Abstract
Experimental infection of mice with a virulent strain of Mycobacterium avium leads to a slowly progressive disease, which we have previously shown culminates in loss of gamma interferon (IFN-γ) production by T lymphocytes and death of the animals approximately 40 weeks after infection. Here we investigated the changes in T-cell activation, the production of interleukin-2 (IL-2), and the response to IL-2 throughout M. avium infection as a possible explanation for this loss. We found that there is a steady increase in the percentage of T cells expressing activation markers right to the end of infection. However, in vivo T-cell proliferation, measured as a percentage of CD4+ and CD8+ cells incorporating 5-bromo-2′-deoxyuridine, initially increased but then remained constant. In the final stages of infection there was a decline in proliferation of activated (CD62L−) T cells. Since IL-2 is a major driver of T-cell proliferation, we asked whether this was due to loss of IL-2 responsiveness or production. However, CD25 (IL-2Rα) continued to be highly expressed in the terminal stages of infection, and although IL-2 production declined, addition of recombinant IL-2 to cultures could not rescue the final loss of IFN-γ production.
Mycobacterial infections are known to lead to a decline in the immunological function of the host (1). In fulminating tuberculosis, this decline has been measured by determining the loss of delayed-type hypersensitivity responses to mycobacterial antigens (16, 27). Infection of mice with a virulent strain of the facultatively intracellular pathogen Mycobacterium avium results in a slowly progressive disease that is eventually fatal about 40 weeks postinfection (11, 23). This process has also been associated with a terminal decline in immune function attributed to an increase in apoptosis of T cells and a decline in production of the key protective cytokine gamma interferon (IFN-γ) (11). Although a decline in T-cell function in chronic mycobacterial infections has been described for human infections, little is known about the activation status and function of T cells in the later stages of these infections.
Many markers have been used to assess T-cell activation or memory status. We examined the early activation markers CD62L (L-selectin), CD25 (α chain of the interleukin-2 [IL-2] receptor [IL-2Rα]), and CD44 (pgp-1), whose expression has been associated by some with immunological memory (12, 26). CD62L expression is downregulated upon activation of T cells, while CD44 expression is increased. Both molecules regulate cell circulation. CD44 allows binding of cells to hyaluronidate and entry of cells into tissues, while downregulation of CD62L releases cells from continual circulation between lymph nodes.
The role of the IL-2/IL-2R pathway in the decline in immunological function seen in mycobacterial infections has been the subject of extensive speculation (24). We hypothesized that the production of or response to IL-2 might be the underlying cause of immunological unresponsiveness. As CD25 expression is clearly a prerequisite for IL-2-driven proliferation, we investigated expression of this molecule on the surface of T cells during infection.
The results of the present study revealed that continuing expression of activation markers by T cells occurs during infection but there is a final decline in proliferation of activated T cells. Interestingly, although like expression of other activation markers expression of CD25 remained high, the T cells were finally not able to produce or respond to IL-2.
MATERIALS AND METHODS
Bacteria and mice.
M. avium serovar 8, with a smooth transparent colony morphology, was isolated from an AIDS patient (23). For infection, aliquots of M. avium frozen at −70°C were thawed, sonicated to break up clumps, and used to infect female C57BL/10 mice intranasally. The infectious dose, checked retrospectively by viable counting, was always between 1.5 × 105 and 0.5 × 105 CFU/mouse. The numbers of viable bacteria in organs were determined by culturing serial 10-fold dilutions of homogenized organ tissue on Middlebrook 7H11 agar plates, and colonies were counted after 5 to 10 days of incubation at 37°C, as described previously (23). In all experiments we used groups of three to five mice that were analyzed individually. In each case the results of one representative experiment of at least two independent experiments are shown. Age- and sex-matched normal control mice for both the early (young normal mice) and advanced (old normal mice) stages of infection were included in all experiments. The results for young normal mice were plotted at zero time, whereas the results for old normal mice were plotted as if the mice were infected when they were 6 weeks old.
Fluorescence-activated cell sorter analysis.
Mice were killed with CO2 overdoses at different times after infection. The spleens were removed and disrupted by passage through a sieve, and then red blood cells were removed by lysis in an NH4Cl solution (3) or by centrifugation over Ficoll-Hypaque (Sigma, Castle Hill, New South Wales, Australia). The cells were maintained continuously in phosphate-buffered saline (PBS) with 0.01% azide to prevent modulation of markers. All antibodies were obtained from Pharmingen (San Diego, Calif.) unless indicated otherwise. A total of 1 × 106 to 2 × 106 cells were incubated with anti-CD44 (clone IM7), anti-CD62L (Mel-14) (our laboratory), or biotinylated anti-CD25 (clone 7D4). Mel-14 hybridoma (provided by W. Heath, