Abstract
Cell organization is largely orchestrated by extracellular gradients of morphogenetic proteins. VEGF, an essential factor for capillary formation, is stored in the extracellular matrix, but the mechanisms by which it and other matrix-bound morphogens are mobilized to form spatial gradients are poorly understood. Here, we suggest an efficient mechanism for morphogen gradient generation by subtle biophysical forces in an in vitro model of capillary morphogenesis. Using a fibrin-bound VEGF variant that is released proteolytically to mimic the in vivo situation, we report that low levels of interstitial flow act synergistically with VEGF to drive endothelial organization, whereas each stimulus alone has very little effect. To help account for this synergy, we show how these slow flows can bias the distribution of cell-secreted proteases, which leads, interestingly, to the creation of an increasing VEGF gradient relative to the cell and skewed in the direction of flow. In contrast, diffusion alone can only account for symmetric, decreasing autocrine gradients. Indeed, branching of capillary structures was biased in the direction of flow only with the combination of VEGF and flow. This work thus demonstrates a general mechanism of morphogen gradient generation and amplification by small ubiquitous mechanical forces that are known to exist in vivo.
Keywords: fibrin, angiogenesis, lymphatic, biotransport, mechanobiology
Tissue morphogenesis results from the organization of cells into functional structures by means of the actions of biomolecular morphogens. The efficacy of a morphogen depends largely on its local gradient relative to the cell rather than its absolute amount (1). Such morphogens, particularly growth factors and some cytokines, commonly bind to the extracellular matrix (ECM) to be later released by cells via proteolytic enzymes. Although Alan Turing (2) first showed how patterns in morphogenesis could arise from diffusion and interaction kinetics, his arguments were derived for directly cell-secreted morphogens. The question of how cells create morphogen gradients by proteolytically mobilizing matrix-bound factors has not yet been addressed, and we wondered whether small biophysical forces that are ubiquitously present in vivo may help facilitate cell utilization of such factors.
Here we explore how in vitro capillary morphogenesis induced by exposure to exogenous matrix-bound VEGF is augmented by slow interstitial flows. Such slow flows, on the order of microns per second (3), exist between the blood and lymphatic capillaries and mediate the transport of macromolecules from the blood to the tissue. Using a matrix-bound variant of VEGF that is only released by cell-mediated proteolysis, we demonstrate proof-of-principle that very low levels of interstitial flow can synergistically enhance the morphogenetic effects of angiogenic growth factors, such as VEGF, that are typically tightly bound to components of the ECM (4, 5). Specifically, we compare the individual and combined effects of slow interstitial flow and VEGF on the morphology and organization of blood endothelial cells (BECs) and lymphatic endothelial cells (LECs) suspended as single cells in a 3D fibrin matrix by using a model whereby an engineered variant form of VEGF121, α2PI1-8-VEGF121, is crosslinked to the fibrin and can only be released by cell-secreted proteases (6, 7). Fibrin is an important wound repair matrix and a permissive substrate for endothelial cell growth (7), and, because soluble proteins are freely diffusible in the matrix, the incorporation of growth factors in bound form within the fibrin network permits the control of VEGF activity by cells populating the matrix. Moreover, the matrix-bound VEGF more closely mimics the physiological situation, where VEGF is tightly bound to the ECM via interactions with heparan sulfate proteoglycans (8). Slow interstitial flow was induced through the fibrin in custom culture chambers (9). BECs and LECs (10) were examined separately because, in vivo, they maintain different relationships with the ECM according to their different functions (11) and because we recently showed that interstitial fluid flow (which notably becomes lymph) is an important player in lymphatic capillary formation in regenerating mouse skin (12).
We then explore potential mechanisms underlying this phenomenon and demonstrate that, although such small convective influences can yield very small shear stresses on the cell surface, they can lead to large changes in morphogen distribution, even in regimes where convection does not dominate diffusion. This skewed protease gradient causes a skewed release profile of VEGF from the bulk of the matrix primarily downstream of the cell that further convects and diffuses, resulting in an increasing gradient relative to the cell. The fact that directionality of structure organization (parallel to flow) is seen only under the combined flow-plus-VEGF condition strongly corroborates this notion.
Methods
Cell Culture. Human microvascular BECs and LECs from neonatal foreskins were a kind gift from Mihaela Skobe (Mt. Sinai School of Medicine, New York) (10). The BECs and LECs were expanded on gelatin-coated flasks in endothelial cell medium (Cambrex, Walkersville, MD) supplemented with 20% FBS (Invitrogen), 1 μg/ml hydrocortisone acetate, 25 μg/ml dibutyril cAMP, and 1% ampicillin-streptomycin-amphotericin B (all from Sigma).
