalopathy (BSE) in cattle (1, 2). A hallmark of TSE is the accumulation in the nervous tissues of abnormal prion protein (PrPSc), an abnormally folded form of the cellular PrP (PrPc). PrPSc is the only known component of infectious prions and is also assumed to be responsible for the neurodegenerative fatal disorders caused by these agents (3, 4). Diagnosis of a TSE infection largely relies on the detection of the pathological isoform (5), which differs from PrPc by various properties, including an increased resistance to proteolysis (6). However, which abnormal state(s) of PrPSc are relevant to various aspects of TSE transmission and pathogenesis remains unclear (7). Phenotypically distinct strains of prions can be recovered from the same host species (8) and can be distinguished by specific traits, such as the distribution of the spongiform changes and of the PrPSc deposits in the brain, and the molecular profile of protease-resistant PrP (PrPres), the fraction of PrPSc that is detected after a treatment with proteinase K (9-12). The strain of the infecting prion and the PrPc sequence of the recipient host are two major determinants of the intra- or across-species transmission barrier (2, 13-15). In the ovine Prnp gene, three codons at positions 136, 154, and 171 prominently influence the incidence and age of onset of natural and experimental scrapie (16). The V136R154Q171 allele (in short, VRQ, where V, R, and Q stand for valine, arginine, and glutamine) and the ARR allele (where A stands for alanine) have consistently been associated with the highest susceptibility or natural resistance to the clinical disease in the field, respectively (17-22). Prions originating in one species can elicit disease in another species, albeit with a generally low efficiency. Transgenic expression of heterologous PrP is a means of facilitating prion transmission from a foreign species to mouse (13, 23-25).
Like BSE in cattle, scrapie in small ruminants is a notifiable disease. However, it is considered nonpathogenic for humans, because there are no epidemiological data linking scrapie to human disease (see ref. 26). In recent years, a novel situation has developed due to the discovery in Europe of a growing number of so-called atypical scrapie cases. First, a clinical form of TSE with unusual pathological features has been repeatedly identified in Norway since 1998. The PrPSc associated with these so-called Nor98 cases displays a distinctive PrPres electrophoretic profile in immunoblots with the presence of a low molecular band at ≈12 kDa and is barely evidenced through immunohistochemistry (IHC) (27). Second, an obligatory active surveillance program based on large-scale testing of both slaughtered and fallen small ruminants has been implemented since 2002 in the European Union member states (28). This program has led not only to a considerable increase in the number of diagnosed scrapie “cases” throughout Europe but also to the identification of an important proportion of so-called “discordant” cases. Although being diagnosed as positive by World Organisation for Animal Health (OIE)-approved confirmatory methods, including IHC, such discordant cases were initially detected by only one (Platelia, Bio-Rad) of the four rapid tests currently used, all based on the detection of PrPres (29, 30).
The above observations raise the possibility that one or more previously unrecognized TSE strains infect sheep flocks in Europe as well as in other countries. A further concern is that a substantial proportion of Nor98 and discordant cases involved sheep with PrP genotypes associated until now with a marked resistance to scrapie disease (29-31). Missing key information, however, is whether a truly infectious TSE agent is associated with the abnormal PrP detected in the brain tissue of Nor98 and/or discordant cases. Indeed, there is evidence that some TSE diseases in humans may be nontransmissible proteinopathies (32, 33). Here we report that both Nor98 and discordant cases, including those detected in ARR/ARR sheep, can efficiently transmit the disease to transgenic mice expressing sheep PrP. We also show that the two types of disease-causing agents share several unique features when propagated in mice, supporting the contention that they represent one or more closely related strain(s).
Materials and Methods
Scrapie Samples. Nor98-infected sheep-brain tissues were collected by the National Veterinary Institute, Oslo. The discordant brain-stem samples, detected through random testing for scrapie diagnosis of sheep and goats in slaughterhouses or rendering plants during the years 2002-2003, were provided by the French national reference laboratory (Afssa-Lyon). Genotypes at the Prnp locus were determined by single-nucleotide polymorphism detection by using DNA extracted from brain material and Taq-man probes (Labogena, Jouy-en-Josas, France). For ARR/ARR sheep, the complete sequence of the Prnp ORF was established (Millegen, Toulouse, France) by using two PCR-amplified 534- and 585-bp overlapping fragments and did not reveal any other associated polymorphism.
Mouse Transmission Assays. The tg338 mouse line used here is transgenic for the VRQ allele of ovine PrP. The mice are homozygous for the transgene, a bacterial artificial chromosome (BAC) insert comprised of 125 kb of sheep DNA (25, 34) introduced on a mouse PrP0/0 background (35). The PrP expression level in their brain is 8- to 10-fold that in sheep. Strict protocols based on the use of disposable equipment were followed for preparation of all inocula in class 2 microbiological safety cabinet and for mouse inoculation. Individually identified 6- to 8-week-old females were inoculated intracerebrally with 20 μl of a 10% (wt/vol) brain homogenate in 5% glucose. An extensively autolyzed sample (DS10) was supplemented with 1,000 units/ml penicillin and 1 mg/ml streptomycin. Mice showing neurological signs were monitored daily and killed in extremis. The inoculations of Nor98 and discordant samples were performed at six different time points, the first series of discordant isolates (five samples) >1 year after the Nor98 isolates. Control mice inoculated with normal sheep-brain material concomitant with this series remained healthy (22 months postinoculation).
