Abstract
Oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs activate immune cells to produce cytokines. CpG ODNs protect mice against infections with intracellular bacteria by the induction of a T helper 1 (Th1) response. To determine the effect of CpG ODNs in pulmonary tuberculosis, mice were treated with CpG ODNs or control ODNs at the time of intranasal infection. CpG ODNs reduced mycobacterial outgrowth for up to 5 weeks after Mycobacterium tuberculosis infection and were associated with a decrease in inflammation in lung tissue. CpG treatment was also associated with elevated levels of gamma interferon (IFN-γ) and decreased levels of interleukin 4 in the lungs and an increased capacity of splenocytes to secrete Th1-type cytokines. CpG ODNs given 2 weeks after infection were still able to reduce mycobacterial outgrowth and to enhance a Th1 response 5 weeks postinfection. Administration of CpG ODNs to IFN-γ-gene-deficient mice failed to reduce mycobacterial outgrowth. These data suggest that CpG ODNs improve host defense during pulmonary tuberculosis by an IFN-γ-dependent mechanism.
Despite optimism after the introduction of antituberculous agents in the 1950s, tuberculosis (TB) remains the leading cause of death among infectious diseases, accounting for 2 million deaths annually (10). In addition to the increasing incidence of TB, there has been a global emergence of drug-resistant strains, posing a threat to existing therapeutic possibilities (26). The case fatality rate for multidrug-resistant TB is 40 to 60%, which equals the case fatality rate for untreated TB (16). Hence, new therapeutic strategies are required for the control of TB.
Unmethylated CpG dinucleotides within bacterial DNA or synthetic oligodeoxynucleotides (ODNs) can activate immune cells (21). These sequence motifs are underrepresented in vertebrates (29), and it has been proposed that immune activation by CpG DNA has evolved as a result of evolutionary selections, contributing to host defense mechanisms that recognize invading microbial agents (22). CpG motifs can stimulate B cells, NK cells, T cells, and macrophages to secrete cytokines (19). A number of studies indicate that CpG can switch on T helper 1 (Th1) immunity with the production of immunoglobulins of class G2a (IgG2a) (9, 25, 35) and a Th1-dominated cytokine profile (4, 19, 33, 36). Indeed, protective Th1-biased immune responses could be induced by the administration of CpG ODNs in animal models of Listeria and Leishmania infections (11, 23, 36).
It is well known that a Th1 immune response conveys protection against infection with Mycobacterium tuberculosis (6, 13). Therefore, in this study, we investigated the effect of CpG ODNs in a murine model of pulmonary TB. Our results demonstrated that CpG ODNs protect against infection with M. tuberculosis by inducing a Th1 response.
MATERIALS AND METHODS
Mice.
All experiments were approved by the Institutional Animal Care and Use Committee of the Academic Medical Center (University of Amsterdam, Amsterdam, The Netherlands). BALB/c mice (female, 7 to 8 weeks old; Harlan Sprague Dawley Inc., Horst, The Netherlands) were used. Each experimental group consisted of eight mice per time point. In some experiments, gamma interferon (IFN-γ) gene-deficient (IFN-γ−/−) mice (Jackson Laboratory, Bar Harbor, Maine) that had been backcrossed to a BALB/c background were used.
Experimental infection.
A virulent laboratory strain of M. tuberculosis (H37Rv) was grown in liquid Dubos medium containing 0.01% Tween 80 for 4 days. A replicate culture was incubated at 37°C and stirred gently, harvested at midlog phase, and stored in aliquots at −70°C. Before each experiment, a vial was thawed and the contents were washed twice with sterile saline to clear the mycobacteria of medium. Mice were anesthesized by inhalation with isoflurane, and lung infection was induced by intranasal (i.n.) inoculation with mycobacteria (105 CFU in 50 μl of NaCl) as described previously by several laboratories (12, 17, 24, 28). The inoculum was plated immediately after inoculation to determine viable counts. After 2 and 5 weeks, mice were anesthesized with fentanyl citrate (0.079 mg/ml)-fluanisone (2.5 mg/ml)-midazolam (1.25 mg/ml) in H2O. Of this mixture, 7.0 ml/kg of body weight was administered intraperitoneally (i.p.).