VEGF. α2-PI1-8-VEGF121 is described in refs. 6 and 7. This bi-domain fusion protein comprises mature human recombinant VEGF121 and the factor XIII substrate sequence from α2-plasmin inhibitor NQEQVSPL at the N terminus. The N-terminal factor XIII substrate sequence covalently binds to fibrin during coagulation under the influence of the coagulation transglutaminase factor XIII.
Experimental Procedure. Fibrin gel matrices were prepared by combining 2 mg/ml human fibrinogen, 2 units/ml human thrombin, 2.5 mM CaCl2 (all from Sigma), 2 units/ml human factor XIII (Baxter Biosurgery), and, in indicated cases, 100 ng/ml α2-PI1-8-VEGF121 together with LECs or BECs at 1.5 × 106 cells per ml. The solution was pipetted into an interstitial flow culture chamber as described in ref. 9 and immersed in medium supplemented additionally with 200 units/ml aprotinin (Sigma) to inhibit global fibrin degradation. The medium could freely diffuse through the porous polyethylene sides and middle of the chamber, which also acted to mechanically anchor the gel and prevent fluid channeling around it. After 18-24 h, medium was perfused through the gel by using a peristaltic pump yielding an average interstitial fluid flow velocity of 4.2 μm/s and a pressure drop of 1.5 mmHg (1 mmHg = 133 Pa). Static controls were kept in the identical chambers and bathed in medium. To ensure that cells in the static cultures were not nutrient-limited, the static experiments were repeated in an open-gel system and results similar to those seen for static conditions were observed (data not shown). For protease inhibition experiments, medium was supplemented with either 800 units/ml aprotinin (Sigma), a serine protease inhibitor, or 25 μM GM6001 (Chemicon, Temecula, CA), a global matrix metalloproteinase (MMP) inhibitor. To visualize the matrix relative to cells with confocal microscopy (see Fig. 2A), the prepolymerized fibrinogen solution was augmented with Alexa Fluor 647 fibrinogen (Molecular Probes) in a 1:25 ratio of labeled:unlabeled fibrinogen. Free VEGF was measured in the outlet medium by ELISA (R & D Systems) after 24 h.
Fig. 2.
Structures formed with the combination of flow plus VEGF are capillary-like. Fluorescently labeled fibrin (red) reveals ECM fibers (cells are green) and shows lumen-containing structures in three examples of BECs. (Scale bars, 20 μm.)
Cell and Structure Quantification. Static and flow cultures were maintained for 6 days at 37°C and 5% CO2, at which time the gels were fixed in 4% paraformaldehyde (Fisher) and stained with 200 nM Alexa Fluor 488-conjugated phalloidin for f-actin and TOTO-3 for nuclei (both from Molecular Probes). Images of the LECs and BECs in the fibrin gels were collected using a laser scanning confocal microscope (Leica LCS SP2). Maximum projections of 3D image stacks were used for cell and structure quantification. Groups of two or more conjoined cells were counted as structures; from these counts, the percentage of cells in structures, the number of structures per area, and the number of cells per area were quantified. To calculate the structure orientation, images were imported into imagej (National Institutes of Health, Bethesda), and each branch of each structure was measured for angle relative to flow direction. An overall angle for each structure represented the average angle of all branches, and the structures were classified as “parallel” (0-45°) or “perpendicular” (45-90°) to the general flow direction. The structures in each bin were then pooled to determine general trends. Finally, we determined the ratio of the number of parallel to perpendicular structures, which was weighted for branch number; a ratio of 1 signifies a random distribution of structure orientations.
Statistics. For all experimental data, ANOVA with Tukey post-analysis was used to compare samples with the static, plain fibrin controls for each cell type group, except in the protease inhibitor studies, for which comparisons were made with static VEGF-fibrin samples. Five independent experiments were performed for each condition on each cell type. Directedness in structure orientation was determined by comparing the population sets to 1 (i.e., random) using a z test. In all figures, error bars indicate standard error (*, P < 0.05; **, P < 0.01).