Immunoblots for PrPres. All tissues collected were identified by a database code with highly resistant thermal labels (Brady, Milwaukee) designed for long-term storage. Brains and spleens were homogenized at 20% (wt/vol) in 5% glucose with a Rybolyser (Hybaid, Middlesex, U.K.). PrPres was extracted by the Bio-Rad test protocol (36), by using 200 μg/ml proteinase K (PK) for 10 min at 37°C, unless stated otherwise. In some experiments, sodium phosphotungstic acid precipitation was performed as described (37), with concentrations of PK varying from 0 to 5 μg/ml for 1 h. After denaturation, the samples were run on 12% NuPAGE gels (Invitrogen), electrotransferred onto nitrocellulose membranes, and immunoblotted with 0.1 μg/ml biotinylated anti-PrP antibody ICSM18 (38). Immunoreactivity was visualized by chemiluminescence (Amersham Pharmacia Biosciences).
Histoblotting. Histoblots were prepared as described (39). Frozen brain sections (10 μm) were mounted onto nitrocellulose membranes. After digestion with 12.5 μg/ml PK for 1 h at 37°C and denaturation with 3 M guanidium thiocyanate, PrPres was detected with 12F10 antibody (40). Alkaline phosphatase activity was revealed by using NBT/BCIP substrate (GIBCO/BRL).
Vacuolation Profiles. All procedures regarding tissue processing have been described (41). Briefly, samples were fixed in neutral-buffered 10% formalin (4% formaldehyde) before paraffin embedding. After deparaffinization, 2-μm-thick tissue sections were stained with hematoxylin/eosin. Vacuolation profiles were established, following the standard method described by Fraser and Dickinson (9), by using two to three brains per isolate.
Results
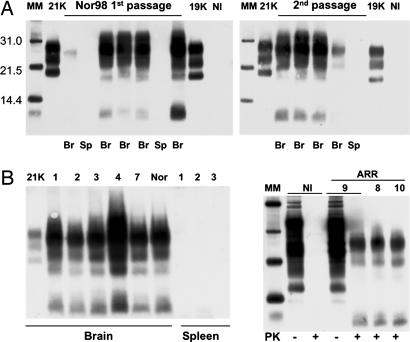
Nor98 Atypical Cases Are Associated with a Truly Infectious Agent Causing Disease in Ovine PrP Transgenic Mice. Inoculation of Nor98 (Lindås isolate) to various inbred mouse lines (RIII, C57BL, and VM) failed to transmit the disease after 850 days (data not shown). Two isolates originating from Nor98-affected Norwegian sheep were inoculated intracerebrally into tg338 mice. These mice overexpressing the ovine PrP VRQ allele on a mouse PrP null background were previously found to greatly enhance transmission of classical sheep scrapie as compared with conventional mice (ref. 25 and our unpublished data). All of the inoculated animals developed a neurological disease after relatively homogenous incubation periods ranging from ≈200 to ≈300 days (Table 1). With both isolates, the clinical symptoms were dominated by a progressive paralysis essentially affecting the hind limbs. Immunoblots were performed to determine whether PK-resistant PrPSc had accumulated in the brain and spleen of the diseased tg338 mice (Fig. 1A). All of the brain homogenates examined (five per isolate) showed the presence of PrPres with an unusual banding pattern due to the presence of additional short fragments migrating at 10-12 kD. This pattern is reminiscent of the atypical PrPres profile observed in the brain of Nor98-affected sheep (27). No PrPres signal could be detected in the spleen homogenates tested (two per isolate) even after prolonged exposure of the blot membrane.
Table 1. Primary and secondary transmissions of Nor98 agent to PrPVRQ transgenic mice.
| Isolate (PrP genotype) | Passage number | Brain tissue* | Mean survival time, days (SEM) | n/n′† |
|---|---|---|---|---|
| Lindås (AHQ) | First | 2 mg | 295 (18) | 7/7 |
| Second | 2 mg | 235 (6) | 7/7 | |
| Third | 2 mg | 190 (10) | 6/6 | |
| Fourth | 0.2 mg | 222 (6) | 5/5 | |
| Rauland (ARQ) | First | 2 mg | 224 (10) | 6/6 |
| Second | 2 mg | 202 (7) | 7/7 | |
| Second | 200 ng | 251 (8) | 6/6 | |
| Second | 20 ng | 294 (17) | 6/6 | |
| Second | 2 ng | 329 (14) | 6/6‡ |
Animals were inoculated intracerebrally with the indicated amount of brain tissue equivalent
Diseased/inoculated
All six mice tested PrPres-positive by immunoblotting
Fig. 1.
