Abstract
The oxytocin receptor (OXTR) and its ligand, oxytocin (OXT), regulate reproductive physiology (i.e., parturition and lactation) and sociosexual behaviors. To define the essential functions of OXTR, we generated mice with a null mutation in the Oxtr gene (Oxtr-/-) and compared them with OXT-deficient (Oxt-/-) mice. Oxtr-/- mice were viable and had no obvious deficits in fertility or reproductive behavior. Oxtr-/- dams exhibited normal parturition but demonstrated defects in lactation and maternal nurturing. Infant Oxtr-/- males emitted fewer ultrasonic vocalizations than wild-type littermates in response to social isolation. Adult Oxtr-/- males also showed deficits in social discrimination and elevated aggressive behavior. Ligand Oxt-/- males from Oxt-/- dams, but not from Oxt+/- dams, showed similar high levels of aggression. These data suggest a developmental role for the OXT/OXTR system in shaping adult aggressive behavior. Our studies demonstrate that OXTR plays a critical role in regulating several aspects of social behavior and may have important implications for developmental psychiatric disorders characterized by deficits in social behavior.
Keywords: lactation, maternal behavior, ultrasonic vocalization, social discrimination, aggressive behavior
Oxytocin (OXT), a nonapeptide hormone, was the first peptide hormone whose structure was determined and the first to be chemically synthesized in a biologically active form (1, 2). OXT is produced primarily in the paraventricular and supraoptic nuclei of the hypothalamus (3) and secreted mainly from the posterior pituitary gland. In addition, OXT fibers project to various brain regions (4) where OXT functions as a neurotransmitter or neuromodulator. Besides its classical functions (i.e., induction of labor and milk ejection), OXT plays an important role in social behavior (i.e., sexual behavior, maternal behavior, affiliation, and social memory), the estrous cycle, penile erection, and ejaculation (4–7).
The actions of OXT are mediated via binding to the OXT receptor (OXTR). OXTR contains seven transmembrane domains and belongs to the class 1 family of G protein-coupled receptors. In response to ligand binding, OXTR mainly leads to stimulation of phospholipase C by interacting with Gαq/11. OXTR is widely expressed in the reproductive tract (i.e., uterus, mammary gland, ovary, testis, and prostate), brain, and kidney in mammals (4), suggesting that signaling through OXTR could elicit a wide variety of effects.
OXT-deficient (Oxt-/-) mice displayed impairments in milk ejection (8, 9) and social recognition (10) but no obvious defects in parturition (8, 9). Although OXT is a strong uterotonin and is considered to drive parturition, the previous observations unexpectedly and clearly prove that OXT is not essential for labor in mice. In contrast, Oxtr mRNA expression in the uterus is up-regulated dramatically at term (11), uterine sensitivity to OXT increases just before parturition (12, 13), and OXTR antagonists delay parturition in mice (14), suggesting an indispensable role for OXTR in mouse parturition. We therefore suspected that a redundant signaling through OXTR during parturition might compensate for the absence of OXT in Oxt-/- mice. Furthermore, growing evidence suggests a role for OXTR in the modulation of social behaviors. To further study the functions of the OXT/OXTR system, we generated mice lacking OXTR (Oxtr-/-) and evaluated their reproductive functions and social behaviors. In addition, we further compared their maternal behavior and male aggressive behavior with those of ligand Oxt-/- mice.
Materials and Methods
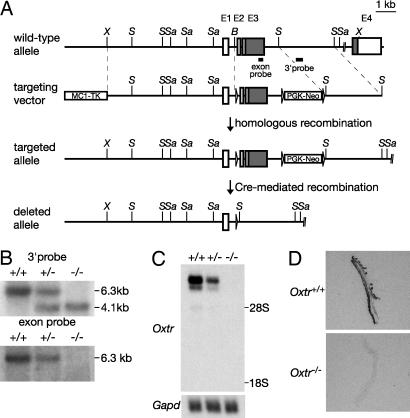
Generation of Oxtr-/- Mutant Mice and Genotyping. To construct the targeting vector, mouse 129/Sv strain-derived genomic clones (11) were used. The Oxtr gene targeting vector was designed to insert a phosphoglycerate kinase promoter-neomycin resistance cassette (PGK-Neo) into intron 3 and three loxP sites around exons 2 and 3 and the PGK-Neo (Fig. 1A). A 6.3-kb XhoI-BamHI fragment was used as the 5′ homology region, a 2.2-kb BamHI-SphI fragment containing exons 2 and 3 was inserted between two loxP sites, and a 2.8-kb SphI-SphI fragment was used as the 3′ homology region. An MC1 promoter–herpes simplex virus-thymidine kinase cassette (MC1TK) was used for negative selection. We linearized this construct with SalI and electroporated it into E14TG2a embryonic stem cells. G418 and FIAU (Moravek Biochemicals, Brea, CA) doubly resistant clones were screened by Southern blot analysis. We generated chimeric mice by microinjection of heterozygous embryonic stem cell clones into C57BL/6J blastocysts. We mated chimeric males to CAG-cre transgenic female mice (15) to recombine the loxP sites and yield mice with an Oxtr deleted allele (Fig. 1 A). Offspring from intercrosses of heterozygous (Oxtr+/-) littermates were genotyped by Southern blot analysis (Fig. 1B). The care and use of mice in this study were approved by the Institutional Animal Care and Use Committee of Tohoku University.