Computational Estimation of Shear Stress. The computational fluid dynamics model was based on a spherical cell embedded in a fibrin network that was idealized as straight, intersecting cylindrical fibers of 400 nm in diameter spaced 5.6 μm apart, as determined from confocal images of a fluorescently labeled fibrin network. A 48-μm cubic domain centered around an 18-μm-diameter cell was designed by using the acis-based solid modeler gambit (Fluent Inc., Lebanon, NH); one-fourth of the model was used because of symmetry. A four-node tetrahedral mesh was constructed with 2,800,000 elements with a density gradient that increased toward the cell and fibers, and the following conditions were imposed: (i) rigid and impermeable solid surfaces with no fluid slip; (ii) a flat velocity profile of 4.2 μm/s at the inlet and zero total stress at the outlet, with symmetry conditions at the lateral sides of the fluid domain; and (iii) incompressible, homogeneous, Newtonian fluid with a density of 1 g/cm3 and a viscosity of 0.748 cP (which was that of the culture medium measured in an Ubbelodhe viscometer at 37°C). The steady state Navier-Stokes equations were solved by the commercial finite-element solver fluent (Fluent Inc.) with a segregated solver.
Computational Estimation of Extracellular Protease Distribution. To compare the generic effects of interstitial fluid flow on the shape of secreted protease (and/or protease activator) gradients, the protease distribution was modeled with a steady-state coupled convection-diffusion equation, Dp∇2Cp - v∇Cp - Rp = 0, where Cp is the protease concentration, v is fluid velocity, Dp is the diffusion coefficient (assumed constant), and Rp is protease decay. The problem was considered around a spherical cell with an 18-μm diameter and with an idealized uniform and constant (dimension-less) cell surface concentration of protease Co = 1. MMPs can be inactivated by tissue inhibitors of metalloproteinases and enzymatic processing or cleared by endocytocis after binding or being entrapped by other molecules, such as thrombospondin 2 (reviewed in ref. 13). Therefore, to account for such effects, we considered a first-order protease decay, Rp = kpCp, and compared no degradation (kp = 0) with a constant decay term kp = 0.2 s-1 (chosen such that kpCp was on the same order as the convection term). Because the fluid phase of the fibrin gel was >99%, convective hindrance by the matrix was neglected as the partition coefficient was 0.999 (14). Cell-secreted MMPs and plasmin activators, all within 52-92 kDa, were assumed to have a Dp of 7 × 10-7 cm2/s (15). In addition, the fibrin matrix was estimated to mechanically hinder diffusivity in this size range by <10% (14). The velocity field was modeled as Brinkman flow (16) with a far-field velocity of either 0 (static) or 4.2 μm/s (flow). The equation was discretized and solved in two dimensions by using a finite volume simulation employing the tridiagonal matrix algorithm performed in custom code written for matlab 6.5.1 (MathWorks, Natick, MA), with a solution domain of 7 and 16 cell diameters in the transverse and longitudinal directions, respectively, in 43,624 discretized control volumes.
Computational Estimation of Free VEGF Distribution. VEGF distribution was estimated by using the steady-state coupled convection-diffusion-reaction equation, Dv∇2Cv - v∇Cv + Rv = 0, where Cv is the free VEGF concentration, Dv is the diffusion coefficient of the liberated VEGF (assumed to be a dimer of 64 kDa) = 7 × 10-7 cm2/s, v is the Brinkman fluid velocity field (16), and Rv is the liberation rate of VEGF from the matrix. Three different boundary conditions at the cell surface rcell were considered: (i) no binding; (ii) first-order binding of VEGF, i.e.,
 |
where kcell = 0.1 μm/s; and (iii) first-order binding with kcell = 1 μm/s. The first condition represented one limiting case of saturated receptor occupancy, whereas the others assumed that receptor occupancy was very small compared with total receptor number. The kcell values were chosen as brackets of a previous estimation of 0.4 μm/s (17) to examine the qualitative effects of including this term. To estimate Rv, we assumed that the release rate of VEGF was linearly proportional to the concentration of protease, that the matrix-bound concentration of VEGF remained constant, and that there were no interactions with the matrix. These assumptions allow a steady-state solution to be obtained but render the model realistic only for early times and only on a first-order scale. However, because the amounts and kinetics of protease secretion by the cell as well as the kinetics of VEGF release are complex and largely unattainable experimentally, the use of limiting cases in this model allows us to demonstrate that our conclusions (i.e., that interstitial flow causes skewed and increasing VEGF gradients relative to the cell in the downstream direction) are robust across a wide range of reasonable assumptions. The same solution method was used here to solve the concentration profiles as that described for protease distribution.