Western blot analysis of PrPres in the brain or spleen of tg338 mice inoculated with brain material from Nor98 sheep (A) or discordant sheep and goat (B). Tissues collected from terminally diseased mice were homogenized, PK-treated, and analyzed individually. (A) Transmission of Nor98 (Lindås isolate; see Table 1). Data obtained with brain (Br) or spleen (Sp) from several primary (Left) or secondary (Right) infected mice are presented. PrPres, detected only in the brain, shows a profile that is unique compared with that seen in mice of this same line inoculated with natural scrapie isolates (designated 21K or 19K). NI, noninfected brain control. (B) Primary transmission of discordant isolates. The isolates are identified by a number as in Table 2 and include one from goat (7) and three from ARR/ARR sheep (Right, 8-10). Typical data obtained with tg338 mouse brain or spleen material are shown. (Left) The PrPres profiles observed are all similar to those produced by the Nor98 agent (Rauland: Nor). (Right) PK treatment (+) of healthy (NI) or discordant (9) ARR/ARR sheep-brain material leads to disappearance and shifting of the PrP signal, respectively, whereas PrP signals of similar intensities are seen with PK-untreated (-) samples. MM, molecular markers.
Subpassages were performed to further investigate the transmission barrier of Nor98 agent to tg338 mice, because the latter express a PrP allele that has not been found yet in the Nor98-affected sheep (31). The survival times on second passage were close to those seen on first passage for the Rauland isolate and on further passaging for both isolates (Table 1). The PrPres retained a Nor98-like profile in the brain and remained undetectable in the spleen over four passages for the Lindås isolate (Fig. 1B and data not shown). An endpoint titration of mousepassaged Nor98 agent was also undertaken (Rauland first passage, Table 1). To date, a 100% attack rate was observed at a 10-6 dilution of 10% homogenate, leading to a provisional probably underestimated infectious titer of ≈1 ID50 per ng of brain tissue, as calculated by the Kärber method.
Altogether, these data showed that Nor98 atypical scrapie is caused by a transmissible agent that exhibits a preferential tropism for the nervous tissue, where it can replicate at fairly high infectivity levels.
Sheep and Goat Discordant Cases Are Also Transmissible and Show a Nor98-Like Molecular Signature in Mice. Ten brain samples (nine sheep, one goat) originating from independent French flocks found to be PrPres-positive through the Bio-Rad rapid test only were inoculated to tg338 mice (Table 2). Three of these discordant isolates derived from ARR homozygous sheep. Remarkably, all of the isolates tested transmitted efficiently, with close mean survival times ranging from 183 to 248 days. Data from ongoing experiments indicate no substantial reduction of the survival time on secondary transmission (187 ± 2 days for DS2 isolate). The diseased mice exhibited obvious neurological signs, strikingly similar to those observed in the Nor98-inoculated mice. PrPres immunoblot analyses confirmed the accumulation of abnormal PrP in the brain for every isolate (44 brains examined) and also revealed the presence of fast-migrating bands identical in size to those for the Nor98 agent (Fig. 1B). No PrPres signal was seen in the brain of two control mice killed at 7-9 months postinoculation with normal sheep brain (not shown). Moreover, no PrPres could be detected in the spleen (18 samples, 9/10 isolates tested; Fig. 1B Left) or other lymphoid organs (not shown).
Table 2. Disease transmission to PrPVRQ transgenic mice by inoculation of French discordant case materials.
| Isolate | Origin | PrP genotype | Mean survival time, days (SEM) | n/n′* |
|---|---|---|---|---|
| DS 1 | Slaughterhouse | ARQ/ARR | 183 (12) | 5/5 |
| DS 2 | Slaughterhouse | ARQ/ARR | 199 (8) | 6/6 |
| DS 3 | Slaughterhouse | ARQ/ARQ | 210 (5) | 6/6 |
| DS 4 | Rendering plant | ARH/ARH | 220 (3) | 7/7 |
| DS 5 | Rendering plant | ARQ/ARQ | 248 (8) | 6/6 |
| DS 6 | Rendering plant | AHQ/ARR | 221 (5) | 10/10 |
| DS 7 | Rendering plant | Goat (AHQ/AHQ) | 236 (8) | 5/5 |
| DS 8 | Slaughterhouse | ARR/ARR | 184 (3.5) | 10/10 |
| DS 9 | Slaughterhouse | ARR/ARR | 203 (4) | 10/10 |
| DS 10 | Rendering plant | ARR/ARR | 196 (4) | 9/9 |
Animals were inoculated intracerebrally with 2 mg of brain material.
Diseased/inoculated
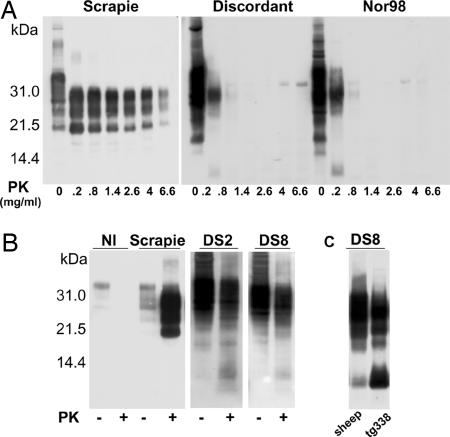
The finding that all of the discordant isolates studied shared several peculiarities with the Nor98 agent upon transmission to mice incited us to further analyze the molecular properties of the PrPSc induced by the two types of agent. Because an unusual sensitivity to proteolysis appears to account at least partially for the incongruous results of the discordant cases in rapid tests (29), their degree of resistance to PK digestion was first compared with that of the Nor98 agent. Complete disappearance of the PrPres signal was observed after a 10-min treatment with 1.4 mg/ml PK of brain homogenates from mice infected by either Nor98 or discordant isolates, whereas a strong typical PrPres signal was still detected at 5-fold higher PK doses when a homogenate from a mouse infected by a typical scrapie isolate was used (Fig. 2A). We next examined whether PrPres in the brain of discordant donor sheep would exhibit a profile similar to that in recipient diseased mice. Sheep-brain PrPSc was enriched through sodium phosphotungstic acid (NaPTA) precipitation (37) before mild PK digestion, to enhance its detection by Western blot. As a result, short PrPres fragments at 10-12 K were consistently generated with each discordant case tested (8/10), including the three ARR homozygous sheep (Fig. 2B and data not shown). Such fragments were similar in size to that in the brain of recipient mice (Fig. 2C).
