Abstract
Understanding how existing antivector immunity impacts live vaccine delivery systems is critical when the same vector system may be used to deliver different antigens. We addressed the impact of antivector immunity, elicited by immunization with attenuated actA-deficient Listeria monocytogenes, on the CD8+-T-cell response to a well-characterized lymphocytic choriomeningitis virus epitope, NP118-126, delivered by infection with recombinant L. monocytogenes. Challenges of immune mice with actA-deficient and with wild-type recombinant L. monocytogenes generated similar numbers of CD8+ T cells specific for the NP118-126 epitope. High-dose immunization with actA-deficient L. monocytogenes resulted in substantial numbers of CD8+ T cells specific for the L. monocytogenes LLO91-99 epitope in the effector and memory stages of the T-cell response. Challenge of these immune mice with recombinant L. monocytogenes resulted in rapid control of the infection and decreased CD8+-T-cell responses against both the secreted and nonsecreted form of the recombinant antigen compared to the response of naïve mice. In contrast, mice immunized with a low dose of actA-deficient L. monocytogenes had ∼10-fold fewer effector and memory T cells specific for LLO91-99 and a substantially higher CD8+-T-cell response against the recombinant antigen after challenge with recombinant L. monocytogenes. Although mice immunized with low-dose actA-deficient L. monocytogenes had a substantial recall response to LLO91-99, which reached the same levels by 5 to 7 days postchallenge as that in high-dose-immunized mice, they exhibited decreased ability to control L. monocytogenes replication. Thus, the level of antivector immunity impacts the control of infection and efficiency of priming responses against new antigens introduced with the same vector.
A substantial effort to identify attenuated microorganisms that can serve as vaccine delivery vehicles has been made. This approach takes advantage of the biology of the microbial vector to prolong antigen display, deliver antigens to relevant host cell compartments, and induce appropriate types of immune responses (1, 24). Bacterial vectors for antigen delivery are attractive for several reasons. Unlike viral vectors, large quantities of genetic material may be added to a bacterial vector without affecting the production of infectious progeny. Since multiple bacterial gene products (virulence factors) are required to induce disease, redundant mutations in the bacterial vector can be introduced to ensure that no virulent progeny are obtained by a back-mutation without interfering with bacterial replication or the capacity to establish a limited infection. However, one drawback to the use of microbial vectors of any type is that antivector immunity may limit specific vector systems to a single immunization. This is an important consideration when the existing immune status against the vector is unknown for the general population or when the same vector is utilized to deliver different antigens. Therefore, we sought to determine the impact of existing antivector immunity on the CD8+-T-cell response against a new antigen delivered by the same microbial vector.
Listeria monocytogenes, a gram-positive facultative intracellular bacterial pathogen, has been proposed and tested in mouse models as a vector to deliver recombinant antigens (13, 14, 23, 25, 26, 30). Infection of mice with L. monocytogenes results in the generation of major histocompatibility complex (MHC) class I-restricted CD8+ T cells, which transfer protective immunity to naïve animals (18). Infection of mice with a virulent strain of recombinant L. monocytogenes designed to express a well-characterized CD8+-T-cell epitope from lymphocytic choriomeningitis virus (LCMV) (NP118-126) leads to priming of CD8+ T cells against the recombinant epitope at levels which are comparable to levels of priming against endogenous L. monocytogenes antigens and which provide protection from subsequent viral challenge (25, 26). These results indicate that recombinant L. monocytogenes can serve as an antigen delivery system that is sufficient to generate protective CD8+-T-cell responses. Furthermore, an attenuated actA-deficient strain of L. monocytogenes has been shown to be safely administered to immunocompromised mice that lack the gamma interferon (IFN-γ) structural gene while still inducing substantial CD8+-T-cell priming against known epitopes from virulent L. monocytogenes and protection against challenge infection (5, 15, 28). Therefore, attenuated L. monocytogenes may be an attractive vaccine vector that is able to elicit immune responses against recombinant proteins even in severely immunocompromised hosts.
Previous studies have yielded conflicting results regarding the impact of prior immunization with virulent L. monocytogenes on priming of CD8+ T cells specific for a newly introduced antigen. One study, which assessed CD8+-T-cell responses by limiting dilution assay 5 days after in vitro stimulation with concanavalin A, found that prior immunization with virulent L. monocytogenes had no effect on CD8+-T-cell priming against an epitope introduced during challenge with L. monocytogenes (9). However, a second study, which assessed CD8+-T-cell responses by enzyme-linked immunospot (ELISPOT) assay for IFN-γ production by cells stimulated directly ex vivo, found that prior immunization with virulent L. monocytogenes decreases the number of CD8+ T cells primed against an epitope introduced during challenge with L. monocytogenes (29). It is likely that the differences observed in these studies result from the use of different experimental conditions (28). In both previous studies, virulent L. monocytogenes was utilized for the initial vaccination and challenge. Since it is unlikely that virulent strains of L. monocytogenes would be used for human vaccination, this study was designed to determine what impact prior immunization with an attenuated strain of L. monocytogenes had on CD8+-T-cell priming against secreted and nonsecreted bacterial antigens introduced in a secondary immunization with either a virulent or an attenuated strain of L. monocytogenes.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d MHC) mice were purchased from the National Cancer Institute. H-2d MHC perforin knockout (PKO) mice with a BALB/c background have been previously described (31).
Bacteria.
Bacteria used in this study were virulent L. monocytogenes strain 10403s (8); virulent recombinant L. monocytogenes strain XFL303 (referred to as L. monocytogenes NPs), expressing a secreted fusion protein containing the LCMV NP118-126 epitope (25); recombinant L. monocytogenes XFL304 (referred to as L. monocytogenes NPns), expressing a nonsecreted fusion protein containing the LCMV NP118-126 epitope (25); attenuated recombinant actA-deficient L. monocytogenes, which carries an in-frame deletion in the actA gene (11); and an attenuated recombinant L. monocytogenes strain that was created from L. monocytogenes NPs by introducing an in-frame deletion of the actA gene (referred to as actA-deficient L. monocytogenes NPs). Growth and maintenance of all L. monocytogenes strains were as described previously (15). The actual number of CFU injected was determined for each experiment by plate count.
Virus.
LCMV strain Armstrong was used as previously described (7). Mice were challenged with approximately 2 × 105 PFU.
Infection with L. monocytogenes.