Results and Discussion
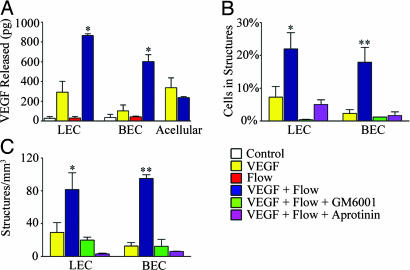
Interstitial Flow Synergizes with VEGF to Direct Capillary Morphogenesis. We observed that the combination of interstitial flow and α2PI1-8-VEGF121 (forthwith referred to simply as VEGF) induced a substantial and synergistic effect on endothelial cell networking, organization, and capillary formation in 3D suspensions as opposed to either condition alone (Fig. 1A). Without flow, very little cell organization was induced.†† When quantified as the fraction of cells present in multicellular (two or more cells) structures (Fig. 1B) and density of structures (Fig. 1C), only the double-positive combination of flow plus VEGF caused statistically significant increases over the double-negative control of static conditions in plain fibrin gels, for LECs and BECs. This response could be attributed to increased cell networking rather than proliferation because total cell density did not differ significantly among any groups (Fig. 1D). Synergy between flow and VEGF in capillary structure formation, as quantified by the percentage of cells in structures, was confirmed by comparing the sum of the averages for VEGF alone and flow alone with the array of values for flow plus VEGF using a z test (P = 4 × 10-6 for BECs and P = 0.019 for LECs).
Fig. 1.
Slow interstitial flow acts in synergy with VEGF to orchestrate capillary morphogenesis. (A) BEC and LEC organization markedly increased when exposed to the combination of flow and VEGF as shown by representative low-magnification (low mag) and high-magnification (high mag) confocal images (projections of 3D stacks). Green indicates f-actin, and red indicates nuclei. (Scale bars: low magnification, 160 μm; high magnification, 40 μm). (B and C) The percentage of cells in structures (B) and the density of structures in each condition (C). For LECs and BECs, the percentage of cells involved in structures and the structure densities were significantly higher with combined stimuli. (D) After 6 days, no significant differences in total cell number were seen.
The morphology of the structures that formed differed between LECs and BECs (Fig. 1 A). Interstitial flow imposed through a VEGF-laden matrix caused LECs to organize into slender, elongated structures with an overlapping pattern reminiscent of a typical lymphatic capillary morphology seen in vivo (19). In contrast, the combined cues induced BECs to organize into thick branching structures with a higher density of conjoined cells within the capillary networks that formed.
We then examined the capillary-like structures formed by the combined cues using red fluorescent fibrin, allowing the ECM to be visualized relative to the cells. Representative sections through BEC structures (Fig. 2) reveal a wide matrix-free lumen and a well defined lumen-cell interface.
Flow Plus VEGF Yields Directionality in Capillary Structures. Because the structures that formed in the flow-plus-VEGF condition appeared qualitatively to be aligned with flow, we estimated the overall orientation of each structure. In Fig. 3A, the total numbers of structures classified as either parallel or perpendicular to flow show that only those structures in the flow-plus-VEGF condition have a skewed distribution toward flow. In Fig. 3B, the ratios of frequencies of parallel versus perpendicular structures (directionality ratio) reveal that only those in the combined condition have ratios considerably greater than 1 (note that only LECs show significant differences because of the large variability in the BEC data). Therefore, when exposed to the combination of flow plus VEGF, structures are more often oriented in the direction of flow.
Fig. 3.
Structure organization is directional only with flow plus VEGF. (A) Frequency histograms of structure orientation (either parallel or perpendicular to flow) are biased in the flow direction only when flow and VEGF are present. Equal frequencies of orientations are seen in all other conditions. (B) Directionality ratios (a ratio of 1 means a random orientation) complement the frequency histograms and show that, for LECs, the biased directionality in structures in flow plus VEGF is significant (P = 0.1 for BECs).
Interstitial Flow Increases Cell-Directed VEGF Liberation from the ECM by Means of Soluble MMPs. The synergistic effects of interstitial flow and VEGF on capillary morphogenesis correlated with increases in VEGF liberation from the matrix by the cells (Fig. 4A). Of note, neither LECs nor BECs produced significant levels of endogenous VEGF. Furthermore, there was no appreciable “shearing” of VEGF from the matrix by flow directly, because acellular controls for static and flow conditions showed the same levels of free VEGF, which was presumably unincorporated during fibrin clotting. Thus, flow-induced increases in VEGF liberation from the matrix were truly cell-directed.
Fig. 4.
Flow induces cell-mediated VEGF release via matrix proteolysis. (A) Liberated VEGF after 24 h normalized to the static acellular control (n = 3). Only the cell-containing matrices show a significant flow-induced difference in VEGF release, and the insignificant VEGF decrease by cell-containing versus acellular static gels is presumably due to cell consumption. (B and C) Inhibition of either fibrinolytic pathway reversed the synergistic effects for endothelial cell organization of flow and VEGF. The number of cells involved in structures (B) and the density of such structures (C) were substantially reduced upon inhibition of MMPs (with GM6001) or plasmin (with aprotinin), both of which interact. Inhibition of MMPs completely abrogated any cell organization, suggesting that the MMP system dominates in fibrin invasion.