Fig. 2.
Biochemical characterization of PrPSc associated with discordant sheep isolates. (A) Comparative PK digestion assay performed on brain material from tg338 mice infected with one of the following isolates: conventional scrapie [PG127 (25)], discordant (DS2), or Nor98 Rauland (see Tables 1 and 2). The homogenates were treated for 10 min at 37°C with PK at the indicated concentrations and analyzed by Western blotting. (B) Atypical Nor98-like PrPres profiles observed in the brain material from discordant sheep (DS2 and DS8). PrPSc prepared by NaPTA precipitation was subjected (+)ornot (-) to mild PK digestion (37). Note the lack of PrPres signal in PK-treated brain material from a healthy sheep (NI) and the typical profile of PrPres detected in brain material from a conventional scrapie-diseased sheep [Langlade (25)]. (C) Similarity of the PrPres patterns observed in brain materials from the donor (ARR/ARR sheep DS8) and from tg338 recipient mice.
These data led us to conclude that the discordant cases studied here likely involved a common infectious agent, whose biochemical properties were both unprecedented in tg338 mice and similar to those of Nor98 agent.
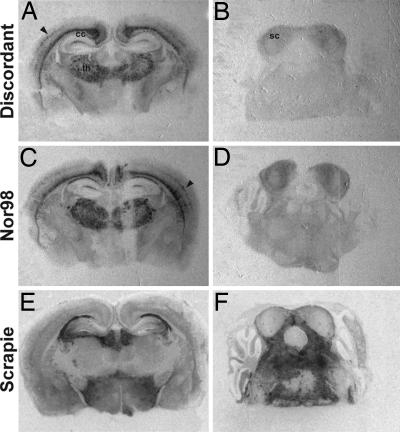
PrP Deposition and Lesion Patterns in Discordant- and Nor98-Infected Mice Are Similar. Histopathological analyses were performed on infected mouse brains to further document and compare the pathobiological characteristics of the two categories of agents. The neuroanatomical distribution of PrPSc in discordant and Nor98-infected mice at the terminal stage of the disease was examined by histoblotting on several anteroposterior coronal brain sections (Fig. 3). Remarkably, the PrPSc deposition pattern was similar for all of the isolates studied. Immunoreactivity was pronounced in the thalamus, the corpus callosum, the cingulum, and the dorsal hippocampal commissure (Fig. 3 A and C), as well as in the striatum and the lateral olfactory tract (not shown). A weaker staining was observed in one layer of the cerebral cortex, the lacunosum molecular layer of the hippocampus, and in the superior colliculus (Fig. 3 A-D). No PrPSc was detected in the hypothalamus, midbrain, brain stem, or cerebellum (Fig. 3 A-D).
Fig. 3.
Regional distribution of protease-resistant PrPSc in the brain of tg338 mice infected with discordant (A and B) and Nor98 (C and D) agent. Histoblots of coronal sections were immunostained for PrPres as described in Materials and Methods. Sections through the thalamus/hippocampus (A and C) and the pons/cerebellum (B and D) are presented. Note the concordant patterns of PrPres accumulation for the two types of agent, with the preferential involvement of the thalamus (th), corpus callosum (cc), cerebral cortex (arrow), and, to a lesser extent, of the superior colliculus (sc; B-D). Histoblots performed on the brain of a tg338 mice inoculated with a conventional scrapie isolate (PG127) are shown for comparison (E and F).
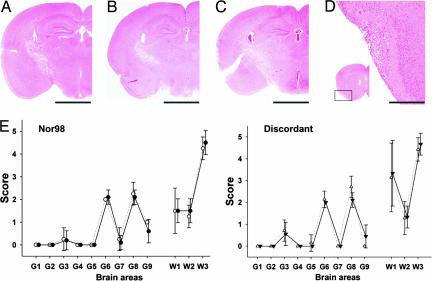
The distribution of vacuolar degeneration, as represented by the lesion profiles (Fig. 4E and ref. 9), was also essentially similar among Nor98 and discordant-infected mice (Fig. 4). In gray-matter areas, vacuolation was mainly scored in the cerebral cortex (areas G8 and G9), the hippocampus (G6), and, to a lesser extent, in the superior colliculus (G3). The lateral olfactory tract in the piriform cortex region was also vacuolated (Fig. 4D). White matter areas and more particularly the pyramidal tract (Fig. 4 A-C; W3 in Fig. 4E) were highly vacuolated. Vacuolation in the cerebellar white matter appeared to be more intense in the discordant-infected mice (W1 area), yet the significance of this difference remains uncertain. Overall, there was a good correlation between PrPSc and vacuolation distribution in the brain, except in the cerebellum. Interestingly, a severe paresis was observed in clinically affected tg338 challenged with either Nor98 or a discordant agent, consistent with the marked PrPSc accumulation in most brain structures involved in motor coordination (thalamus, cerebral cortex, pyramidal tract, and cerebellum).