Fig. 1.
Generation of Oxtr-/- mice. (A) The wild-type and mutant Oxtr loci and gene targeting constructs. Exons (E) are indicated by boxes (white boxes, 5′ and 3′ UTRs; gray boxes, coding regions). Positions of restriction enzyme sites and the probes used for Southern blot analysis are shown (X, XhoI; S, SphI; Sa, SacI; B, BamHI). The loxP sites are represented by arrows (not to scale). (B) Southern blot analysis of genomic DNA from littermate progeny from Oxtr+/- crosses. SacI-digested tail DNA was hybridized with the radiolabeled probes indicated in A.(C) Northern blot analysis of poly(A)+ RNA (2 μg per lane) from the pregnant uteri (day 19 of gestation) of Oxtr+/+, Oxtr+/-, and Oxtr-/- mice. The blot was sequentially hybridized with Oxtr and Gapd cDNA probes. (D) OXTR-binding autoradiograms of uteri from pregnant Oxtr+/+ and Oxtr-/- mice (day 19 of gestation).
Maintenance of Mice. In addition to Oxtr-/- mice generated in the present study, we also used Oxt-/- mice generated previously (8) to distinguish between the role of the ligand, OXT, and that of the receptor, OXTR, in the regulation of maternal and aggressive behaviors. Oxtr-/-, Oxtr+/-, and Oxtr+/+ mice were maintained on a mixed 129 × C57BL/6J genetic background. Oxt-/- and Oxt+/+ mice used in this study were maintained on a mixed 129 × C57BL/6J genetic background, as described (8). In the maternal behavior and aggressive behavior tests, we used Oxtr-/- and Oxtr+/+ mice or Oxt-/- and Oxt+/+ mice from heterozygous intercrosses. For analysis of the potential effects of maternal OXT, we also used intercrosses of homozygous Oxt-/- and Oxt+/+ mice to generate Oxt-/- and Oxt+/+ mice from homozygous parents followed by crossfostering with C57BL/6J females.
Southern and Northern Blot Analyses. For Southern blot analysis, 3 μg of genomic DNA extracted from the tail was digested with SacI and loaded on 1% agarose gels. For Northern blot analysis, total RNA from tissues was isolated with TRIzol Reagent (Invitrogen), and poly(A)+ RNA was purified by using oligotex-dT30/super (Takara Bio, Otsu, Japan) and loaded on formaldehyde-agarose gels. These DNA and RNA samples were subjected to electrophoresis and transferred to Byodyne B nylon membranes (Pall). The membranes were hybridized to 32P-labeled probes. Probes for Southern blots were obtained by digestion with restriction enzyme, and probes for Northern blots were obtained by RT-PCR [Oxtr probe, spanning from 170 to 429 nucleotides (259 base pairs) in the mouse Oxtr mRNA (GenBank accession no. D86599) coding region; glyceraldehyde-3-phosphate dehydrogenase (Gapd) probe, spanning from 39 to 1,111 nucleotides (1,073 base pairs) of the mouse Gapd mRNA (GenBank accession no. NM_008084)], and labeled with Megaprime DNA labeling systems (Amersham Biosciences) with [32P]-dCTP. Membranes were stripped and reprobed for Gapd to ensure equal loading.
Maternal Behavior Test. Maternal behavior was tested on both postpartum (13–18 weeks old) and virgin females (7–9 weeks old). Pregnant females were individually housed a few days before parturition and tested for maternal behavior on the morning of parturition. Nesting material was provided 1 day before testing. Each new mother was observed for 20 min with minimal disturbance, and the following data points were recorded: time crouching over pups and percentage of newborns scattered. One hour after the removal of her pups, each female was exposed to three 1- to 3-day-old foster pups from a wild-type dam, which were placed in each corner of the cage distant from her nest. During the next 30 min, each female was continuously observed, and the following data points were recorded: latency to sniff and retrieve each pup and latency and duration of crouching over all three pups in the nest. Different pups were used in each test. Virgin females were individually housed for 2 days before testing. For 2 consecutive days, each female was exposed to three 1- to 3-day-old foster pups for 30 min as described above. Only the second test was scored.