Age- and sex-matched BALB/c or PKO mice 8 to 12 weeks old were immunized by intravenous injection with various numbers of actA-deficient L. monocytogenes CFU (50% lethal dose [LD50] ≈ 107 CFU) and were challenged 28 to 33 days later by intravenous injection with 1.5 × 105 to 3 × 105 virulent L. monocytogenes NPs or L. monocytogenes NPns (∼0.1 LD50 for immune mice) or 1.5 × 107 to 3 × 107 CFU of attenuated actA-deficient L. monocytogenes NPs (∼0.1 LD50 for immune mice). Naïve mice were infected with 1 × 103 to 3 × 103 CFU of L. monocytogenes NPs or L. monocytogenes NPns (∼0.1 LD50 for naïve mice).
51Cr release assays.
NP118-126- and LLO91-99-specific responses were determined in a 21-h direct ex vivo 51Cr release assay using P815 (H-2d MHC) targets with or without 100 nM NP118-126 or LLO91-99 peptide. A total of 104 51Cr-labeled target cells were plated in each well of 96-well microtiter plates in 100 μl of RP10. Effector cells were added in 100 μl to generate a series of effector-to-target ratios. Total release was determined by incubating targets in 0.5% Triton X-100. Spontaneous release was determined by incubating targets in medium alone. Percent specific release was determined as [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100.
Intracellular cytokine staining of splenocytes.
Intracellular cytokine staining to detect epitope-specific CD8+ T cells was performed as described elsewhere (28). Briefly, approximately 2 × 106 splenocytes were incubated for 6 h at 37°C with medium alone or with 100 nM NP118-126 or LLO91-99 peptide, all in the presence of brefeldin A. Cells were washed in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline supplemented with 1% fetal calf serum and NaN3) and were incubated with antibody directed against the FcγII/III receptors (2.4G2) (1:100) and fluorescein isothiocyanate-labeled anti-CD8 (1:100; PharMingen) on ice for 30 min. The cells were washed, fixed, and permeabilized (Cytofix/Cytoperm; PharMingen) on ice for 15 min. The splenocytes were then washed in Perm/Wash solution (PharMingen) and stained with phycoerythrin-conjugated anti-IFN-γ (XMG1.2) (1:100) for 30 min on ice. Cells were washed and resuspended in 250 μl of FACS buffer for analysis by flow cytometry (FACScan; Becton Dickinson). Lymphocytes were gated by forward and side scatter and analyzed with Flowjo (Treestar). The total number of antigen-specific CD8+ T cells obtained for each condition was determined by using the percent CD8+ splenocytes, the percent IFN-γ+ CD8+ splenocytes, and the total splenocyte number. The total number of cytokine-positive cells obtained in the unstimulated samples was subtracted from the total number of cytokine-positive cells obtained in the stimulated sample from each mouse. The average response ± standard deviation (SD) for each treatment group was then calculated.
CFU assays.
Immune BALB/c or PKO mice were infected with 1.5 × 105 to 2 × 105 CFU of L. monocytogenes NPns. At various times following infection the numbers of L. monocytogenes CFU in homogenates of spleen and livers were determined as described previously (15). The results are expressed as mean CFU per organ ± SDs.
RESULTS
Immune mice generate equivalent CD8+-T-cell responses following challenge with virulent and actA-deficient recombinant L. monocytogenes.
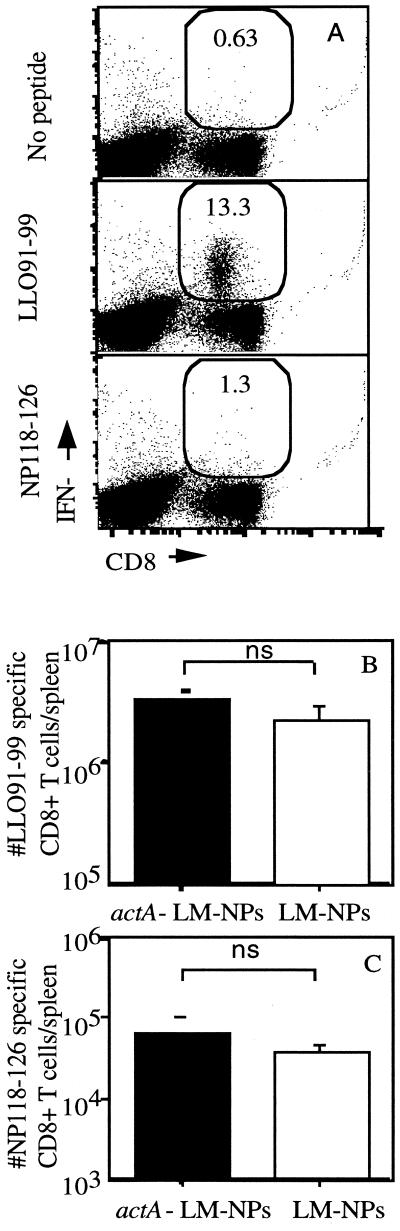
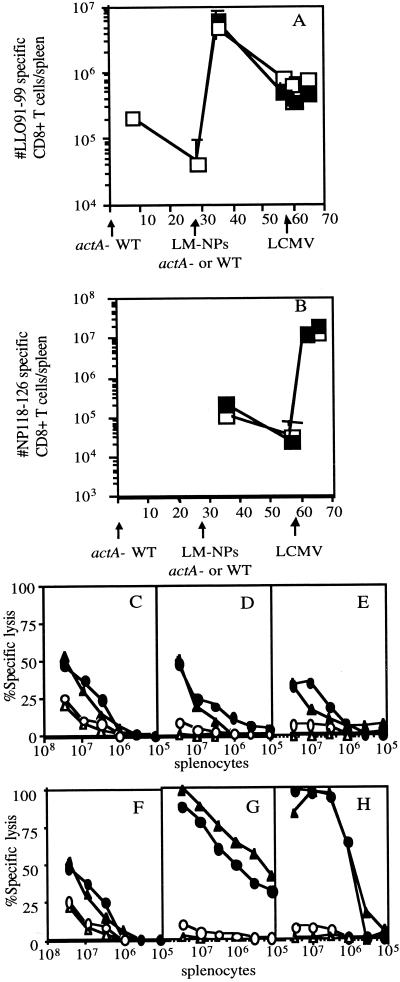
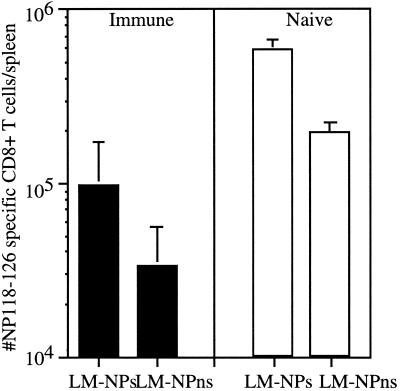
Attenuated L. monocytogenes strains are potentially attractive vaccine delivery vectors to elicit type I CD4+- and CD8+-T-cell responses. It is likely that some individuals in the population will have preexisting immunity to L. monocytogenes that could negatively impact its use as a delivery vector. Thus, we wished to determine whether preexisting immunity affects the CD8+-T-cell response to an antigen introduced in either a virulent or an attenuated strain of recombinant L. monocytogenes. Mice were immunized with 106 CFU of actA-deficient L. monocytogenes and then challenged 30 days later with either virulent L. monocytogenes NPs (105) or actA-deficient L. monocytogenes NPs (6 × 106), both of which express the Ld-restricted CD8+-T-cell epitope NP118-126 from the nucleoprotein of LCMV (26). The secondary response to the endogenous L. monocytogenes epitope LLO91-99 and the primary response to the newly introduced LCMV NP118-126 epitope were determined by intracellular cytokine staining for IFN-γ. IFN-γ intracellular cytokine staining provides an estimate of the number of antigen-specific CD8+ T cells similar to those obtained by either ELISPOT assay or MHC class I tetramer staining (6, 7, 21, 27) without underestimating the number of antigen-specific CD8+ T cells, as occurs with limiting dilution assays (6, 7, 21, 27). The recall response to LLO91-99 at day 7 postchallenge is vigorous (Fig. 1A), while the response to the newly introduced NP118-126 is much lower (Fig. 1A). Immune mice challenged with approximately 0.1 LD50 (for immune mice) of L. monocytogenes NPs and those challenged with the same relative