Fig. 4.
Brain vacuolar degeneration in discordant- and Nor98-infected tg338 mice. (A-D) Spongiform changes in mice inoculated with Nor98 (A), ARQ/ARQ (B), or ARR/ARR (C) discordant sheep isolates. Note the similar distribution of the vacuolated areas, mainly involving the tractus pyramidalis and the corpus callosum (A-C; Scale bar = 2 mm). The lateral olfactory tract in the anterior piriform cortex was also consistently vacuolated (D, showing a higher magnification of the boxed area; Scale bar = 0.2 mm). (E) Lesion profiles in mice infected with Nor98 or discordant agent. The gray (G1-G8) and white matter (W1-W3) scoring positions used to construct the profiles are indicated. The mean scores with SEM are shown. (Left) Profiles as obtained with Nor98 scrapie-inoculated mice (two isolates), distributed into two groups: primary transmissions (four mice; open circles) and all subsequent passages (12 mice; filled circles). (Right) Profiles obtained with discordant isolate-inoculated mice distributed into two groups: ARR/ARR isolates (three isolates, nine mice; filled triangles) and isolates of other genotypes (five isolates, 10 mice; open triangles).
From these results, it appears that discordant and Nor98 isolates share both typical and similar neuropathological features upon transmission to tg338 mice. Moreover, no change was noted during serial propagation of the Nor98 agent.
Discussion
Atypical Scrapie and Discordant Cases in Small Ruminants Involve a Truly Infectious Agent. Whether a transmissible agent is actually involved in the so-called “atypical” or “unclassified” scrapie cases recurrently detected in sheep, notably in individuals with resistant PrP genotypes, has become a growing concern during the last few years. Here we show that inoculation to transgenic mice of 12 isolates of such types consistently led to a distinctive fatal TSE disease. These isolates originated from two Nor98 clinically affected sheep and from nine sheep and one goat that produced a discordant response upon rapid test screening. This result demonstrates that PrPSc deposition in the brain of such animals, hardly evidenced through conventional scrapie diagnosis techniques, is associated with the multiplication of a bona fide infectious agent. Hence, such atypical scrapie forms or cases must now be considered as true TSE, involving a prion rather than simply arising from a PrP protein disorder, whatever their origin might be, acquired or spontaneous.
Nor98 Efficiently Replicates in PrPVRQ Transgenic Mice, Reproducing Unusual Histopathological Features Observed in Sheep. Unlike conventional mice, tg338 mice expressing the VRQ allele of sheep PrP uniformly developed disease after exposure to the Nor98 agent and died within ≈7 months (for the fastest of the two isolates). Two lines of evidence argue that the sheep-to-tg338 mice transmission barrier for this agent was rather low. First, secondary transmissions did not lead to a dramatic shortening of the survival time. Second, propagation of the Nor98 agent into these mice appeared to faithfully recapitulate several unusual features previously reported in sheep: (i) brain PrPres exhibited a similar atypical profile; and (ii) no PrPres could be detected in the spleen, consistent with a preferential neurotropism. In addition, the distribution of PrPSc deposits and spongiform changes in the mouse and sheep brains largely overlapped. One noticeable exception, however, was that cerebellar pathology was restricted to vacuolar degeneration in tg338 mice, whereas both PrPSc and vacuoles are present in the cerebellum of Nor98-infected sheep (27). This host-related discrepancy might result, among other factors, from the artificial route of infection used in our experiments.
Taken together, the above data support the view that the Nor98 agent essentially retained its strain-specific characteristics after transmission to tg338 mice, implying that PrPVRQ provides a compatible substrate for its propagation. Furthermore, the agent multiplied to fairly high infectivity levels in these mice. The brain tissue from a primary inoculated terminally diseased mouse contained ≥1ID50 infectious unit per ng. Such a high titer is merely a feature of fast-replicating strains that kill tg338 mice within ≈2 months (our unpublished data). This efficient transmission and multiplication of Nor98 agent in tg338 mice may look surprising, given that the VRQ allele has so far not been found in Nor98 sheep, although being well represented in the Norwegian ovine population, thus suggesting that it may confer a substantial resistance to Nor98 scrapie (31). However, this postulated resistance may have been overcome here by direct introduction of the agent into the brain tissue, similar to that reported for ARR sheep inoculated with the BSE agent by the intracerebral route (42).
Discordant and Nor98 TSE Agents Might Be a Unique Strain. Another striking finding of this study is that all of the discordant isolates tested (nine sheep and one goat) apparently involved a unique transmissible agent, whose characteristics in terms of incubation duration (≈200 days), PrPres molecular profile, susceptibility to PK digestion, and tissue tropism turned out to be remarkably similar to that of the Nor98 agent transmitted to the same line of mice. It is to be emphasized that the strain phenotype produced by both sources of agent, including the apparent lack of tropism for the lymphoreticular tissue, was overall unprecedented within the panel of natural scrapie isolates already transmitted to tg338 mice by the intracerebral route, which includes ≈50 sheep and goat isolates and BSE agent (our unpublished data). This strengthens the view that an agent closely related, if not identical, to Nor98 was present in the discordant sheep tested.