Because VEGF121 is only liberated by cell-induced proteolysis, our findings imply a causal increase in fibrin proteolysis by interstitial flow. Fibrinolysis can be mediated through two interacting pathways: the plasminogen activator (PA) and MMP systems. In the PA system, cell-secreted tissue-type PA and urokinase-type PA cleave plasminogen to generate plasmin, which in turn digests fibrin. The MMPs are secreted in latent zymogen forms and are activated extracellularly by plasmin or other MMPs; the exceptions are membrane-type MMPs that can be activated intracellularly (20). Furthermore, MMPs play a governing role in cell invasion and angiogenesis in fibrin (21, 22). Thus, VEGF in our system can be liberated by plasmin, which is activated by cell-secreted urokinase-type PA and tissue-type PA, or by the action of cell-secreted MMPs that can cleave fibrin, such as MMP-3, MMP-9, and the membrane-bound MT1-MMP, (23) which intersect the subsets of MMPs known to degrade fibrin and to be secreted by human endothelial cells (24).
In probing these proteolytic pathways experimentally we found that the synergistic effects of flow plus VEGF on endothelial organization were completely abrogated by adding 25 μM GM6001, a broad-spectrum MMP inhibitor, or 800 units/ml aprotinin, a serine protease inhibitor (Fig. 4 B and C). Furthermore, although aprotinin prevented only the synergistic effects of flow plus VEGF (i.e., levels were the same as those seen with VEGF alone), GM6001 completely eliminated all organization. These findings are consistent with the notion that, even in the absence of any active cell response, interstitial flow should change the extracellular distribution of soluble, but not membrane-bound, proteases that release VEGF. Aprotinin plays two important roles: (i) It blocks plasmin-mediated fibrinolysis, and (ii), by inhibiting plasmin, it inhibits plasmin's role in activating soluble MMPs but not necessarily MT1-MMP (25). Thus, with high levels of aprotinin, the primary means of liberating VEGF from the fibrin matrix is via membrane-bound MMPs whose spatial distribution should be unaffected by flow. Indeed, Fig. 4 B and C show that the degree of organization seen with aprotinin is the same as that seen with VEGF alone. In contrast, GM6001 blocks the activity of all MMPs, leaving only plasmin to free VEGF from the matrix, and, in our system, resulting in complete blockage of all cell organization. Because aprotinin and GM6001 reduce the overall availability of soluble MMPs, these data suggest that soluble MMPs are the dominant player in flow-enhanced fibrinolysis. Other studies have demonstrated that membrane-bound MMPs are critical for invasion in static cultures where matrix-bound growth factors are not involved (21, 22); however, under such static conditions, the distribution of soluble MMPs does not affect the distribution of growth factors; thus, they would not appear to play a role in cell migration. In our dynamic and potentially more physiological model, the role of diffusible MMPs becomes more apparent because matrix-bound growth factors and flow are present, both of which, we propose, are necessary to create biased gradients of the morphogenic factors.
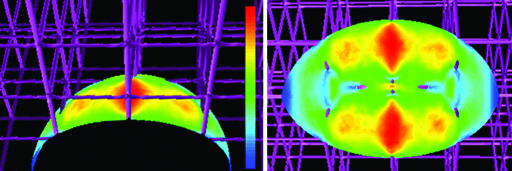
Mechanisms of Synergy: Shear Stress Versus Directionally Biased VEGF Release. We considered two possible mechanisms for the synergy seen between VEGF and flow in capillary formation: shear stress activation and directional biasing of VEGF release. We computationally evaluated the shear stress imposed on a cell in a 3D fibrillar matrix by interstitial flow of 4.2 μm/s and found that it averaged only 0.033 dyne/cm2 (1 dyne = 10 μN) with a maximum value of 0.065 dyne/cm2 (Fig. 5). Although higher levels of shear stress can stimulate gene expression (26) and affect cell differentiation pathways (27), the levels we predict in our system are very low compared with those reported to induce gene or protein expression in endothelial cells (28, 29). Thus, it is unlikely that shear-induced mechanotransduction is the primary mechanism responsible for the significant change in the networking effects observed.
Fig. 5.