amount of actA-deficient L. monocytogenes NPs generated similar numbers of CD8+ T cells specific for LLO91-99 (2.2 × 106 for L. monocytogenes NPs challenged versus 3.3 × 106 for actA-deficient L. monocytogenes NPs) (Fig. 1B). Infection of immune mice with L. monocytogenes NPs and with actA-deficient L. monocytogenes NPs also resulted in similar numbers of CD8+ T cells specific for the NP118-126 epitope (3.5 × 104 for L. monocytogenes NPs versus 5.4 × 104 for actA-deficient L. monocytogenes NPs) (Fig. 1C). Reduced levels of NP118-126-specific memory CD8+ T cells were present at 28 days postinfection. In order to further characterize the functional characteristics of the memory NP118-126-specific CD8+ T cells generated by the attenuated and virulent recombinant L. monocytogenes, mice were challenged 28 days after recombinant L. monocytogenes immunization with LCMV. In order to determine if each of these strains of recombinant L. monocytogenes is able to act as an effective immunization vector, the CD8+-T-cell response to the newly introduced NP118-126 epitope was also monitored. The kinetics of the CD8+-T-cell response and the number of IFN-γ-producing NP118-126-specific CD8+ T cells was the same in actA-deficient- and wild-type (WT)-immunized mice challenged with LCMV (Fig. 2B) and WT L. monocytogenes NPs (data not shown). The NP118-126-specific CD8+ T cells also demonstrate a similar ability to lyse NP118-126-expressing targets directly ex vivo 3, 5, and 9 days following LCMV challenge (Fig. 2F to H) and 3 and 5 days following L. monocytogenes NPs challenge (data not shown). The number of LLO91-99-specific CD8+ T cells does not differ in either group of mice after LCMV challenge (Fig. 2A). Furthermore, the lytic ability of the LLO91-99-specific CD8+ T cells does not differ in mice immunized with actA-deficient L. monocytogenes NPs or WT L. monocytogenes NPs (Fig. 2C to E). These results indicate that challenge of immune mice with either virulent or with attenuated recombinant L. monocytogenes can generate similar recall responses and that the two are equally effective at generating CD8+ T cells that are specific for an antigen introduced in the context of a previously encountered pathogen. The functional ability of these CD8+ T cells is indistinguishable in mice immunized with virulent and attenuated recombinant L. monocytogenes. Since challenge of immune mice with either WT or attenuated L. monocytogenes resulted in no appreciable differences in the CD8+-T-cell response to either the recall or new antigen, the remaining experiments used only virulent recombinant L. monocytogenes as the challenge organism.
FIG. 1.
Challenge of immune mice with actA-deficient listeriae and that with WT listeriae generate similar numbers of antigen-specific CD8+ T cells. BALB/c mice were immunized with 106 CFU of actA-deficient L. monocytogenes and challenged 28 days later with either 1.5 × 105 CFU of L. monocytogenes NPs or 6 × 106 CFU of actA-deficient L. monocytogenes NPs. The number of LLO91-99-specific (A and B) or NP118-126-specific (A and C) CD8+ T cells per spleen was determined 7 days after challenge by intracellular cytokine staining for IFN-γ. (A) Data are from a representative mouse challenged with actA-deficient L. monocytogenes NPs and stimulated with no peptide (top), LLO91-99 (middle), or NP118-126 (bottom). The number within the gate is the percentage of CD8+ T cells producing IFN-γ. Data are from one of three independent experiments with three mice per group. ns, no significant difference between values.
FIG. 2.
Challenges of immune mice with actA-deficient and WT L. monocytogenes NPs generate functionally similar antigen-specific CD8+ T cells. BALB/c mice were immunized with 105 CFU of actA-deficient L. monocytogenes and challenged 28 days later with either 1.3 × 105 CFU of L. monocytogenes NPs or 1.1 × 105 CFU of actA-deficient L. monocytogenes NPs. The mice were challenged 28 days later with 2 × 105 LCMV particles. The numbers of LLO91-99-specific (A) and NP118-126-specific (B) CD8+ T cells per spleen were determined by intracellular cytokine staining for IFN-γ on the indicated days after immunization. (A and B) Open symbols, mice challenged with actA-deficient L. monocytogenes NPs; closed symbols, mice challenged with WT L. monocytogenes NPs. Three mice per group were analyzed at each time point. The lytic ability of antigen-specific CD8+ T cells was tested in a 21-h, ex vivo 51Cr release assay at 3 (C and F), 5 (D and G), and 9 (E and H) days after LCMV challenge of immunized mice. Specific lysis of P815 (C through H, open symbols) and P815 targets coated with LLO91-99 (C through E, filled symbols) or NP118-126 (F through H, filled symbols) was analyzed for mice immunized with actA-deficient L. monocytogenes NPs (circles, C through H) or WT L. monocytogenes NPs (triangles, C through H). For analysis, splenocytes from three mice were pooled at each time point.
Impact of actA-deficient L. monocytogenes dose on the magnitude and kinetics of the LLO91-99-specific CD8+-T-cell response.