The infectivity of the 10 discordant samples looked similarly high and comparable with that found in the brain of clinically affected Nor98 sheep (see Results and below), suggesting that this agent may be present at elevated titers in the nervous tissue of preclinical animals. Although contamination by a Nor98 agent at the slaughterhouse or the rendering plant cannot be formally excluded, only a massive contamination of every discordant sample would account for our findings. The various geographical origins of the samples and the method and policy of sample collection render this possibility extremely remote. On the other hand, (i) the time schedule of the inoculations of the discordant and Nor98 isolates and (ii) the concomitant injection of other inocula, including noninfected sheep brain and nondiscordant samples with longer incubation times, of which none produced a Nor98 phenotype, rule out a potential Nor98 crosscontamination at the laboratory.
Because the number of discordant specimens tested here remains limited, the question arises as to which extent the present observation can be generalized. In this regard, it is worth noting that the VRQ allele seems to be underrepresented in sheep diagnosed as discordant in various countries (29, 30), similar to what was reported for Nor98 scrapie (31). Moreover, it appears that, in a majority of discordant sheep brains, the molecular profile of PrPres resembles that of Nor98 scrapie, as was the case for the discordant isolates studied here. Collectively, these and our data lend support to the view that the discordant cases identified through active surveillance in sheep and possibly goat, currently representing at least 20% of the animals recorded as positive (29), may be predominantly attributable to Nor98 or a closely related agent.
This Agent Has a Different Allelic PrP Host Preference as Compared with Classical Scrapie. An important outcome from the present study is that the supposedly resistant ARR homozygous sheep may be fully susceptible to infection by a Nor98-like agent. Indeed, there was no significant difference in terms of survival time, spongiform changes, or PrPSc deposition in tg338 mice between the three ARR/ARR-derived isolates and the other discordant isolates tested. Furthermore, the incubation times observed on primary transmission were overall remarkably homogenous, arguing that the infectious agent may be present at relatively high titers in the obex, the brain area sampled from these animals. Data from ongoing titration experiments show that the infectious titer in the obex of such sheep can actually reach 108 ID50 unit/gram (DS8 isolate; not shown). Other brain regions might be even more heavily infected, considering both the close relationship between this and the Nor98 agent and the anatomical distribution of PrPSc in Nor98 sheep brains (27). It thus appears that PrPARR not only confers susceptibility to natural infection by a Nor98-like agent but also allows its fairly efficient multiplication in the nervous tissue. These data, along with the finding made in BSE-inoculated sheep (42), firmly establish that the resistance to infection afforded by arginine 171 in homozygous sheep or in genetically engineered mice (43) is TSE agent-dependent.
Whether ARR homozygous individuals infected by Nor98-like prion may be silent carriers or will exhibit clinical signs remains, however, an open question. Accumulation of PrPSc or infectivity at high levels in the brain does not lead ineluctably to overt clinical manifestations in some mouse TSE models (44, 45). As of now, there is no unquestionable evidence for natural scrapie disease involving an ARR homozygous animal (20, 46), and no Nor98-affected sheep of this genotype has been reported (31). Experimental infection of ARR homozygous sheep and of mice expressing PrPARR as a transgene may help to clarify this issue, which has obvious implications in terms of TSE surveillance in sheep. Because of the unprecedented biochemical features of the abnormal PrP produced in these atypical TSE forms, studies in such models could also provide valuable insights about molecular states of PrPSc that may play a pivotal role in TSE pathogenesis.
Conclusion
Our study demonstrates that an authentic TSE infectious agent is responsible in sheep and goats of sporadic atypical infections that remained unnoticed until recently. This raises important issues with regard to control of scrapie infection in small ruminants. Of major concern, ARR/ARR sheep can no longer be regarded as free of natural TSE infection. This finding challenges, at least to some extent, the foundation of the selective breeding programs engaged in several European Union member states (47, 48) and may call for a reappraisal of possible consequences of this strategy in the long term. Finally, more information about this newly discovered type of TSE agent, its prevalence in countries free of scrapie or BSE disease, and its potential to across-species transmission would be needed for a comprehensive evaluation of its implications in terms of public health.
Acknowledgments
We thank S. Hawke (Imperial College School of Medicine, London), and J. Grassi (Commissariat à l'Energie Atomique, Saclay, France) for kindly providing antibodies ICSM18 and 12F10, respectively; the staff of the Animalerier Rongeurs (Institut National de la Recherche Agronomique) for mouse care; our colleagues of the French scrapie strain-typing network (chaired by M. Eloit) for discussions; and Anne-Lise Haenni for carefully reading the manuscript. This study was supported by national grants from the Ministries of Research (Groupement d'Intérêt Scientifique Infections à Prion) and Agriculture (Direction Générale de l'Alimentation).
Author contributions: H.L. designed research; A.L.D., V.B., O.A., F.R., T.L.L., J.-L.V., and H.L. performed research; T.B., B.B., P.S., and S.L.B. contributed new reagents/analytic tools; V.B., O.A., and H.L. analyzed data; and H.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TSE, transmissible spongiform encephalopathy; PrP, prion protein; PrPSc, abnormal PrP; PrPres, protease-resistant PrP; PK, proteinase K; BSE, bovine spongiform encephalopathy.