Shear stress imposed on the cell by 4.2 μm/s flow is likely to be very low. Computational estimates of the maximum and average shear stress at the cell surface were 0.065 and 0.033 dyne/cm2; the linear color scale ranges from blue (0) to red (0.065 dyne/cm2).
In contrast, interstitial flow may significantly alter the distribution of secreted proteases, which will in turn alter the distribution of VEGF liberation from the bulk matrix, even when convective effects are smaller than or on the same scale as diffusive effects (30), as was the case with the experimental conditions used here (Peclet number ≈ 0.5). To explore these potential effects, we first computationally estimated the relative steady-state distribution of soluble MMPs secreted from a hypothetical cell under static versus flow conditions using simplifying assumptions. The resulting concentration profiles show a skewing of protease in the direction of flow, and, as expected, shape and magnitude are reduced when a decay term is included (Fig. 6 A and C).
Fig. 6.
Proposed effects of 4.2 μm/s flow versus static conditions on VEGF distribution around a cell. (A) Computational estimates of cell-secreted protease transport shows directional biasing of diffusion gradients by interstitial flow compared with a symmetric gradient under static conditions. Colors represent normalized concentration contours from surface conditions Co (dark red) to 0 (blue). (B) Computational estimates of initial relative free-VEGF distribution (liberated by the pattern shown in A and further biased by convection and diffusion) shows an upward gradient of VEGF. (C and D) Effects of first-order decay on protease distribution (C) and the corresponding free-VEGF distribution resulting from that decaying protease profile (D). Despite the decreased broadcast distance and different shape of the gradient, the VEGF concentration is still increasing relative to the cell and dramatically biased downstream. (E) Comparison of free-VEGF profiles (from B and D) along the cell midline in the upstream and downstream directions shows an increasing slope in VEGF concentration for conditions in B because of the skewing effects of convection on both the VEGF-liberating proteases and the VEGF itself. Curves B* and B** refers to additional simulations that include first-order VEGF binding terms on the cell surface of 0.1 and 1 μm/s.
Because the proteases liberate VEGF from the matrix, the shape of VEGF release is patterned after that of the extracellular protease gradient and further skewed by diffusion and convection. This skewing effect is particularly consequential considering that the levels of soluble VEGF present in the system are very low at any given time (Fig. 4A). We estimated the relative steady-state distribution of free VEGF for early times (i.e., before the concentration of matrix-bound VEGF decreased significantly), assuming a linear relationship between protease concentration and VEGF liberation. Surprisingly, free VEGF concentration increased downstream of the cell under flow conditions such that an upward gradient is seen by the cell in the direction of flow (Fig. 6B). This increasing and biased gradient contrasts with free VEGF profiles under static conditions, where a decreasing and symmetric gradient surrounds the cell. Furthermore, this effect also was seen in VEGF profiles resulting from decaying protease gradients (Fig. 6D) as well as those resulting from the cases for which cell surface binding of VEGF was considered (Fig. 6E). Thus, regardless of the details of protease decay and VEGF binding to the cell surface, the same overall conclusions can be made: that interstitial flow, coupled with matrix-bound growth factor, instills directional bias in the system, leads to an increased broadcast distance of liberated factor, and creates increasing gradients in the downstream direction. Without flow, only symmetric gradients can form and usually only decreasing gradients (although we note that when binding rates exceed diffusive flux toward the cell, a slightly increasing VEGF concentration is seen just outside the cell surface that then decreases with distance as shown in Fig. 6E, curve B**, static). Such changes in the free VEGF gradient may strongly impact gradient-sensitive endothelial cell organization (31, 32).
If this hypothesized mechanism is even partly responsible for the synergistic effects seen between VEGF and interstitial flow, we would expect that cell-cell networking or structure formation would be directionally biased only when flow and VEGF are combined. Indeed, the data in Fig. 3 A and B clearly corroborates this notion. This effect could not simply be attributed to hydrodynamic forces alone, because cells in the flow-alone condition organized with random orientations. The fact that structures align with flow only when matrix-bound VEGF is present strongly suggests directionality in VEGF release profiles.
As for cell-secreted proteases, either an increase in transport or an increase in total amount released could contribute to the increased VEGF levels seen in Fig. 4A. Furthermore, although protease amounts might increase as a direct response to mechanical stress, e.g., shear-induced protease up-regulation, the amount of active protease might also change by convection-driven redistribution, which may change the balance of cell-produced enzyme with inhibitors in the extracellular milieu. To this end, we measured secreted levels of MMP-3, MMP-9, urokinase-type PA, and tissue-type PA in the medium after 24 h and saw a trend of increased levels of each under flow conditions, although none of the differences were statistically significant (see Supporting Methods and Fig. 7A, which are published as supporting information on the PNAS web site). To further clarify whether these effects were due to shear stress alone, we exposed BEC monolayers to 2D shear stress of 0.033 dyne/cm2 and compared protease release to those under static conditions. Similar trends were seen in the 2D shear experiments for all proteases and activators tested except tissue-type PA (see Supporting Methods and Fig. 7B). Thus, both mechanisms may slightly increase protease production but not in a significant way.