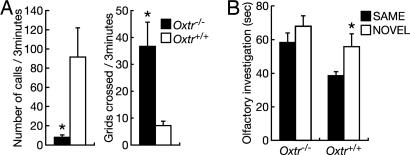
Ultrasonic Vocalization Test and Measurement of Locomotor Activity in Pups. Seven-day-old male pups from seven Oxtr+/- breeding pairs were tested between 1 and 4 h before the dark phase. The parents were removed from the home cage 20 min before testing, and the cage was placed on a heated surface at 35°C until testing was completed. Each pup was placed into a Plexiglass recording chamber (40 × 40 cm) for 3 min. Vocalizations were recorded by using an ultrasonic detector and analyzed as WAV files (16). The number of 4.5 × 4.5-cm grids crossed during the test was also noted.
Social Discrimination Test. Before testing, males (4–7 months old) were individually housed and exposed to ovariectomized females for varying periods of time (15–40 min) for 2 days to reduce mating bouts during test sessions. The social discrimination test (17) consisted of placing an ovariectomized C57BL/6J stimulus female into the home cage of the experimental male for 5 min. After a 30-min interexposure interval, the female (SAME, Fig. 4) was then placed back into the cage along with another C57BL/6J ovariectomized stimulus female (NOVEL, Fig. 4) for 5 min. The amount of time spent investigating each female's anogenital or perioral area was then scored from the video-recorded session. Interactions including sexual behavior were excluded from the analysis. Females were exposed to only one male per day to reduce male odor contamination.
Fig. 4.
Infant ultrasonic vocalization and adult social discrimination. (A) Measurements of social isolation-induced ultrasonic vocalizations (Left) and locomotor activity (Right) in Oxtr-/- (n = 8) and Oxtr+/+ (n = 10) male pups from heterozygous intercrosses. (B) Test for social discrimination test. After the first exposure to a female and an interexposure interval, this female (SAME) was placed back along with another female (NOVEL). Oxtr-/- (n = 14) and Oxtr+/+ (n = 10) males were examined for investigation times directed to the SAME or NOVEL females. *, P < 0.05 (Mann–Whitney U test). Error bars, standard error.
Resident-Intruder Aggression Test. Ten-week-old resident males were individually housed for ≈4 weeks before testing. Ten-week-old C57BL/6J mice, housed in groups, were used as intruders. Two tests of 5 min each with a 5-min interval were performed. New intruder mice were used in each test. The following data points were recorded: attack duration, frequency and latency, and latency to first attack.
Additional Details. For further details, see Supporting Text, which is published as supporting information on the PNAS web site.
Results
Generation of Oxtr-/- Mutant Mice. To define the roles of OXTR in the reproductive and central nervous systems, we generated OXTR-deficient mice by gene targeting (Fig. 1 A). The disruption of the Oxtr gene locus (Fig. 1B), the absence of Oxtr transcripts (Fig. 1C), and the absence of OXTR binding (Fig. 1D) in Oxtr-/- mice confirmed that the recombined allele is null. A 1:2:1 Mendelian distribution of the progeny from heterozygous intercrosses was observed [(Oxtr+/+:Oxtr+/-:Oxtr-/-), 65:133:69 (males); 79:133:78 (females)], indicating that OXTR is not required for embryonic development.
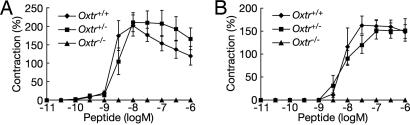
Reproductive Functions in Oxtr-/- Mice. In Oxtr-/- mice, OXT-induced contractions in pregnant myometrium were not evident (Fig. 2A). Because arginine vasopressin (AVP), another nonapeptide hormone synthesized in the paraventricular and supraoptic nuclei and secreted from the posterior pituitary, acts as a partial agonist of OXTR (18, 19) and also stimulates uterine contractions (20, 21), we also examined AVP-induced myometrium contractions in Oxtr-/- mice. The myometrium of pregnant Oxtr-/- mice did not respond to AVP (Fig. 2B). Receptor autoradiography confirmed the absence of OXTR binding in pregnant myometrium of Oxtr-/- mice (Fig. 1D). We previously reported the inability to detect AVP receptor 1A (Avpr1a) mRNA in the wild-type mouse uterus by using RT-PCR (21). Taken together, these findings prove that AVP-induced uterine contractions in pregnant wild-type mice are mediated solely by OXTR, consistent with our previous report (21).
Fig. 2.
Reproductive functions in Oxtr -/- mice. (A and B) The amplitude of OXT-(A) or AVP-(B) stimulated contractions of myometrial strips isolated from pregnant mice (day 19 of gestation) of each genotype. These investigations were performed as described (21).