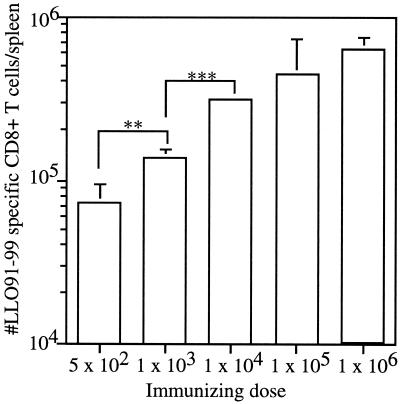
In order to determine the quantitative impact of previous immunization with attenuated L. monocytogenes on the generation of CD8+-T-cell responses against a new antigen, we evaluated CD8+-T-cell priming against the dominant H-2Kd-restricted LLO91-99 epitope (22) 7 days after infection of BALB/c mice with various doses of the actA-deficient L. monocytogenes strain using intracellular cytokine staining for IFN-γ. Significant levels of LLO91-99-specific CD8+-T-cell priming were observed after infection with doses of actA-deficient L. monocytogenes varying from 500 to 106 CFU, and the magnitude of CD8+-T-cell response correlated with the infecting dose (Fig. 3). Mice that received an immunizing dose of 500 CFU of actA-deficient L. monocytogenes had approximately 10-fold fewer (7 × 104 versus 6 × 105) total LLO91-99-specific CD8+ T cells per spleen than did mice that were immunized with 106 CFU of actA-deficient L. monocytogenes (Fig. 3). These results indicate that CD8+-T-cell priming against the actA-deficient L. monocytogenes strain occurs over a wide range of doses and that the level of CD8+-T-cell priming can be modulated by the immunizing dose.
FIG. 3.
The number of antigen-specific CD8+ T cells generated during an immune response correlates with the immunizing dose of actA-deficient L. monocytogenes. BALB/c mice were immunized with the indicated doses (in CFU) of actA-deficient L. monocytogenes; 7 days later the number of LLO91-99-specific CD8+ T cells per spleen was determined by intracellular cytokine staining for IFN-γ. Representative data are from one of three independent experiments and are means plus SDs for three mice/group. Statistical analysis performed with Student’s t test resulted in P values of <0.005. Statistical analysis was also performed to compare the CD8+-T-cell priming observed with each of the lower doses against priming at 106 CFU. Values obtained with 500 (P < 0.005), 103 (P < 0.005), and 104 (P < 0.01) CFU were all significantly different from that obtained with 106 CFU. **, P < 0.005; ***, P < 0.01.
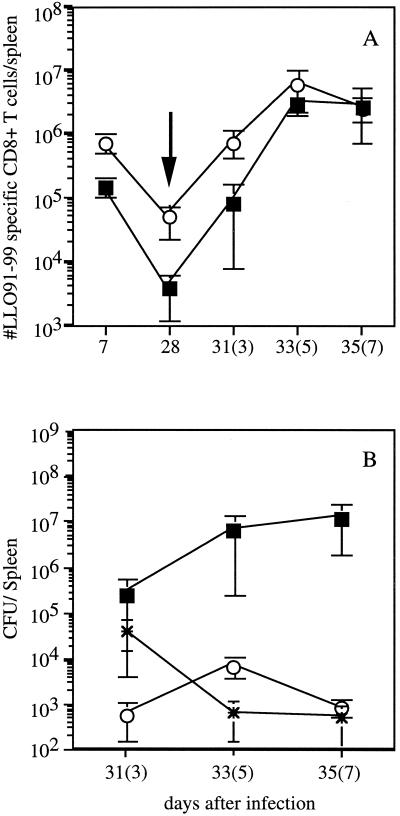
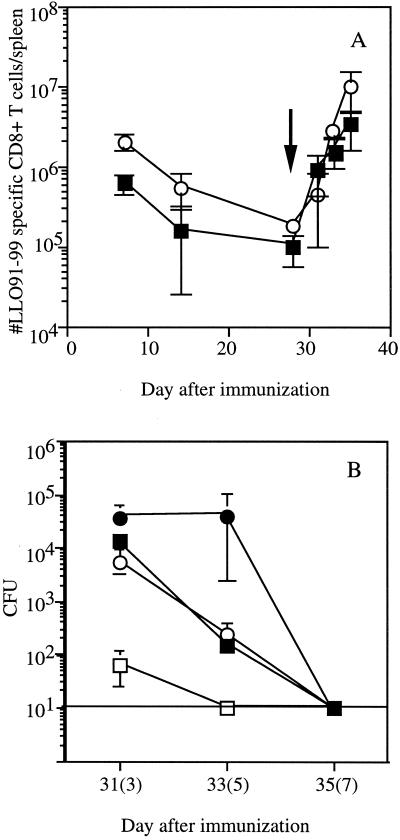
The CD8+-T-cell response against L. monocytogenes can be divided into three phases: (i) activation and expansion of effector CD8+ T cells; (ii) a death phase, in which more than 80% of antigen-specific CD8+ T cells are eliminated by apoptosis; and (iii) a memory phase, in which the number of remaining antigen-specific CD8+ T cells is maintained. Previous studies showed that the activation and expansion phase generally occurs through day 7 to 9 of infection, the death phase is essentially complete by day 14, and the memory phase lasts from approximately day 14 up to 2 years (7, 21). Recent studies have demonstrated that the magnitude of the primary CD8+-T-cell response to infection influences the number of CD8+ T cells present in the stable memory pool, which in turn influences the number of CD8+ T cells generated during a recall response (12, 21). To address these issues in mice immunized with actA-deficient L. monocytogenes, the magnitude of the LLO91-99-specific response was analyzed at various times following immunization. Again, immunization with 106 CFU of actA-deficient L. monocytogenes resulted in significantly more LLO91-99-specific CD8+ T cells at 7 days postinfection of BALB/c mice than immunization with 500 CFU (Fig. 3 and 4A). As previously shown with virulent L. monocytogenes (12), the number of LLO91-99-specific CD8+ T cells decreases by day 28 in mice immunized with either dose of L. monocytogenes (Fig. 4A). Since the number of LLO91-99-specific CD8+ T cells per spleen at 28 days postimmunization in mice given 500 CFU of actA-deficient L. monocytogenes approaches the reliable limit of detection of the assay (∼5 × 103 antigen-specific CD8+ T cells/spleen), the difference in the number of LLO91-99-specific memory CD8+ T cells in mice given 500 and 106 CFU of attenuated L. monocytogenes is at least 10-fold. These results are consistent with the notion that there is a direct relationship between the number of antigen-specific CD8+ effector T cells obtained at the peak of expansion and the number of antigen-specific memory CD8+ T cells (12, 21).
FIG. 4.