References
- 1.Detwiler, L. A. & Baylis, M. (2003) Rev. Sci. Technol. 22, 121-143. [DOI] [PubMed] [Google Scholar]
- 2.Collinge, J. (2001) Annu. Rev. Neurosci. 24, 519-550. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner, S. B. (1982) Science 216, 136-144. [DOI] [PubMed] [Google Scholar]
- 4.Weissmann, C. (2004) Nat. Rev. Microbiol. 2, 861-871. [DOI] [PubMed] [Google Scholar]
- 5.Soto, C. (2004) Nat. Rev. Microbiol. 2, 809-819. [DOI] [PubMed] [Google Scholar]
- 6.Bolton, D. C., McKinley, M. P. & Prusiner, S. B. (1982) Science 218, 1309-1311. [DOI] [PubMed] [Google Scholar]
- 7.Mallucci, G. & Collinge, J. (2005) Nat. Rev. Neurosci. 6, 23-34. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, M. E. (2003) Br. Med. Bull. 66, 99-108. [DOI] [PubMed] [Google Scholar]
- 9.Fraser, H. & Dickinson, A. G. (1968) J. Comp. Pathol. 78, 301-311. [DOI] [PubMed] [Google Scholar]
- 10.Bruce, M. E., McConnell, I., Fraser, H. & Dickinson, A. G. (1991) J. Gen. Virol. 72, 595-603. [DOI] [PubMed] [Google Scholar]
- 11.Safar, J., Wille, H., Itri, V., Groth, D., Serban, H., Torchia, M., Cohen, F. E. & Prusiner, S. B. (1998) Nat. Med. 4, 1157-1165. [DOI] [PubMed] [Google Scholar]
- 12.Hill, A. F., Joiner, S., Wadsworth, J. D., Sidle, K. C., Bell, J. E., Budka, H., Ironside, J. W. & Collinge, J. (2003) Brain 126, 1333-1346. [DOI] [PubMed] [Google Scholar]
- 13.Scott, M., Foster, D., Mirenda, C., Serban, D., Coufal, F., Walchli, M., Torchia, M., Groth, D., Carlson, G., DeArmond, S. J., et al. (1989) Cell 59, 847-857. [DOI] [PubMed] [Google Scholar]
- 14.Telling, G. C., Parchi, P., DeArmond, S. J., Cortelli, P., Montagna, P., Gabizon, R., Mastrianni, J., Lugaresi, E., Gambetti, P. & Prusiner, S. B. (1996) Science 274, 2079-2082. [DOI] [PubMed] [Google Scholar]
- 15.Asante, E. A., Linehan, J. M., Desbruslais, M., Joiner, S., Gowland, I., Wood, A. L., Welch, J., Hill, A. F., Lloyd, S. E., Wadsworth, J. D., et al. (2002) EMBO J. 21, 6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter, N. (1997) Trends Microbiol. 5, 331-334. [DOI] [PubMed] [Google Scholar]
- 17.Goldmann, W., Hunter, N., Smith, G., Foster, J. & Hope, J. (1994) J. Gen. Virol. 75, 989-995. [DOI] [PubMed] [Google Scholar]
- 18.Westaway, D., Zuliani, V., Cooper, C. M., Da Costa, M., Neuman, S., Jenny, A. L., Detwiler, L. & Prusiner, S. B. (1994) Genes Dev. 8, 959-969. [DOI] [PubMed] [Google Scholar]
- 19.Belt, P. B., Muileman, I. H., Schreuder, B. E., Bos-de Ruijter, J., Gielkens, A. L. & Smits, M. A. (1995) J. Gen. Virol. 76, 509-517. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda, T., Horiuchi, M., Ishiguro, N., Muramatsu, Y., Kai-Uwe, G. D. & Shinagawa, M. (1995) J. Gen. Virol. 76, 2577-2581. [DOI] [PubMed] [Google Scholar]
- 21.Elsen, J. M., Amigues, Y., Schelcher, F., Ducrocq, V., Andreoletti, O., Eychenne, F., Khang, J. V., Poivey, J. P., Lantier, F. & Laplanche, J. L. (1999) Arch. Virol. 144, 431-445. [DOI] [PubMed] [Google Scholar]
- 22.Baylis, M., Chihota, C., Stevenson, E., Goldmann, W., Smith, A., Sivam, K., Tongue, S. & Gravenor, M. B. (2004) J. Gen. Virol. 85, 2735-2740. [DOI] [PubMed] [Google Scholar]
- 23.Telling, G. C., Scott, M., Hsiao, K. K., Foster, D., Yang, S. L., Torchia, M., Sidle, K. C., Collinge, J., DeArmond, S. J. & Prusiner, S. B. (1994) Proc. Natl. Acad. Sci. USA 91, 9936-9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collinge, J., Sidle, K. C., Meads, J., Ironside, J. & Hill, A. F. (1996) Nature 383, 685-690. [DOI] [PubMed] [Google Scholar]