To examine reproductive function, Oxtr-/- and Oxtr+/+ mice were mated in all possible combinations. Contrary to our prediction, the onset and duration of labor were normal in Oxtr-/- females (Table 1). Furthermore, Oxtr-/- mice exhibited normal rates of mating and pregnancy and litter sizes, demonstrating that OXTR is not essential for either male or female reproductive function. However, all offspring from Oxtr-/- dams died within 24 h after birth, regardless of the genotype of the offspring (Table 1). This mortality was likely due to defects in lactation, because milk was not observed in the digestive tracts of pups from Oxtr-/- dams. All offspring from Oxtr-/- mice were successfully fostered to Oxtr+/+ mice, and thus the survival defect in the pups lies entirely with the Oxtr-/- dams. Histological analysis of mammary glands in Oxtr-/- females indicated that the development of mammary tissues during gestation and milk production were normal except for the accumulation of milk in ducts of the postpartum mammary glands (data not shown). Thus, Oxtr-/- females failed to lactate, similar to Oxt-/- females (8).
Table 1. Effect of genotype on reproductive functions.
| Fertility and sexual behavior in male and female mice
|
Parturition and maternal behavior in female mice
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pairing
|
No. of mating trials
|
Mating rate, %
|
Pregnancy rate, %
|
Average litter size
|
No. of pregnant females
|
Onset of labor, day
|
Duration of labor, hour
|
Postnatal survivors per total births, %
|
|
| Male | Female | ||||||||
| Oxtr+/+ | × Oxtr+/+ | 79 | 35 | 71 | 7.5 ± 0.7 | 11 | 19.1 ± 0.1 | 3.8 ± 0.4 | 96 |
| Oxtr–/– | × Oxtr+/+ | 63 | 41 | 65 | 8.2 ± 0.9 | 10 | 19.2 ± 0.1 | 4.8 ± 0.9 | 92 |
| Oxtr+/+ | × Oxtr–/– | 43 | 42 | 67 | 8.6 ± 0.5 | 10 | 19.2 ± 0.1 | 4.8 ± 0.9 | 0 |
| Oxtr–/– | × Oxtr–/– | 82 | 33 | 59 | 8.4 ± 0.8 | 13 | 19.3 ± 0.1 | 3.2 ± 0.2 | 0 |
The profile of reproductive functions in Oxtr+/+ and Oxtr–/– mice (male, 10–25 weeks old; female, 10–15 weeks old). Each genotype was mated and females were selected without reference to ovulatory cycle. Mating rate denotes the ratio of plugged females to matings, and pregnancy rate denotes the ratio of pregnant females to plugged females. The morning when the copulation plug was observed is designated as day 0.5 of gestation. The data represent mean ± SEM.
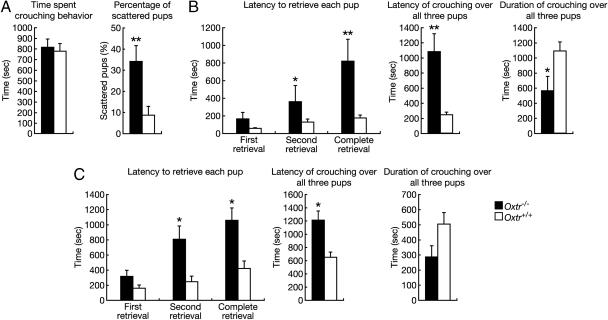
Oxtr-/- Female Mice Display Defects in Maternal Nurturing. Because OXT plays a role in maternal behavior (7), we examined maternal behavior in Oxtr-/- mice. Initially, the behavior of postpartum females was observed for 20 min in their home cages. Both Oxtr-/- and Oxtr+/+ females built nests and spent the majority of this period crouching over their pups (P > 0.05), but pups of Oxtr-/- females were often found scattered around the cage (P < 0.01) (Fig. 3A). Following this initial observation, we monitored the dams' responses to three pups placed in different corners of the cage for 30 min. Oxtr-/- dams displayed a significantly longer latency to retrieve the pups (first retrieval, P > 0.05; second retrieval, P < 0.05; and complete retrieval, P < 0.01) or to crouch over the pups (P < 0.01) and spent less time crouching over the pups (P < 0.05) than Oxtr+/+ females (Fig. 3B). The impairment of retrieval was not due to the failure of the mothers to detect the pups, because latency to approach and sniff the pups was similar to wild-type mothers (11.7 ± 5.0 s compared with 9.9 ± 4.2 s; P > 0.05). Additionally, virgin Oxtr-/- females displayed a similar phenotype (Fig. 3C), suggesting that OXTR is required for nurturing responses to pups outside the physiological context of pregnancy and parturition. In contrast, both postpartum and virgin Oxt-/- females displayed normal maternal behavior (data not shown), consistent with an earlier study (22). This could be explained by other ligands, such as AVP, activating this receptor.
Fig. 3.