Conclusions. Two major conclusions arise from this work. First, we experimentally demonstrate the strong morphogenetic potential of slow interstitial flow, here in the example of inducing and directing capillary structures in vitro by enhancing access to matrix-bound growth factors. Because VEGF exists primarily in a matrix-bound form in vivo and is complexed with heparan sulfate proteoglycans in the ECM (8), this conclusion is highly relevant to the physiological condition; it is also relevant to many other growth factors and chemokines with heparin-binding domains.
Second, and more generally, we introduce a potential mechanism for autologous morphogen gradient generation: Cells secrete a matrix-binding form of the morphogen, and, when they subsequently secrete a soluble liberating protease in a dynamic environment, a morphogen gradient is created that increases away from the cell in the direction of flow. In other words, low levels of interstitial flow allow cells to create their own increasing morphogen gradient. This biased and increasing gradient is not possible without flow, and it is not possible without matrix binding of the morphogen. Such an exquisite system may have evolved as a way for cells to create far-field gradients of morphogens by using interstitial fluid forces; in contrast, were cells only able to secrete the active forms of morphogens or did not have a biasing flow environment, self-regulation of those gradients would be impossible. This ability to form increasing gradients raises the intriguing possibility that interstitial flow may facilitate autocrine chemotaxis in cells that express chemokine receptors and their ligands, such as dendritic cells (33).
Supplementary Material
Acknowledgments
We thank M. Skobe and S. Podgrabinska (Mount Sinai School of Medicine) for the BECs and LECs; J. Hubbell and C. Weber for assistance with the fibrin chemistry and VEGF fusion protein; C. Yong for performing 2D shear studies and quantifying structure directionality; J. Pedersen for help with image analysis; and M. Glucksberg, S. Hirosue, and D. Trono for critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01 HL075217-01 (to M.A.S.), National Institutes of Health Training Grant T32 GM 08449 (to C.-L.E.H.), the Arnold and Mabel Beckman Foundation (M.A.S.), and the Gebert Ruef Foundation (A.H.Z.).
Author contributions: C.-L.E.H., A.H.Z., M.E.F., and M.A.S. designed research; C.-L.E.H., M.E.F., and F.B. performed research; A.H.Z. contributed new reagents/analytic tools; C.-L.E.H., M.E.F., F.B., and M.A.S. analyzed data; and C.-L.E.H., M.E.F., and M.A.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BEC, blood endothelial cell; ECM, extracellular matrix; LEC, lymphatic endothelial cell; MMP, matrix metalloproteinase; PA, plasminogen activator.
Footnotes
Three-dimensional cultures of endothelial cells in single-cell suspensions, unlike those in sandwich culture, typically do not spontaneously organize without exogenous factors like phorbol 12-myristate 13-acetate, VEGF, and basic fibroblast growth factor (18). In addition, the concentration of free VEGF in our system is very low (≈1 ng/ml) compared with that used in other experiments (typically 20-100 ng/ml).