- 25.Vilotte, J. L., Soulier, S., Essalmani, R., Stinnakre, M. G., Vaiman, D., Lepourry, L., Da Silva, J. C., Besnard, N., Dawson, M., Buschmann, A., et al. (2001) J. Virol. 75, 5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prusiner, S. B. & Scott, M. R. (1997) Annu. Rev. Genet. 31, 139-175. [DOI] [PubMed] [Google Scholar]
- 27.Benestad, S. L., Sarradin, P., Thu, B., Schonheit, J., Tranulis, M. A. & Bratberg, B. (2003) Vet. Rec. 153, 202-208. [DOI] [PubMed] [Google Scholar]
- 28.Moynagh, J. & Schimmel, H. (1999) Nature 400, 105. [DOI] [PubMed] [Google Scholar]
- 29.Buschmann, A., Biacabe, A. G., Ziegler, U., Bencsik, A., Madec, J. Y., Erhardt, G., Luhken, G., Baron, T. & Groschup, M. H. (2004) J. Virol. Methods 117, 27-36. [DOI] [PubMed] [Google Scholar]
- 30.Orge, L., Galo, A., Machado, C., Lima, C., Ochoa, C., Silva, J., Ramos, M. & Simas, J. P. (2004) J. Gen. Virol. 85, 3487-3491. [DOI] [PubMed] [Google Scholar]
- 31.Moum, T., Olsaker, I., Hopp, P., Moldal, T., Valheim, M. & Benestad, S. L. (2005) J. Gen. Virol. 86, 231-235. [DOI] [PubMed] [Google Scholar]
- 32.Tateishi, J., Kitamoto, T., Hoque, M. Z. & Furukawa, H. (1996) Neurology 46, 532-537. [DOI] [PubMed] [Google Scholar]
- 33.Chiesa, R. & Harris, D. A. (2001) Neurobiol. Dis. 8, 743-763. [DOI] [PubMed] [Google Scholar]
- 34.Laude, H., Vilette, D., Le Dur, A., Archer, F., Soulier, S., Besnard, N., Essalmani, R. & Vilotte, J. L. (2002) C. R. Biol. 325, 49-57. [DOI] [PubMed] [Google Scholar]
- 35.Bueler, H., Aguzzi, A., Sailer, A., Greiner, R. A., Autenried, P., Aguet, M. & Weissmann, C. (1993) Cell 73, 1339-1347. [DOI] [PubMed] [Google Scholar]
- 36.Deslys, J. P., Comoy, E., Hawkins, S., Simon, S., Schimmel, H., Wells, G., Grassi, J. & Moynagh, J. (2001) Nature 409, 476-478. [DOI] [PubMed] [Google Scholar]
- 37.Wadsworth, J. D., Joiner, S., Hill, A. F., Campbell, T. A., Desbruslais, M., Luthert, P. J. & Collinge, J. (2001) Lancet 358, 171-180. [DOI] [PubMed] [Google Scholar]
- 38.Beringue, V., Mallinson, G., Kaisar, M., Tayebi, M., Sattar, Z., Jackson, G., Anstee, D., Collinge, J. & Hawke, S. (2003) Brain 126, 2065-2073. [DOI] [PubMed] [Google Scholar]
- 39.Taraboulos, A., Jendroska, K., Serban, D., Yang, S. L., DeArmond, S. J. & Prusiner, S. B. (1992) Proc. Natl. Acad. Sci. USA 89, 7620-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krasemann, S., Groschup, M. H., Harmeyer, S., Hunsmann, G. & Bodemer, W. (1996) Mol. Med. 2, 725-734. [PMC free article] [PubMed] [Google Scholar]
- 41.Andreoletti, O., Berthon, P., Levavasseur, E., Marc, D., Lantier, F., Monks, E., Elsen, J. M. & Schelcher, F. (2002) J. Histochem. Cytochem. 50, 1357-1370. [DOI] [PubMed] [Google Scholar]
- 42.Houston, F., Goldmann, W., Chong, A., Jeffrey, M., Gonzalez, L., Foster, J., Parnham, D. & Hunter, N. (2003) Nature 423, 498. [DOI] [PubMed] [Google Scholar]
- 43.Perrier, V., Kaneko, K., Safar, J., Vergara, J., Tremblay, P., DeArmond, S. J., Cohen, F. E., Prusiner, S. B. & Wallace, A. C. (2002) Proc. Natl. Acad. Sci. USA 99, 13079-13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill, A. F., Joiner, S., Linehan, J., Desbruslais, M., Lantos, P. L. & Collinge, J. (2000) Proc. Natl. Acad. Sci. USA 97, 10248-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Race, R., Meade-White, K., Raines, A., Raymond, G. J., Caughey, B. & Chesebro, B. (2002) J. Infect. Dis. 186, S166-S170. [DOI] [PubMed] [Google Scholar]
- 46.Buschmann, A., Luhken, G., Schultz, J., Erhardt, G. & Groschup, M. H. (2004) J. Gen. Virol. 85, 2727-2733. [DOI] [PubMed] [Google Scholar]
- 47.Arnold, M., Meek, C., Webb, C. R. & Hoinville, L. J. (2002) Prev. Vet. Med. 56, 227-249. [DOI] [PubMed] [Google Scholar]
- 48.Baylis, M. & McIntyre, K. M. (2004) Nature 432, 810-811. [DOI] [PubMed] [Google Scholar]