Maternal nurturing in female Oxtr-/- mice. (A) Observation of newly postpartum Oxtr-/- (n = 9) and Oxtr+/+ (n = 10) females before tests for maternal behavior. (B and C) Tests for maternal behavior. Pup retrieval and crouching behaviors in Oxtr-/- (n = 9) and Oxtr+/+ (n = 10) postpartum females (B), and Oxtr-/- (n = 15) and Oxtr+/+ (n = 7) virgin females (C) from heterozygous intercrosses. Failure to retrieve or crouch was assigned as 30 min, the length of the observation period. *, P < 0.05 and **, P < 0.01 (Mann–Whitney U test). Error bars, standard error.
The decrease in maternal behavior of postpartum Oxtr-/- females could be explained as a reflection of the inability to lactate. However, our data showed that virgin Oxtr-/- females that have not experienced lactation displayed an impairment of maternal behavior (Fig. 3C), and Oxt-/- females showed normal maternal behavior despite their inability to lactate (data not shown). These results strongly suggest that any deficits in maternal behavior in these mice would be a clear indication of a specific behavioral deficit.
Decreased Ultrasonic Vocalizations and Increased Locomotor Activity in Infant Oxtr-/- Males. Next, we examined isolation-induced ultrasonic vocalizations and locomotor activity of infant Oxtr-/- males. Oxtr-/- males, like Oxt-/- males (16), emitted significantly fewer calls than did wild-type littermates (P < 0.05) (Fig. 4A), while displaying significantly higher levels of locomotor activity during the test (P < 0.05) (Fig. 4A). These results suggest that perhaps Oxtr-/- males are less distressed by social isolation and shift their behavior toward more exploratory activity than do wild-type littermates.
Social Amnesia in Oxtr-/- Mice. Because Oxt-/- mice display social amnesia (10), we examined social discrimination in adult Oxtr-/- males. Males were initially exposed to an ovariectomized C57BL/6J female. After a 30-min separation, the male was simultaneously exposed to this same female and a novel female of the same strain. As expected, Oxtr+/+ males spent significantly more time investigating the novel compared with the familiar female (P < 0.05) (Fig. 4B) and therefore were able to discriminate between the two. However, Oxtr-/- males spent a similar amount of time investigating both females (P > 0.05) (Fig. 4B), suggesting an impairment of social discrimination. There was no difference between the two genotypes in the amount of time spent investigating the initial female (P > 0.05), indicating that the Oxtr-/- males' deficit was not due to a difference in exposure time or motivation to investigate a female (data not shown). However, Oxtr-/- males were able to discriminate outbred CD-1 stimulus females (data not shown), suggesting that the deficit represents an impairment rather than a complete disruption in social recognition.
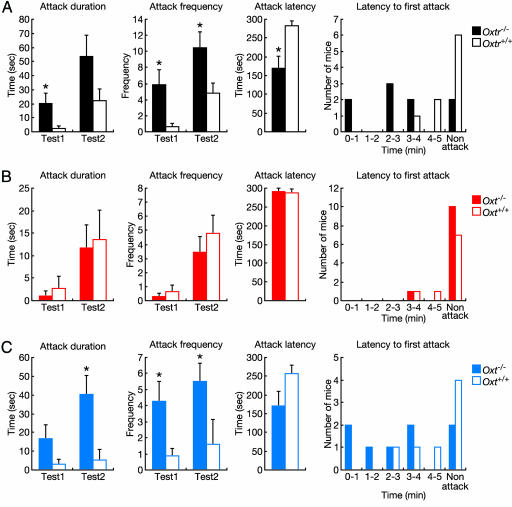
Oxtr-/- Males Display High Levels of Aggression Due to the Lack of OXTR Activation During Prenatal Development. Because we observed more wounded mice in group-housed males from cages containing Oxtr-/- mice than cages containing only Oxtr+/+ mice (Table 2), we assessed aggressive behavior using the resident-intruder test. Oxtr-/- resident males attacked the intruder with shorter latency (P < 0.05), for longer duration (test1, P < 0.05; and test2, P > 0.05), and with higher frequency (test1, P < 0.05; and test2, P < 0.05) compared with Oxtr+/+ residents (Fig. 5A). In contrast, in adjacent cages containing male Oxt-/- mice, the rate of wounded mice was similar to that in Oxt+/+ mice (Table 2). Furthermore, aggressive behavior of Oxt-/- mice in the resident-intruder test was indistinguishable from Oxt+/+ males (Fig. 5B).
Table 2. The number of wounded mice (3–9 months old) in cages, including each genotype.
| Genotypes of members in the same cage | No. of observed mice | Wounded mice | Unwounded mice | Wounded per total, % | Mice per cage |
|---|---|---|---|---|---|
| Oxtr+/+ | 37 | 1 | 36 | 2.7 | 4.1 |
| Oxtr+/+, Oxtr+/–, Oxtr–/– | 54 | 18 | 36 | 33.3 | 4.0 |
| Oxt+/+ | 21 | 0 | 21 | 0.0 | 3.5 |
| Oxt+/+, Oxt+/–, Oxt–/– | 62 | 2 | 60 | 3.2 | 4.1 |
Fig. 5.