Kinetics of CD8+-T-cell responses in mice immunized with low and high doses of actA-deficient L. monocytogenes. (A) BALB/c mice were immunized with either 500 (squares) or 106 (circles) CFU of actA-deficient L. monocytogenes. Mice were challenged 28 days later with 2 × 105 CFU of L. monocytogenes NPns (arrow). The number of LLO91-99-specific CD8+ T cells per spleen was determined by intracellular cytokine staining for IFN-γ on the indicated days (numbers of days after challenge are in parentheses). The reliable limit of detection is ∼5 × 103 antigen-specific CD8+ T cells. Data are from a representative experiment from three independent experiments and are means ± SDs for three mice/group. (B) Following the challenge, the ability of mice to eliminate bacteria was determined by measuring the number of L. monocytogenes present in the spleen at the indicated time points. Mice were immunized with 500 (squares) or 106 (circles) actA-deficient L. monocytogenes organisms. Asterisks indicate naïve mice challenged with 2 × 103 CFU of L. monocytogenes NPns. Time points shown are days 3, 5, and 7 after infection of naïve mice and after challenge of immune mice (in parentheses). Statistical analysis by Student’s t test indicates that P is <0.05 for all time points.
We next determined whether the size of the memory pool induced by immunization with actA-deficient L. monocytogenes influenced the magnitude of the epitope-specific recall CD8+-T-cell response to secondary challenge with virulent recombinant L. monocytogenes (2 × 105 CFU, ∼0.1 LD50 for immune mice). Challenge of mice immunized with 500 CFU of actA-deficient L. monocytogenes generated LLO91-99-specific CD8+ T cells with early kinetics that were similar to those of mice immunized with 106 CFU of actA-deficient L. monocytogenes (Fig. 4A). As expected, the mice immunized with 500 CFU of actA-deficient L. monocytogenes had fewer LLO91-99-specific CD8+ T cells at day 3 (∼8-fold) following challenge than mice which had been immunized with 106 CFU of attenuated L. monocytogenes (Fig. 4A). However, by day 5 after challenge, only a twofold difference was observed. At 7 days after challenge, the numbers of LLO91-99-specific CD8+ T cells were the same in mice immunized with low and high doses of actA-deficient L. monocytogenes. Similar results were obtained in mice challenged with another virulent L. monocytogenes strain (data not shown). In order to determine the impact of immunization dose on control of the challenge infection, bacterial numbers were measured at various times postchallenge. The number of L. monocytogenes present in the spleens (Fig. 4B) and livers (data not shown) of mice immunized with 500 CFU of actA-deficient L. monocytogenes was higher than that in mice immunized with 1 × 106 CFU of actA-deficient L. monocytogenes or in naïve mice given 2 × 103 CFU of recombinant L. monocytogenes (∼0.1 LD50) at all times after challenge. The mice immunized with 500 CFU of L. monocytogenes had 450-fold more L. monocytogenes in the spleen 3 days after challenge, and the difference increased to 1,000-fold by 5 days after challenge and to 15,000-fold by 7 days after challenge (Fig. 4B). These results indicate that the mice immunized with the low dose of actA-deficient L. monocytogenes have a decreased ability to eliminate bacteria following challenge with virulent L. monocytogenes despite numbers of LLO91-99-specific CD8+ T cells by day 7 postchallenge similar to those found in high-dose-immunized mice (Fig. 4A). However, the mice immunized with low doses of attenuated L. monocytogenes do demonstrate protective immunity, since naïve mice infected with the dose of virulent L. monocytogenes used in the challenge rapidly succumb to this level of infection (data not shown). Thus, the presence of low numbers of CD8+ memory T cells at the time of challenge correlates with reduced efficiency of bacterial control following challenge infection.
The response to antigens introduced during a recall response is reduced compared to a primary response against the same antigen.
Previous studies with recombinant L. monocytogenes strains expressing the same model antigens in secreted or nonsecreted form demonstrated that antigen compartmentalization has a relatively small but reproducible impact on the magnitude of the primary CD8+-T-cell response (25, 28). While the exact route by which nonsecreted L. monocytogenes antigens are presented to prime the CD8+-T-cell response is unknown, it is possible that cross-presentation of necrotic or apoptotic L. monocytogenes-infected cells by dendritic cells (DC) is required (3, 4, 32). Antivector immunity results in more rapid clearance of infected cells and may reduce the number of L. monocytogenes-infected cells that proceed to apoptosis or necrosis. Therefore, we wished to determine if antivector immunity differentially impacted CD8+-T-cell responses to a new antigen expressed as either a secreted or a nonsecreted epitope. To this end, BALB/c mice were immunized with actA-deficient L. monocytogenes (1 × 106 CFU) and challenged 30 days later with virulent recombinant L. monocytogenes (3 × 105 CFU, ∼0.1 LD50 for immune WT mice) expressing the CD8+-T-cell epitope NP118-126 as a secreted (L. monocytogenes NPs) or nonsecreted (L. monocytogenes NPns) fusion protein (25). The response against NP118-126 was determined 7 days later by intracellular cytokine staining for IFN-γ. The immune BALB/c mice exhibited a decreased ability to respond to new secreted (sixfold) and nonsecreted (sixfold) bacterial antigens compared to the response generated in naïve mice infected with 103 CFU of virulent recombinant L. monocytogenes (∼0.1 LD50 for naïve WT mice) (Fig. 5). Following sublethal infection of naïve WT mice, CD8+-T-cell priming against the nonsecreted NP118-126 epitope is three- to fourfold less than the response against the secreted form of the same epitope (25, 28). This relationship is also observed in WT mice previously immunized with attenuated L. monocytogenes (Fig. 5). Since the naïve and the immune mice were challenged with the same relative amount (∼0.1 LD50), the decreased level of CD8+-T-cell priming in the immune mice is not due to their receiving a smaller dose of recombinant L. monocytogenes. Although naïve mice had greater numbers of bacteria present at 3 days after infection, by 5 days after infection the number is less than that in mice immunized with 1 × 106 CFU of L. monocytogenes and challenged with 2 × 105 CFU of recombinant L. monocytogenes (Fig. 4B). These results indicate that antivector immunity has a significant impact on, but does not eliminate, the CD8+-T-cell responses against new epitopes (secreted or nonsecreted) delivered by recombinant L. monocytogenes.
FIG. 5.
Previous immunization with attenuated L. monocytogenes decreases the response against newly introduced bacterial antigens. Naïve BALB/c mice were challenged with recombinant L. monocytogenes NPs or L. monocytogenes NPns. Previously immunized mice had received 106 CFU of actA-deficient L. monocytogenes 28 days earlier and were then challenged with 3 × 105 CFU of L. monocytogenes NPs or L. monocytogenes NPns. The number of antigen-specific CD8+ T cells per spleen was determined by intracellular cytokine staining for IFN-γ. Data are from a representative experiment of six independent experiments and are means ± SDs for three mice/group.