References
- 1.Gurdon, J. B. & Bourillot, P. Y. (2001) Nature 413, 797-803. [DOI] [PubMed] [Google Scholar]
- 2.Turing, A. M. (1952) Philos. Trans. R. Soc. London B 237, 37-72. [Google Scholar]
- 3.Chary, S. R. & Jain, R. K. (1989) Proc. Natl. Acad. Sci. USA 86, 5385-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park, J. E., Keller, G. A. & Ferrara, N. (1993) Mol. Biol. Cell 4, 1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara, N., Gerber, H. P. & LeCouter, J. (2003) Nat. Med. 9, 669-676. [DOI] [PubMed] [Google Scholar]
- 6.Zisch, A. H., Schenk, U., Schense, J. C., Sakiyama-Elbert, S. E. & Hubbell, J. A. (2001) J. Controlled Release 72, 101-113. [DOI] [PubMed] [Google Scholar]
- 7.Ehrbar, M., Djonov, V. G., Schnell, C., Tschanz, S. A., Martiny-Baron, G., Schenk, U., Wood, J., Burri, P. H., Hubbell, J. A. & Zisch, A. H. (2004) Circ. Res. 94, 1124-1132. [DOI] [PubMed] [Google Scholar]
- 8.Fairbrother, W. J., Champe, M. A., Christinger, H. W., Keyt, B. A. & Starovasnik, M. A. (1998) Structure (London) 6, 637-648. [DOI] [PubMed] [Google Scholar]
- 9.Ng, C. P., Helm, C. L. & Swartz, M. A. (2004) Microvasc. Res. 68, 258-264. [DOI] [PubMed] [Google Scholar]
- 10.Podgrabinska, S., Braun, P., Velasco, P., Kloos, B., Pepper, M. S., Jackson, D. G. & Skobe, M. (2002) Proc. Natl. Acad. Sci. USA 99, 16069-16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz, M. A. (2001) Adv. Drug Delivery Rev. 50, 3-20. [DOI] [PubMed] [Google Scholar]
- 12.Boardman, K. C. & Swartz, M. A. (2003) Circ. Res. 92, 801-808. [DOI] [PubMed] [Google Scholar]
- 13.Sternlicht, M. D. & Werb, Z. (2001) Annu. Rev. Cell. Dev. Biol. 17, 463-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogston, A. G., Preston, B. N. & Wells, J. D. (1973) Proc. R. Soc. London Ser. A 333, 297-316. [Google Scholar]
- 15.Young, M. E., Carroad, P. A. & Bell, R. L. (1980) Biotechnol. Bioeng. 22, 947-955. [Google Scholar]
- 16.Barman, B. (1996) Ind. J. Pure Appl. Math. 27, 1249-1256. [Google Scholar]
- 17.MacGabhann, F. & Popel, A. S. (2004) Am. J. Physiol. 286, H153-H164. [DOI] [PubMed] [Google Scholar]
- 18.Madri, J. A., Pratt, B. M. & Tucker, A. M. (1988) J. Cell Biol. 106, 1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leak, L. V. (1976) Fed. Proc. 35, 1863-1871. [PubMed] [Google Scholar]
- 20.Lijnen, H. R. (2001) Ann. N.Y. Acad. Sci. 936, 226-236. [DOI] [PubMed] [Google Scholar]
- 21.Hotary, K. B., Yana, I., Sabeh, F., Li, X. Y., Holmbeck, K., Birkedal-Hansen, H., Allen, E. D., Hiraoka, N. & Weiss, S. J. (2002) J. Exp. Med. 195, 295-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafleur, M. A., Handsley, M. M., Knauper, V., Murphy, G. & Edwards, D. R. (2002) J. Cell Sci. 115, 3427-3438. [DOI] [PubMed] [Google Scholar]
- 23.Bini, A., Wu, D., Schnuer, J. & Kudryk, B. J. (1999) Biochemistry 38, 13928-13936. [DOI] [PubMed] [Google Scholar]
- 24.Pepper, M. S. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1104-1117. [DOI] [PubMed] [Google Scholar]
- 25.Mazzone, M., Baldassarre, M., Beznoussenko, G., Giacchetti, G., Cao, J., Zucker, S., Luini, A. & Buccione, R. (2004) J. Cell Sci. 117, 6275-6287. [DOI] [PubMed] [Google Scholar]
- 26.Ingber, D. E. (2003) Ann. Med. 35, 564-577. [DOI] [PubMed] [Google Scholar]
- 27.Estes, B. T., Gimble, J. M. & Guilak, F. (2004) Curr. Topics Dev. Biol. 60, 91-126. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler, T., Bouzourene, K., Harrison, V. J., Brunner, H. R. & Hayoz, D. (1998) Arterioscl. Thromb. Vasc. Biol. 18, 686-692. [DOI] [PubMed] [Google Scholar]
- 29.Barakat, A. & Lieu, D. (2003) Cell Biochem. Biophys. 38, 323-343. [DOI] [PubMed] [Google Scholar]
- 30.Swartz, M. A. (2003) Curr. Opin. Biotechnol. 14, 547-550. [DOI] [PubMed] [Google Scholar]
- 31.Ruhrberg, C., Gerhardt, H., Golding, M., Watson, R., Ioannidou, S., Fujisawa, H., Betsholtz, C. & Shima, D. T. (2002) Genes Dev. 16, 2684-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozawa, C. R., Banfi, A., Glazer, N. L., Thurston, G., Springer, M. L., Kraft, P. E., McDonald, D. M. & Blau, H. M. (2004) J. Clin. Invest. 113, 516-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randolph, G. J., Angeli, V. & Swartz, M. A. (2005) Nat. Rev. Immunol. 5, 1-12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.