Aggressive behavior as measured by the resident-intruder test. (A and B) Aggressive behavior in the resident-intruder test of Oxtr-/- (n = 9) and Oxtr+/+ (n = 9) mice (A) and Oxt-/- (n = 11) and Oxt+/+ (n = 9) mice (B) from heterozygous intercrosses. (C) Aggressive behavior in the resident-intruder test of Oxt-/- (n = 8) and Oxt+/+ (n = 7) mice from intercrosses of homozygous Oxt-/- and Oxt+/+ mice and fostering by C57BL/6J females. *, P < 0.05 (Mann–Whitney U test). Error bars, standard error.
To investigate this discrepancy in aggression phenotypes between Oxtr-/- and Oxt-/- mice, we examined possible compensatory effects of the Oxtr gene mutation. OXTR and AVPR1a autoradiography in the brain showed no OXTR binding in Oxtr-/- mice and the density of AVPR1a binding was similar between Oxtr+/+ (5,826 ± 602.3 dpm/mg) and Oxtr-/- (4,787 ± 463 dpm/mg) mice (Fig. 6, which is published as supporting information on the PNAS web site). Northern blot analysis failed to detect any RNA products with sequence similarity to Oxtr mRNA in the brain (Fig. 7, which is published as supporting information on the PNAS web site). In addition, Oxtr-/- mice showed no differences in Oxt and Avp mRNA expression in the hypothalamus (data not shown), pituitary OXT (Oxtr+/+, 202.6 ± 10.7 ng per pituitary, n = 9; Oxtr-/-, 222.8 ± 9.3 ng per pituitary, n = 7) and AVP (Oxtr+/+, 471.6 ± 70.9 ng per pituitary, n = 9; Oxtr-/-, 453.3 ± 28.3 ng per pituitary, n = 7), plasma OXT (Oxtr+/+, 36.3 ± 5.2 pg/ml, n = 5; Oxtr-/-, 39.3 ± 6.1 pg/ml, n = 5), or plasma testosterone levels (Oxtr+/+, 1,329.4 ± 277.7 pg/ml, n = 10; Oxtr-/-, 1,148.7 ± 225.5 pg/ml; n = 10). These results indicated that there were no apparent dysregulations of AVP, AVPR1a, or testosterone, each of which is known to influence aggression.
OXT in the dam can transfer through the placental barrier (23–25), and Oxtr mRNA is present in the mouse brain during embryonic development (Fig. 8, which is published as supporting information on the PNAS web site). Furthermore, perinatal injections of OXT in prairie voles have an impact on adult behavior (26). Therefore, we hypothesized that in utero exposure to OXT might have rescued the aggression phenotype in Oxt-/- mice derived from heterozygous matings. Therefore, we analyzed aggression in Oxt-/- mice generated from homozygous matings (Fig. 5C), creating an OXT-free developmental environment. Like Oxtr-/- males, Oxt-/- males generated from homozygous matings exhibited highly aggressive behavior, as reported (16). Our findings indicate that embryonic exposure to OXT affects the development of aggression in adulthood, although other intrauterine factors in Oxt-/- dams could also influence aggression. However, because increased aggression is seen in Oxtr-/- mice from Oxtr+/- mothers, it suggests that the defect lies in the pup.
Discussion
This study provides a comprehensive analysis of mice lacking the Oxtr gene. Although OXT has been used to induce or augment labor in humans, and the OXTR antagonist delays labor in wild-type outbred mice (14), parturition is initiated and proceeds normally in Oxtr-/- similar to Oxt-/- mice (8). Although this unexpected phenotype may be due to functional redundancy in the OXT signaling system or a compensatory effect resulting from the absence of OXTR throughout development, our results show that the OXT/OXTR signaling pathway is not essential for normal parturition in mice.
In both aggressive behavior and maternal behavior, phenotypes were different between Oxtr-/- and Oxt-/- mice. Oxtr-/- males from Oxtr+/- dams displayed elevated aggressive behavior (Fig. 5A). Oxt-/- males from Oxt-/- dams, but not from Oxt+/- dams, displayed similar high levels of aggression (Fig. 5 B and C). This indicates that in utero exposure to maternal OXT may affect adult aggressive behavior. In addition, maternal behavior in Oxtr-/- females from Oxtr+/- dams was impaired (Fig. 3), but Oxt-/- females from Oxt+/- or Oxt-/- dams showed normal maternal behavior (data not shown). These results suggest that, although prenatal activation of the OXT/OXTR system may significantly affect adult aggressive behavior in males, it is not sufficient for the establishment of maternal behavior, and that the presence of OXT is essential for controlling aggressive behavior, but not maternal behavior. Thus, these causative mechanisms seem different between aggressive behavior and maternal behavior. We speculate that the difference in phenotypes of maternal behavior between Oxtr-/- and Oxt-/- mice can be explained by a possibility that other ligands than OXT that activate OXTR can compensate for the defect of the Oxt gene.