Immunization dose influences CD8+-T-cell priming against a new antigen.
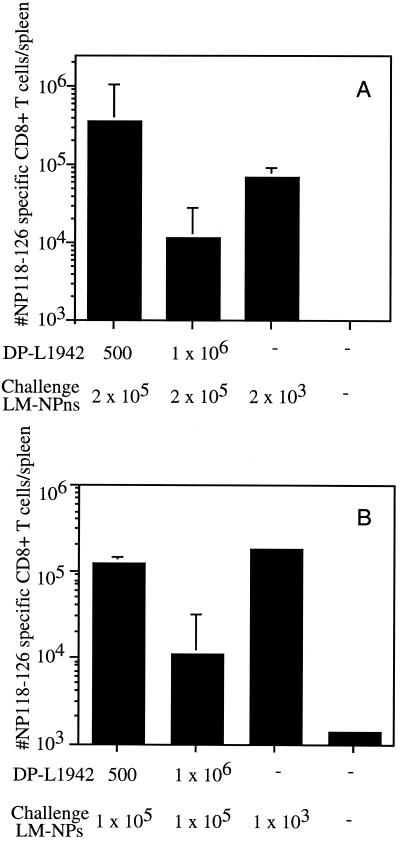
Since the number of L. monocytogenes CFU given to mice during immunization results in different numbers of LLO91-99-specific CD8+ T memory cells (Fig. 4A) as well as a difference in ability to eliminate L. monocytogenes after challenge (Fig. 4B), the impact of immunization dose on the generation of CD8+ T cells in response to a newly introduced antigen was investigated. After challenge with recombinant L. monocytogenes, mice immunized with 500 CFU of actA-deficient L. monocytogenes had >10-fold more CD8+ T cells specific for a newly introduced nonsecreted bacterial antigen than did mice that were immunized with 106 actA-deficient L. monocytogenes (3 × 105 versus 1 × 104) (Fig. 6A). Similar results were observed in the CD8+-T-cell response against the secreted bacterial antigen (105 versus 104) (Fig. 6B), indicating that protein localization does not have an impact on the ability to prime responses against the newly introduced antigen in WT mice. These results demonstrate that the level of preexisting CD8+-T-cell immunity against the vector directly impacts the ability to generate CD8+-T-cell responses against newly introduced antigens.
FIG. 6.
Mice immunized with 500 CFU of actA-deficient L. monocytogenes generate more CD8+ T cells specific for an antigen introduced during challenge than mice immunized with 106 CFU of actA-deficient L. monocytogenes. BALB/c mice were immunized with either 500 or 106 CFU of actA-deficient L. monocytogenes. The mice were challenged 28 days later with 2 × 105 CFU of L. monocytogenes NPns (A) or 1 × 105 CFU of L. monocytogenes NPs (B). Naïve BALB/c mice were immunized with 2 × 103 CFU of L. monocytogenes NPns (A) or 1 × 103 CFU of L. monocytogenes NPs (B). The number of NP118-126-specific CD8+ T cells per spleen was determined by intracellular cytokine staining for IFN-γ 7 days after challenge. Data are from a representative experiment of three (A) or two (B) independent experiments and are means ± SDs for three mice/group.
Delayed clearance of infected cells in PKO mice does not increase CD8+-T-cell priming against newly introduced antigens.
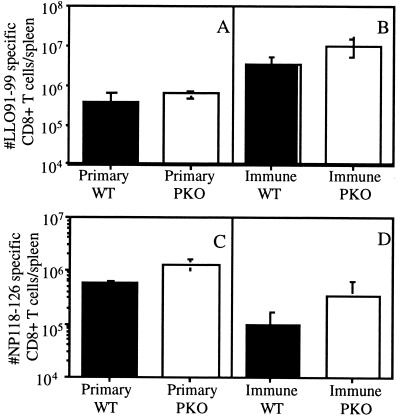
One potential explanation for the reduced ability to generate CD8+ T cells specific for bacterial antigens introduced during a recall response is that the infected cells are rapidly eliminated by the cytolytic activity of activated effector memory CD8+ T cells. If more rapid elimination of antigen-presenting cells accounts for the decreased responses against newly introduced antigens, then delaying the lysis of infected cells may allow the development of a greater CD8+-T-cell response to newly introduced antigens. PKO mice have decreased CD8+-T-cell cytotoxicity (17, 19) and lyse targets with delayed kinetics in vitro (31). Based on these results, we hypothesized that PKO mice would demonstrate delayed clearance of infected host cells and increased priming of CD8+-T-cell responses to newly introduced antigen. Therefore, the ability of PKO mice to generate responses against L. monocytogenes antigens introduced during a recall response was examined. PKO and WT BALB/c mice were immunized with 106 CFU of actA-deficient L. monocytogenes. The mice were rested for 28 to 32 days and then challenged with 1.5 × 105 CFU of L. monocytogenes NPs. The response against LLO91-99 was determined to compare the kinetics of CD8+-T-cell responses during immunization and challenge. PKO mice had greater numbers (∼2- to 3-fold) of LLO91-99-specific CD8+ T cells than WT mice at 7 and 28 days after immunization (Fig. 7A and 8A) (7) and 7 days after challenge with virulent recombinant L. monocytogenes (Fig. 8B) (7). However, PKO mice demonstrated delayed elimination of L. monocytogenes following challenge with 1.5 × 105 CFU of L. monocytogenes NPs compared to WT mice (Fig. 7B). The response generated against the newly introduced NP118-126 epitope was determined by intracellular cytokine staining 7 days after challenge. The PKO mice exhibited a threefold-enhanced response to newly introduced secreted antigens (L. monocytogenes NPs infection) compared to WT mice (Fig. 8D). These results demonstrate that the PKO mice generate enhanced CD8+-T-cell responses to newly introduced antigens. However, PKO mice demonstrate the same increased numbers of CD8+ T cells following a primary infection (Fig. 7A and 8A and C). Since there is not a greater net increase in the number of CD8+ T cells generated against a newly introduced antigen than in the number of CD8+ T cells generated during a primary response to L. monocytogenes, the absence of perforin-dependent cytolysis did not dramatically alter the response of vaccinated mice to a new antigen. These results indicate that increasing the duration of bacterial infection does not enhance the CD8+-T-cell response generated against bacterial antigens.
FIG. 7.
PKO mice have numbers of LLO91-99-specific CD8+ T cells similar to those of WT mice but eliminate bacteria with delayed kinetics after challenge with L. monocytogenes. (A) WT (squares) and PKO (circles) mice were immunized with 1 × 106 CFU of actA-deficient L. monocytogenes. The mice were challenged 28 days later with 1.5 × 105 CFU of L. monocytogenes NPs (arrow). The number of CD8+ T cells specific for LLO91-99 was determined at each time point by intracellular cytokine staining for IFN-γ. Data are means ± SDs. (B) The numbers of CFU in spleen (open symbols) and liver (closed symbols) were determined on the indicated days after immunization. Dashed line, limit of detection. Data are from a representative experiment of three independent experiments and are the averages for three mice at each time point in each group.
FIG. 8.
PKO mice respond to a newly introduced bacterial antigen during recall responses to L. monocytogenes. WT (filled bars) and PKO (open bars) were immunized with 1 × 106 CFU of actA-deficient L. monocytogenes and challenged with 1.5 × 105 CFU of L. monocytogenes NPs 28 days later. Previously unimmunized WT mice were challenged with 1.5 × 103 CFU of L. monocytogenes NPs. The numbers of CD8+ T cells specific for LLO91-99 (A and B) and NP118-126 (C and D) were determined 7 days later by intracellular cytokine staining for IFN-γ. Data are from a representative experiment of three independent experiments and are means ± SDs for three mice/group.
DISCUSSION
Naïve CD8+ T cells must encounter their antigen in the context of professional antigen-presenting cells, likely DC, in order to receive the appropriate costimulatory signals for activation and clonal expansion. However, memory-T-cell activation is less stringent due to increased adhesion molecule expression and perhaps alteration in the sensitivity of T-cell receptor stimulation (2). In a primary infection, the immature DC acquire antigen and mature; the mature DC then migrate to the draining lymph nodes, where they are able to activate naïve T cells. In a recall response, memory T cells, which are capable of circulating through tissue, are recruited to the initial site of infection, where they can be activated to lyse infected host cells or produce cytokines. These effector mechanisms function to limit the challenge infection and thus may reduce the quantity of antigen for priming of naïve CD8+ T cells specific for new antigens introduced with a previously encountered microbial vector. Consistent with this notion, our results (Fig. 5) demonstrate that immunization with an attenuated pathogen reduces the number of CD8+ T cells that respond to a new antigen introduced during a recall response.
The impact of prior immunization on the response to a newly introduced L. monocytogenes antigen has recently been addressed by two groups, with disparate results. Using a frequency analysis after in vitro restimulation with the nonspecific mitogen concanavalin A, Bouwer et al. found that existing immunity to L. monocytogenes did not inhibit the ability to develop a CD8+-T-cell response against a secreted bacterial antigen introduced during a recall response to L. monocytogenes (9). In contrast, Vijh et al. used a peptide-specific direct ex vivo ELISPOT assay and found that the response to newly introduced secreted bacterial antigens was reduced during a recall response compared to the response generated during primary infection (29). It was found previously that in 51Cr release assays performed 5 days after in vitro restimulation, the response against the secreted NP118-126 fusion protein introduced during a recall infection did not differ significantly from that in a primary immune response (28). When the responses to the new secreted epitope were quantitated by IFN-γ intracellular cytokine staining ex vivo, it was found that the response to the NP118-126 epitope was reduced compared to the level of response generated during a primary infection (Fig. 5). The disparity found with these different methods is consistent with the notion that bulk in vitro restimulation assays may underestimate differences in initial precursor frequencies.
Since it is unlikely that virulent L. monocytogenes will be used as a vaccine vector, we compared the CD8+-T-cell responses generated by virulent and attenuated recombinant L. monocytogenes. In mice previously exposed to attenuated L. monocytogenes, the CD8+-T-cell response to WT and actA-deficient L. monocytogenes NPs were similar in magnitude and function (Fig. 2). The mice were given different doses of WT (105 CFU) and actA-deficient (107 CFU) L. monocytogenes NPs; however, these doses both represent approximately 0.1 LD50 for each strain of L. monocytogenes in previously immunized mice. Thus, in mice given similar effective doses of WT and actA-deficient L. monocytogenes NPs, the preexisting antivector immunity has similar impacts on the ability to generate CD8+-T-cell responses against a new antigen.
Studies with virulent L. monocytogenes suggest that infectious dose does not correlate with the level of CD8+-T-cell responses generated (20, 29). However, the use of attenuated L. monocytogenes allowed us to vary the immunizing dose over a much greater range. This larger range of infection clearly indicates that there is a correlation between infection level and the number of antigen-specific CD8+ T cells generated (Fig. 3). The ability to manipulate the level of adaptive immunity allowed us to determine how the level of existing immunity impacts priming against an epitope introduced during the recall response. The number of antigen-specific CD8+ T cells present at both the peak and memory phases of the response correlated with the numbers of attenuated L. monocytogenes given to the mice. In addition to having a decreased number of LLO91-99-specific CD8+ T cells following challenge with recombinant L. monocytogenes, mice immunized with low doses of bacteria had an increase in the number of bacteria present. These results indicate that the number of bacteria used for immunization directly impacts the ability of mice to clear a subsequent infection. This is due to the fact that the number of antigen-specific CD8+ T cells present in the low-dose-immunized mice does not reach the memory level of the high-dose-infected mice until 3 days after infection. During this time, bacteria continue to replicate and create new foci of infection. However, the continued presence of bacteria leads neither to increased expansion of antigen-specific CD8+ T cells nor to a delay in the contraction of CD8+-T-cell numbers (20). Therefore, by the time the T-cell numbers are equivalent in the low- and high-dose-immunized mice, the death phase of the response has begun. It is unlikely that the high-dose-immunized mice have an effector population of CD8+ T cells which do not produce IFN-γ, as it has been previously shown that estimates of the number of antigen-specific CD8+ T cells obtained from intracellular cytokine staining for IFN-γ, tetramer staining, and ELISPOT assay are very similar (6, 7, 21, 27). Thus, the inability of the low-dose-immunized mice to eliminate bacteria is not due to functional differences in the antigen-specific CD8+ T cells but rather due to the decreased number of CD8+ T cells that are present early after challenge.
Consistent with the notion that the level of CD8+-T-cell memory influences priming against a new antigen introduced with the same vector, mice immunized with 500 CFU of L. monocytogenes generated greater numbers of CD8+ T cells specific for newly introduced secreted and nonsecreted bacterial antigens than did mice immunized with 106 CFU of L. monocytogenes (Fig. 5). Together, these results suggest that the presence of higher numbers of memory CD8+ T cells may limit the activation of naïve CD8+ T cells specific for the newly introduced antigen by controlling the infection and decreasing the antigen load. In individuals with preexisting immunity to the vector used to introduce new antigens, substantial immune responses may not be generated or may require higher antigen doses or more immunizations in order to develop an effective memory pool of antigen-specific CD8+ T cells.