ODNs.
Phosphorothioate ODNs, which are resistant to nucleases, were obtained from Eurogentec (Seraing, Belgium). The immunostimulatory CpG ODN had the sequence 5′-TCCATGACGTTCCTGATGCT-3′. The control ODN, in which the CpG motif was inverted, had the sequence 5′-TCCATGAGCTTCCTGATCCT-3′. Thirty micrograms of CpG ODNs or control ODNs was dissolved in 200 μl of NaCl and injected i.p. 2 h prior to and 6 h after M. tuberculosis infection. This treatment schedule was based on a regimen that was found to be protective against murine leishmaniasis (36). For the postponed-treatment experiment, mice received 40 μg of CpG ODNs or control ODNs i.p. 2 weeks postinfection and were sacrificed 5 weeks postinfection.
Enumeration of mycobacteria.
The lungs and livers were harvested and homogenized in sterile saline with a tissue homogenizer (Biospec Products, Bartlesville, Okla.). Tenfold serial dilutions were plated on Middlebrook 7H11 agar plates containing oleic acid-albumin-dextrose-catalase enrichment (Difco, Braunschweig, Germany) and incubated at 37°C in sealed bags. Colonies were counted after 3 weeks.
Preparation of lung tissue for ELISA.
Homogenates of lung tissue were diluted 1:1 with lysis buffer (0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl, 1 mM MgCl; pH 7.4) at 4°C for 20 min. Homogenates were then centrifuged at 1,500 × g for 10 min to remove cell debris, after which the supernatants were stored at −20°C. IFN-γ and interleukin 4 (IL-4) (both from R&D Systems, Abingdon, United Kingdom), as well as tumor necrosis factor alpha (TNF-α; Genzyme, Cambridge, Mass.) were measured by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturer. Detection limits of the assays were 31 pg/ml.
Spleen cell stimulation assays.
Single-cell suspensions were obtained by crushing spleens through a 40-μm-pore-size cell strainer (Becton Dickinson, Mountain View, Calif.). Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, 100 mM EDTA; pH 7.4), and the remaining cells were washed twice. Splenocytes were suspended in medium (RPMI 1640 [BioWhittaker, Verviers, Belgium], 10% fetal calf serum, 1% antibiotic-antimycotic [Gibco BRL Life Technologies, Gaithersburg, Md.]). Round-bottom plates were coated overnight with anti-CD3 (clone no. 145.2c11) and washed with sterile phosphate-buffered saline. Splenocytes were seeded in 96-well round-bottom culture plates at a cell density of 106 cells in triplicate diluted with RPMI 1640 containing anti-CD28 (Pharmingen, San Diego, Calif.). Splenocytes were also stimulated with 20 μg of tuberculin purified protein derivative (PPD; Statens Seruminstitut, Copenhagen, Denmark)/ml. Supernatants were harvested after a 48-h incubation at 37°C in 5% CO2, and cytokine levels were analyzed by ELISA.
Histological analysis.
Lung tissue samples were fixed in 10% neutral buffered formalin. After the samples were embedded in paraffin, 4-μm-thick sections were stained with hematoxylin and eosin or Ziehl-Neelsen stain for acid-fast bacilli. All slides were coded and semiquantitatively scored by a pathologist for the total area of inflammation (percentage of surface of the slide) and granuloma formation. Granulomas were defined as collections of 10 or more macrophages and lymphocytes widespread in the peripheral lung parenchyma (3).
Statistical analysis.
Differences were compared by use of the Mann-Whitney U test. For comparison of survival curves, Kaplan-Meier analysis with a log rank test was used. Results were considered statistically significant at a P of <0.05.
RESULTS
CpG ODNs reduce mycobacterial outgrowth.
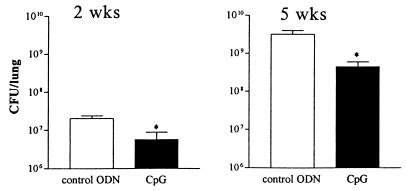
The inoculations with CpG ODNs were not associated with adverse effects. Treatment with CpG ODNs at the time of infection resulted in a reduction of the mycobacterial burden in the lungs, compared to that of controls, which was present at 2 weeks (P = 0.001) and lasted until at least 5 weeks (P < 0.001) after infection (Fig. 1). The infection disseminated to the livers of the mice in both treatment groups. At 2 weeks, the mycobacterial loads (means ± standard errors of the means [SEM]), in the livers of CpG ODN-treated and control mice were similar [(2.1 ± 2) × 103 and (4.3 ± 1.8) × 103, respectively]. At 5 weeks, CpG ODN-treated mice displayed fewer M. tuberculosis CFU in their livers than did the control mice [(309 ± 139) × 103 and (859 ± 317) × 103, respectively] although this difference did not reach statistical significance (P = 0.059).
FIG. 1.
Treatment with CpG ODNs reduces outgrowth of M. tuberculosis in lungs. Mice were treated twice with 30 μg of CpG ODNs or control ODNs, 2 h before and 6 h after i.n. infection with 105 CFU of M. tuberculosis. CFU were counted 2 and 5 weeks after infection. Data are mean ± SEM of results for eight mice per group for each time point. *, P < 0.05 versus results for control.
Cytokine concentrations in lung tissue.
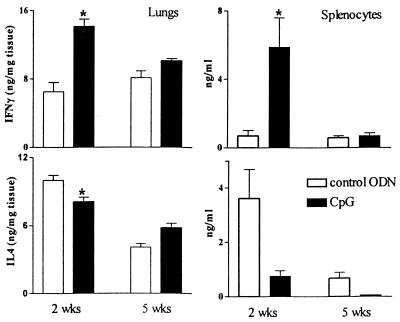
A number of studies have demonstrated the Th1 stimulatory effect of CpG motifs (4, 19, 33, 36). IFN-γ is considered the main Th1 cytokine, responsible for the activation of macrophages and early T-cell activation. To obtain insight into mechanisms contributing to the inhibitory effect of CpG on mycobacterial outgrowth, IFN-γ as the principal Th1 cytokine and IL-4 as the principal Th2 cytokine were measured in homogenates of lung tissue from the mice used for bacterial counts in this study (Fig. 2). CpG ODN-treated mice had higher IFN-γ concentrations in lung tissue than did mice treated with control ODNs. This difference was most obvious at 2 weeks (P < 0.05) and with treated mice when compared to controls (P < 0.05). Again, this difference appeared early in the course of infection with M. tuberculosis and had disappeared at 5 weeks. Therefore, the protective effect of CpG in murine pulmonary TB seems to be associated with an enhanced Th1 response and a relatively diminished Th2 response at the site of infection. Macrophages are important sources of TNF-α, which is essential for the formation of granulomas (18). Therefore, we measured TNF-α levels in the lungs of mice. M. tuberculosis induced high levels of TNF-α, but no differences between CpG ODN-treated mice and mice receiving control ODNs were found (data not shown).
FIG. 2.
CpG ODN treatment enhances IFN-γ concentrations in lungs and increases the IFN-γ production capacity of splenocytes. Mice were treated twice with 30 μg of CpG ODNs or control ODNs, 2 h before and 6 h after i.n. infection with 105 CFU of M. tuberculosis. (Left panels) IFN-γ and IL-4 concentrations in lung homogenates; (right panels) IFN-γ and IL-4 concentrations in supernatants of 106 splenocytes stimulated ex vivo with anti-CD3/CD28. Data are mean ± SEM of results for eight mice per group for each time point. ★, P < 0.05 versus results for control.
CpG increases the capacity of splenocytes to produce a Th1 cytokine profile.
Th1 response was further studied by harvesting splenocytes during M. tuberculosis infection, and the ability to release cytokines after stimulation with the non-antigen-specific T-cell stimulator anti-CD3/CD28 was analyzed. Splenocytes of CpG ODN-treated mice produced more IFN-γ than splenocytes from controls after 2 weeks (P < 0.05) but not after 5 weeks (Fig. 2). Levels of IL-4 tended to be lower in splenocytes of CpG ODN-treated mice after 2 weeks but the difference did not reach statistical significance due to a large variation (P = 0.11). This suggests that CpG results in an enhancement of the ability of splenocytes to mount a Th1 response.
CpG administration results in less inflammation in the lungs of M. tuberculosis-infected mice.
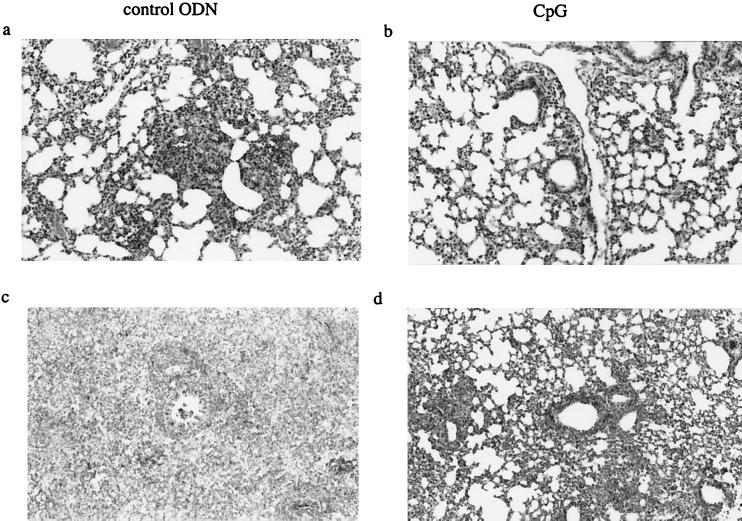
Two weeks after M. tuberculosis infection, control mice developed small, well-defined granulomas composed primarily of lymphocytes and macrophages throughout the lung parenchyma (Fig. 3). Moreover, peribronchiolar and perivascular lymphocytic infiltrates were present. CpG ODN-treated mice displayed fewer lymphocytic infiltrates. In accordance with the degree of inflammation, acid-fast stain-positive bacilli were much more abundant in control mice than in CpG ODN-treated animals. After 5 weeks, the lungs of both groups showed more inflammation, with confluent granulomas composed primarily of macrophages, granulocytes, and lymphocytes. Still, CpG ODN-treated mice displayed less extensive inflammation of the lung parenchyma (50%) than did the untreated mice (70%).
FIG. 3.
Histopathology (hematoxylin and eosin staining) of lungs of mice sacrificed 2 weeks (a and b) (original magnification, ×110) and 5 weeks (c and d) (original magnification, ×70) after M. tuberculosis infection. Mice treated with CpG ODN (b and d) show less inflammation and granuloma formation than control ODN-treated mice (a and c). Slides are representative of at least five mice per group for each time point.
Survival experiments.
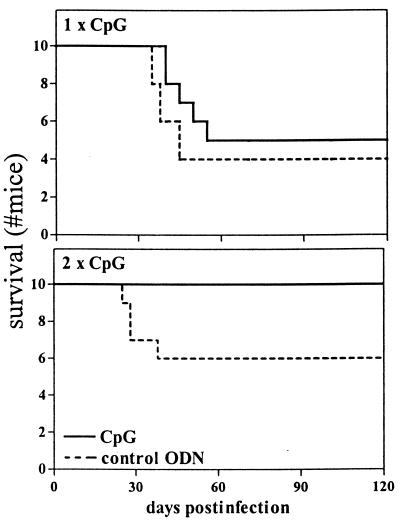
To determine whether CpG had an effect on survival of M. tuberculosis infection, 10 mice injected with CpG ODNs and 10 mice injected with control ODNs were monitored for 4 months. During this observation period, 50% of the CpG ODN-treated mice died versus 60% of the control mice (not significant) (Fig. 4). Considering that the enhancement of a protective Th1 response after a single CpG ODN treatment was diminished after 5 weeks in comparison to the more profound effect after 2 weeks, another survival experiment was carried out in which CpG ODN administration was repeated 2 weeks after infection with M. tuberculosis. After 4 months, 40% of the control mice had died, whereas all CpG ODN-treated mice were still alive (P = 0.03) (Fig 4).
FIG. 4.
Effect of CpG ODN treatment on survival. All mice received 105 CFU M. tuberculosis i.n. at time zero. (Upper panel) Effect of CpG ODN treatment at the time of infection only (30 μg twice, 2 h before and 6 h after infection); (lower panel) effect of repeated CpG treatment (30 μg twice, 2 h before and 6 h after infection, and again 2 weeks thereafter). Experimental groups consisted of 10 mice per group for each treatment schedule. Repeated CpG ODN treatment significantly improved the rates of survival (P = 0.03 versus control ODN results by Kaplan-Meier analysis with log rank test).
Protection by CpG is abrogated in IFN-γ gene-deficient mice.
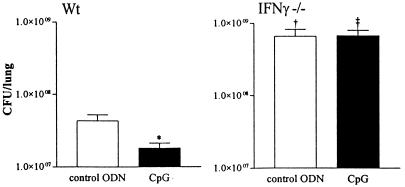
Protection conferred by CpG against M. tuberculosis was associated with elevated levels of IFN-γ in the lungs. Considering that IFN-γ is essential for host defense against TB (13, 27), we wished to determine whether this protection was mediated by IFN-γ. Thus, TB was induced in IFN-γ−/− mice, which were sacrificed 2 weeks after infection. As has been shown previously (7, 19), IFN-γ−/− mice had higher numbers of mycobacteria in their lungs than did wild-type mice (P < 0.001) (Fig. 5). Consistent with previous results, wild-type mice receiving CpG ODNs had lower numbers of mycobacteria in their lungs than did mice receiving control ODNs (P < 0.001). However, in IFN-γ−/− mice, the difference in mycobacterial outgrowth between mice receiving CpG ODNs and those receiving control ODNs was abolished, indicating that protection by CpG is IFN-γ dependent.
FIG. 5.
The effect of CpG ODN treatment on mycobacterial outgrowth is IFN-γ dependent. Wild-type (Wt) BALB/c (left) and IFN-γ−/− BALB/c (right) mice were treated twice with 30 μg of CpG ODNs or control ODNs, 2 h before and 6 h after i.n. infection with 105 CFU of M. tuberculosis. CFU were counted 2 weeks after infection. Data are mean ± SEM of results for eight mice per group for each time point. *, P < 0.05 versus control ODN results; †, P < 0.05 versus results for wild type with control ODN treatment; ‡, P < 0.05 versus results for wild type with CpG ODN treatment.
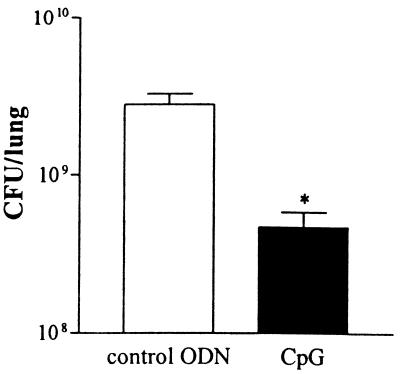
Postponed treatment with CpG still enhances host defense.
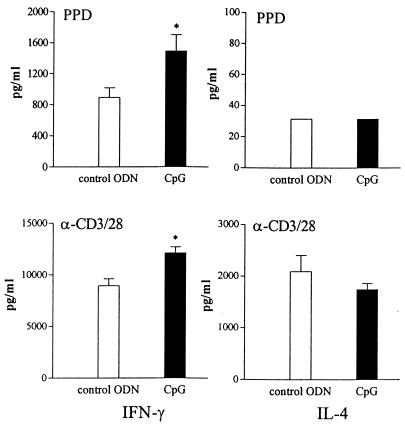
To determine the effects of CpG as a possible therapeutic agent, mice received 40 μg of CpG ODNs or control ODNs i.p. 2 weeks postinfection and were sacrificed 5 weeks postinfection. At this time point, the lungs of CpG ODN-treated mice contained sixfold fewer viable mycobacteria than did the lungs of control ODN-treated animals (P = 0.027) (Fig. 6). To examine the influence of postponed treatment with CpG ODNs on the development of Th1 and Th2 responses, splenocytes harvested from these mice at 5 weeks postinfection were stimulated ex vivo. With these experiments, we also evaluated the ability of CpG ODN treatment to enhance an antigen-specific Th1 response using PPD to stimulate splenocytes. As shown in Fig. 7, splenocytes from CpG ODN-treated mice released more IFN-γ in response to either PPD or anti-CD3/CD28 antibodies (P = 0.027 and P = 0.046, respectively). IL-4 was not detectable in supernatants of splenocytes stimulated with PPD and tended to be lower in supernatants of anti-CD3/CD28 antibody-stimulated splenocytes from CpG ODN-treated animals than in those from control ODN-treated mice (not significant). These data suggest that CpG ODNs given 2 weeks after the infection were still able to promote a protective Th1 response.
FIG. 6.
Postponed treatment with CpG ODNs reduces outgrowth of M. tuberculosis in lungs. Mice were treated with 40 μg of CpG ODNs or control ODNs 2 weeks after i.n. infection with 105 CFU of M. tuberculosis. CFU were counted 5 weeks after infection. Data are mean ± SEM of results for eight mice per group for each time point. *, P < 0.05 versus results for control.
FIG. 7.
Postponed CpG ODN treatment increases the IFN-γ production capacity of splenocytes. Mice were treated with 40 μg of CpG ODNs or control ODNs 2 weeks after i.n. infection with 105 CFU of M. tuberculosis. IFN-γ and IL-4 were measured in supernatants of 106 splenocytes harvested 5 weeks postinfection and stimulated ex vivo with PPD or anti-CD3/CD28. Data are mean ± SEM of results for eight mice per group for each time point. *, P < 0.05 versus results for control.
DISCUSSION
In this study, administration of CpG ODNs to mice infected i.n. with M. tuberculosis resulted in enhanced survival and a reduction in mycobacterial burden in the pulmonary compartment. The beneficial effect of CpG, which was associated with a Th1-type immune response, was mediated by IFN-γ, as indicated by the absence of protection in IFN-γ−/− mice.
The present data are in line with previous reports on the ability of bacterial DNA containing unmethylated CpG motifs or synthetic CpG ODNs to protect against intracellular microorganisms (11, 23, 36). Of interest is also the observation that mice vaccinated with M. bovis BCG and CpG ODNs have an increased Th1 response and a reduced mycobacterial outgrowth after infection with M. tuberculosis (14). It should be noted that CpG-induced protection against different types of microorganisms shows kinetic differences. In infection with Listeria monocytogenes, at least 48 h was required between the time of CpG ODN administration and the time of injection of the pathogen, presumably reflecting the time necessary to upregulate the innate immune responses needed to control this rapidly replicating microorganism (23). In contrast, CpG exerted protective effects against the more indolent pathogen Leishmania major, even when its administration was delayed until 20 days after infection (36). We add to these results that CpG reduced the growth of M. tuberculosis in mouse lungs even when the administration of CpG ODN was delayed for 2 weeks postinfection and that this postponed treatment was associated with an antigen-specific Th1 response. In accordance, the same CpG ODNs as those used in the present investigation directed an antigen-specific immune response toward a Th1 phenotype in mice with L. major infection (36). Together, these findings confirm the notion that CpG ODNs can enhance both nonspecific innate immune defense mechanisms and antigen-specific immune responses (21). Recent evidence indicates that CpG activates immunocompetent cells via Toll-like receptor 9 and that mice deficient in this receptor cannot mount a Th1 response upon exposure to CpG (15).
The CpG-mediated protection we observed was associated with elevated levels of IFN-γ in the lungs, which presumably are produced by both stimulated T cells and NK cells. In addition, protection was related to an increase in the IFN-γ-producing capacity of splenocytes, in both an antigen- and a non-antigen-specific manner. The disappearance of the protective effect of CpG in IFN-γ−/− mice indicates that CpG-induced protection against TB was dependent on IFN-γ. This was also found in mouse models of asthma (33) and listeriosis (23). Although not studied, a protective effect of CpG ODNs on cells of the innate immune system can also be expected to be found in our experiments. Several studies have shown that CpG motifs can activate macrophages (30, 32) and dendritic cells (1, 34) and are able to indirectly activate NK cells (2).
Protection by a single administration of CpG ODN lasted for up to 27 days in cases of murine leishmaniasis (36) and for 2 weeks in subjects infected with L. monocytogenes (11). We found that the protective effect of CpG during TB infection lasted at least 5 weeks. This difference may be explained by the lower replication time of mycobacteria compared to that of other pathogens. When the time course of protection is coupled to the time course of bacterial growth, protection by CpG may be the result of activation of the cells of the innate immune system, which limits mycobacterial replication until an antigen-specific T-cell response has developed. Interestingly, in the experiments in which CpG ODNs were given simultaneously with M. tuberculosis, levels of Th1 cytokine in the lungs as well as levels of the supernatants of stimulated splenocytes were elevated at 2 weeks, but not 5 weeks, after infection. Apparently, an early protective Th1 response is sufficient to reduce the mycobacterial burden at later time points. However, survival was improved only when CpG ODN administration was repeated after 2 weeks. This latter finding is in line with earlier reports demonstrating that repeated administration of CpG ODNs can extend the duration of protection against certain pathogens (21). Importantly, repeated administration of CpG ODN was without overt toxicity, confirming previous studies (20, 21, 36). Further studies to establish the CpG regimen that confers optimal protection against M. tuberculosis are warranted.
In previous studies, exogenous administration of IFN-γ has been shown to be useful as an adjuvant therapy for TB (5, 27). CpG ODN treatment results in endogenous production of IFN-γ, which is subject to regulatory pathways of the innate immune response. Presumably, endogenous IFN-γ production may be associated with toxicity lower than that of exogenous IFN-γ administration. Another promising feature of CpG ODN treatment is its long-term protection, with infrequent dosing required. It should be noted that CpG may initiate inappropiate immune responses and has been reported to induce septic shock in mice and to prime mice for the Shwartzman reaction (8, 31). However, present insights indicate that CpG ODNs can be given relatively safely at doses that increase host defense mechanisms (21). Therefore, the present data may provide the rationale for further development of CpG as a new adjunctive therapy for TB.
Acknowledgments
This work was supported by grants from the Mr. Willem Bakhuys Roozeboom Foundation to N. P. Juffermans and The Netherlands Organization of Scientific Research to J. C. Leemans.
We thank J. Daalhuisen for excellent technical assistance.
N. P. Juffermans and J. C. Leemans contributed equally to this work.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Askew, D., R. S. Chu, A. M. Krieg, and C. V. Harding. 2000. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 165: 6889–6895. [DOI] [PubMed] [Google Scholar]
- 2.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157: 1840–1845. [PubMed] [Google Scholar]
- 3.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162: 3504–3511. [PubMed] [Google Scholar]
- 4.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186: 1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condos, R., W. N. Rom, and N. W. Schluger. 1997. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet 349: 1513–1515. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178: 2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowdery, J. S., J. H. Chace, A.-K. Yi, and A. M. Krieg. 1996. Bacterial DNA induces NK cells to produce IFN-γ in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156: 4570–4575. [PubMed] [Google Scholar]
- 9.Davis, H. L., R. Weeranta, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160: 870–876. [PubMed] [Google Scholar]
- 10.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282: 677–686. [DOI] [PubMed] [Google Scholar]
- 11.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162: 2291–2298. [PubMed] [Google Scholar]
- 12.Erb, K. J., J. W. Holloway, A. Sobeck, H. Moll, and G. Le Gros. 1998. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178: 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freidag, B. L., G. B. Melton, F. Collins, D. M. Klinman, A. Cheever, L. Stobie, W. Suen, and R. A. Seder. 2000. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect. Immun. 68: 2948–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745. [DOI] [PubMed] [Google Scholar]
- 16.Iseman, M. D., and L. A. Madsen. 1989. Drug-resistant tuberculosis. Clin. Chest Med. 10: 341–353. [PubMed] [Google Scholar]
- 17.Juffermans, N. P., S. Florquin, L. Camoglio, A. Verbon, A. H. Kolk, P. Speelman, S. J. H. van Deventer, and T. van der Poll. 2000. Interleukin-1 signaling is essential for host defense during murine tuberculosis. J. Infect. Dis. 182: 902–908. [DOI] [PubMed] [Google Scholar]
- 18.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56: 731–740. [DOI] [PubMed] [Google Scholar]
- 19.Klinman, D. M., A. K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 93: 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67: 5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12: 35–43. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549. [DOI] [PubMed] [Google Scholar]
- 23.Krieg, A. M., L. Love-Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161: 2428–2434. [PubMed] [Google Scholar]
- 24.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166: 4604–4611. [DOI] [PubMed] [Google Scholar]
- 25.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161: 4463–4466. [PubMed] [Google Scholar]
- 26.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, and P. Nunn. 1998. Global surveillance for antituberculosis-drug resistance, 1994–1997. N. Engl. J. Med. 338: 1641–1649. [DOI] [PubMed] [Google Scholar]
- 27.Raad, I., R. Hachem, N. Leeds, R. Sawaya, Z. Salem, and S. Atweh. 1996. Use of adjunctive treatment with interferon-gamma in an immunocompromised patient who had refractory multidrug-resistant tuberculosis of the brain. Clin. Infect. Dis. 22: 572–574. [DOI] [PubMed] [Google Scholar]
- 28.Saunders, B. M., and C. Cheers. 1996. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect. Immun. 64: 4236–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu, T. S., K. Takahashi, and M. Tomita. 1997. CpG distribution patterns in methylated and non-methylated species. Gene 205: 103–107. [DOI] [PubMed] [Google Scholar]
- 30.Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-mediated shock. Eur. J. Immunol. 27: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 31.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Häcker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature 386: 336–337. [DOI] [PubMed] [Google Scholar]
- 32.Stacey, K. J., M. J. Sweet, and D. A. Hume. 1996. Macrophages ingest and are activated by bacterial DNA. J. Immunol. 157: 2116–2122. [PubMed] [Google Scholar]
- 33.Sur, S., J. S. Wild, B. K. Choudhury, N. Sur, R. Alam, and D. M. Klinman. 1999. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 162: 6284–6293. [PubMed] [Google Scholar]
- 34.Tascon, R. E., S. Ragno, D. B. Lowrie, and M. J. Colston. 2000. Immunostimulatory bacterial DNA sequences activate dendritic cells and promote priming and differentiation of CD8+ T cells. Immunology 99: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner, G. J., H. M. Liu, J. E. Wooldridge, C. E. Dahle, and A. M. Krieg. 1997. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc. Natl. Acad. Sci. USA 94: 10833–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Röcken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160: 3627–3630. [PubMed] [Google Scholar]