In contrast to our results, it was reported by another group (27) that Oxt-/- males displayed reduction in the duration of aggressive behavior. This contradiction might be caused by differences in the targeting construct used, which resulted in the elimination of the majority of the Neurophysin but not the OXT coding region of the Oxt gene, or in the testing paradigm used (9, 27). In the study reporting decreased duration of aggressive attack, the tests were performed in a neutral arena, although in our study and in those of others reporting increased aggression (16, 28), a resident intruder paradigm was used. Therefore, the changes in aggression due to disruption of the OXT system may be context-dependent.
The impairment in social discrimination in Oxtr-/- mice in our study is consistent with previous results from ligand Oxt-/- mice. Oxt-/- females as well as Oxt-/- males also show significant deficits in social discrimination (10, 29). The comparison of social discrimination in Oxtr-/- mice between genders would be important for understanding the regulation of social discrimination that is related to estrogen, gonadal steroid (29).
Seminatural environment-housed Oxt-/- females from Oxt+/- dams showed high levels of aggression and infanticidal behaviors, unlike cage-housed Oxt-/- females from Oxt+/- dams (28). These results indicate that postnatal environment also affects the behavior via the OXT/OXTR system. In a seminatural environment, phenotypes of Oxtr-/- mice, such as impaired maternal behavior or elevated aggressive behavior, might be altered, and Oxt-/- males from Oxt+/- dams might be aggressive. These studies suggest an important interaction between the environment and the OXT/OXTR system in regulating social behavior.
Our observations demonstrated that the OXT/OXTR system plays an important role in regulating social behavior and might have important implications for human behavioral disruptions. Further comprehensive investigations of the functions of OXTR, including deleting the Oxtr gene in a spatiotemporal manner in the CNS, using region-specific Cre recombinases, may provide new insights into the neurobiological mechanisms resulting in psychiatric disorders associated with disruptions in social behavior, including autism.
Supplementary Material
Acknowledgments
We thank A. Smith (University of Edinburgh, Edinburgh, U.K.), Y. Katoh-Fukui (National Institute for Basic Biology, Aichi, Japan), J. Miyazaki (Osaka University, Osaka), and T. Higuchi (University of Fukui, Fukui, Japan) for their generous gifts of the embryonic stem cell line E14TG2a, CAG-cre mice, and the anti-OXT antibody; Y. Mishina (National Institute of Environmental Health Sciences, Research Triangle Park, NC) for technical advice on blastocyst injection and encouragement; S. Kato (University of Tokyo, Tokyo) for encouragement; and M. Mitsui-Saito (Tohoku University, Sendai-shi, Japan) for technical assistance with the measurement of uterine contraction. This work was supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (14360046) and by the Sankyo Foundation of Life Science. The contributions of L.J.Y., I.F.B., and H.E.R. were supported by National Institutes of Health Grant MH 56539. M.M.M. was supported in part by National Institutes of Health Grant HD42500. Y.T. was supported by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists.
Author contributions: L.J.Y. and K.N. designed research; Y.T., M.Y., I.F.B., H.E.R., M.K., T.O., and K.N. performed research; T.Y. and M.M.M. contributed new reagents/analytic tools; Y.T., T.O., T.K., L.J.Y., and K.N. analyzed data; Y.T. and K.N. wrote the paper; and T.O., T.K., M.M.M., and L.J.Y. reviewed the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OXT, oxytocin; OXTR, OXT receptor; AVP, arginine vasopressin; AVPR1a, AVP receptor 1A.
References
- 1.Du Vigneaud, V., Ressler, C. & Trippett, S. (1953) J. Biol. Chem. 205, 949-957. [PubMed] [Google Scholar]
- 2.Du Vigneaud, V., Ressler, C., Swan, J. M., Roberts, C. W., Katsoyannis, P. G. & Gordon, S. (1953) J. Am. Chem. Soc. 75, 4879-4880. [Google Scholar]
- 3.Gainer, H. & Wray, W. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 1099-1129.