PKO mice are unable to carry out the granule exocytosis pathway of CD8+-T-cell cytolysis (17, 19). However, they are capable of a delayed lysis of targets, mediated via CD95L/CD95 interaction, during in vitro 51Cr release assays (10). PKO mice also exhibit adaptive immunity to L. monocytogenes infection that is partially dependent on CD8+ T cells, although it is not as potent as that observed in WT mice (16, 31). Since PKO mice exhibit delayed clearance of L. monocytogenes during infection, they were used to determine whether delaying antigen clearance would increase the number of CD8+ T cells generated against a newly introduced antigen. After immunization with attenuated L. monocytogenes, PKO mice reproducibly generate greater numbers of effector and memory LLO91-99-specific CD8+ T cells than WT mice (7; this study). Consistent with previous studies (16, 31), the immune PKO mice had higher bacterial numbers at days 3 and 5 after challenge, but by day 7 no detectable bacteria remained in the spleens or livers of either type of mice (Fig. 7B). Furthermore, immune PKO mice generated a more vigorous CD8+-T-cell response against a newly introduced secreted bacterial antigen than WT mice. However, this response was no greater than the three- to fourfold increase in antigen-specific CD8+ T cells seen in PKO mice following primary infection with actA-deficient L. monocytogenes (Fig. 7A and 8) (7). Therefore, increasing the time between infection and clearance of bacteria does not increase the number of CD8+ T cells specific for the newly introduced antigen. These results also indicate that perforin is not required for generating CD8+-T-cell responses against a new antigen.
Furthermore, these data are consistent with recent findings that demonstrated that the number of CD8+ T cells generated in response to a bacterial infection is not decreased when mice are treated with antibiotics 24 h after infection (20). Administration of antibiotics starting 24 h after infection resulted in clearance of bacteria within 12 h of initiation of antibiotic treatment, indicating that the first 36 h of infection are important for determining the size of the CD8+-T-cell response. In view of these results, it is not surprising that extending the duration of infection in PKO mice did not result in substantially greater numbers of antigen-specific CD8+ T cells. Together these results suggest that the most reliable method of increasing CD8+-T-cell priming in individuals with preexisting antivector immunity may involve an increase in the infectious dose or the number of immunizations.
Acknowledgments
We thank D. Portnoy for the actA deletion construct, Lori Gorton, Kate Slade, and Julia Vera for excellent technical assistance, and Stanley Perlman and Vladimir Badovinac for critical review of the manuscript.
This work was supported by NIH grants AI46653 and AI42767 to J.T.H. A.R.T. was supported by USPHS training grant T32 AI07511 and 5 T32 AI07260-15.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ada, G. 1991. Strategies for exploiting the immune system in the design of vaccines. Mol. Immunol. 28: 225–230. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272: 54–60. [DOI] [PubMed] [Google Scholar]
- 3.Albert, M. L., S. F. A. Pearce, L. M. Francisco, B. Sauter, R. Pampa, R. L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392: 86–89. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., and J. T. Harty. 2000. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-γ. J. Immunol. 164: 6444–6452. [DOI] [PubMed] [Google Scholar]
- 6.Badovinac, V. P., and J. T. Harty. 2000. Intracellular staining for TNF and IFN-γ detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Methods 238: 107–117. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and IFN-γ. Science 290: 1354–1358. [DOI] [PubMed] [Google Scholar]
- 8.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139: 2005–2009. [PubMed] [Google Scholar]
- 9.Bouwer, H. G. A., H. Shen, X. Fan, J. F. Miller, R. A. Barry, and D. J. Hinrichs. 1999. Existing antilisterial immunity does not inhibit the development of a Listeria monocytogenes-specific primary cytotoxic T-lymphocyte response. Infect. Immun. 67: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, M. Y., B. Lowin, L. French, H. Acha-Orbea, and J. Tschopp. 1996. Cytotoxic T cells deficient in both functional fas ligand and perforin shown residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J. Exp. Med. 183: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Pro. Natl. Acad. Sci. USA 90: 11890–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch, D. H., I. M. Pilip, S. L. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8: 353–362. [DOI] [PubMed] [Google Scholar]
- 13.Goossens, P. L., and G. Milon. 1992. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int. Immunol. 4: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 14.Goossens, P. L., G. Milon, P. Cossart, and M. F. Saron. 1995. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int. Immunol. 7: 797–805. [DOI] [PubMed] [Google Scholar]
- 15.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3: 107–119. [DOI] [PubMed] [Google Scholar]
- 16.Kagi, D., B. Ledermann, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1994. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 24: 3068–3072. [DOI] [PubMed] [Google Scholar]
- 17.Kagi, D., B. Ledermann, K. Burki, P. Seller, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369: 31–37. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11: 129–163. [DOI] [PubMed] [Google Scholar]
- 19.Lowin, B., F. Beermann, A. Schmidt, and J. Tschopp. 1994. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 91: 11571–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol 165: 6833–6839. [DOI] [PubMed] [Google Scholar]
- 21.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. D. Sourdive, A. J. Zajac, J. D. Miller, J. Stansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8: 177–187. [DOI] [PubMed] [Google Scholar]
- 22.Pamer, E. G., J. T. Harty, and M. J. Bevan. 1991. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer, R., D. A. Portnoy, S. A. Brassell, and Y. Paterson. 1992. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J. Immunol 149: 53–59. [PubMed] [Google Scholar]
- 24.Shanta, M. T., L. Stevceva, S. Agwale, G. K. Lewis, and D. M. Hone. 2000. Recent advances with recombinant bacterial vaccine vectors. Mol. Med. Today 6: 66–71. [DOI] [PubMed] [Google Scholar]
- 25.Shen, H., J. F. Miller, X. Fan, D. Kolwyck, R. Ahmed, and J. T. Harty. 1998. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92: 535–545. [DOI] [PubMed] [Google Scholar]
- 26.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92: 3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164: 208–216. [DOI] [PubMed] [Google Scholar]
- 28.Tvinnereim, A. R., and J. T. Harty. 2000. CD8+ T cell priming against a non-secreted Listeria monocytogenes antigen is independent of the anti-microbial activities of IFN-γ. Infect. Immun. 68: 2196–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijh, S., I. M. Pilip, and E. G. Pamer. 1999. Noncompetitive expansion of cytotoxic T lymphocytes specific for different antigens during bacterial infection. Infect. Immun. 67: 1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiskirch, L. M., and Y. Paterson. 1997. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol. Rev. 158: 159–169. [DOI] [PubMed] [Google Scholar]
- 31.White, D. W., and J. T. Harty. 1998. Perforin-deficient CD8(+) T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-γ but requires TNF-α. J. Immun. 160: 898–905. [PubMed] [Google Scholar]
- 32.Yrlid, U., and M. J. Wick. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]