- 4.Gimpl, G. & Fahrenholz, F. (2001) Physiol. Rev. 81, 629-683. [DOI] [PubMed] [Google Scholar]
- 5.Kimura, T. & Ivell, R. (1999) Results Probl. Cell Differ. 26, 135-168. [DOI] [PubMed] [Google Scholar]
- 6.Insel, T. R., O'Brien, D. J. & Leckman, J. F. (1999) Biol. Psychiatry 45, 145-157. [DOI] [PubMed] [Google Scholar]
- 7.Argiolas, A. & Gessa, G. L. (1991) Neurosci. Biobehav. Rev. 15, 217-231. [DOI] [PubMed] [Google Scholar]
- 8.Nishimori, K., Young, L. J., Guo, Q., Wang, Z., Insel, T. R. & Matzuk, M.M. (1996) Proc. Natl. Acad. Sci. USA 93, 11699-11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young, W. S., 3rd, Shepard, E., Amico, J., Hennighausen, L., Wagner, K. U., LaMarca, M., McKinney, C. & Ginns, E. I. (1996) J. Neuroendocrinol. 8, 847-853. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, J. N., Young, L. J., Hearn, E. F., Matzuk, M. M., Insel, T. R. & Winslow, J. T. (2000) Nat. Genet. 25, 284-288. [DOI] [PubMed] [Google Scholar]
- 11.Kubota, Y., Kimura, T., Hashimoto, K., Tokugawa, Y., Nobunaga, K., Azuma, C., Saji, F. & Murata, Y. (1996) Mol. Cell. Endocrinol. 124, 25-32. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki, H. & Kuriyama, H. (1975) Jpn. J. Physiol. 25, 345-356. [DOI] [PubMed] [Google Scholar]
- 13.Stepke, M. T., Schwenzer, N. & Eichhorn, W. (1994) Int. J. Oral Maxillofac. Surg. 23, 440-442. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, A. J., Leng, G. & Russell, J. A. (2002) Reproduction 123, 543-552. [DOI] [PubMed] [Google Scholar]
- 15.Sakai, K. & Miyazaki, J. (1997) Biochem. Biophys. Res. Commun. 237, 318-324. [DOI] [PubMed] [Google Scholar]
- 16.Winslow, J. T., Hearn, E. F., Ferguson, J., Young, L. J., Matzuk, M. M. & Insel, T. R. (2000) Horm. Behav. 37, 145-155. [DOI] [PubMed] [Google Scholar]
- 17.Landgraf, R., Frank, E., Aldag, J. M., Neumann, I. D., Sharer, C. A., Ren, X., Terwilliger, E. F., Niwa, M., Wigger, A. & Young, L. J. (2003) Eur. J. Neurosci. 18, 403-411. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, T., Makino, Y., Saji, F., Takemura, M., Inoue, T., Kikuchi, T., Kubota, Y., Azuma, C., Nobunaga, T., Tokugawa, Y., et al (1994) Eur. J. Endocrinol. 131, 385-390. [DOI] [PubMed] [Google Scholar]
- 19.Chini, B., Mouillac, B., Balestre, M. N., Trumpp-Kallmeyer, S., Hoflack, J., Hibert, M., Andriolo, M., Pupier, S., Jard, S. & Barberis, C. (1996) FEBS Lett. 397, 201-206. [DOI] [PubMed] [Google Scholar]
- 20.Mackler, A. M., Ducsay, C. A., Veldhuis, J. D. & Yellon, S. M. (1999) Biol. Reprod. 61, 873-878. [DOI] [PubMed] [Google Scholar]
- 21.Kawamata, M., Mitsui-Saito, M., Kimura, T., Takayanagi, Y., Yanagisawa, T. & Nishimori, K. (2003) Eur. J. Pharmacol. 472, 229-234. [DOI] [PubMed] [Google Scholar]
- 22.Young, L. J., Winslow, J. T., Wang, Z., Gingrich, B., Guo, Q., Matzuk, M.M. & Insel, T. R. (1997) Horm. Behav. 31, 221-231. [DOI] [PubMed] [Google Scholar]
- 23.Noddle, B. A. (1964) Nature 203, 414. [DOI] [PubMed] [Google Scholar]
- 24.Dawood, M. Y., Lauersen, N. H., Trivedi, D., Ylikorkala, O. & Fuchs, F. (1979) Acta Endocrinol. 91, 704-718. [DOI] [PubMed] [Google Scholar]
- 25.Malek, A., Blann, E. & Mattison, D. R. (1996) J. Matern. Fetal Med. 5, 245-255. [DOI] [PubMed] [Google Scholar]
- 26.Carter, C. S. (2003) Physiol. Behav. 79, 383-397. [DOI] [PubMed] [Google Scholar]
- 27.DeVries, A. C., Young, W. S., III, & Nelson, R. J. (1997) J. Neuroendocrinol. 9, 363-368. [DOI] [PubMed] [Google Scholar]
- 28.Ragnauth, A. K., Devidze, N., Moy, V., Finley, K., Goodwillie, A., Kow, L. M., Muglia, L. J. & Pfaff, D. W. (2005) Genes Brain Behav. 4, 229-239. [DOI] [PubMed] [Google Scholar]
- 29.Choleris, E., Gustafsson, J. A., Korach, K. S., Muglia, L. J., Pfaff, D. W. & Ogawa, S. (2003) Proc. Natl. Acad. Sci. USA 100, 6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.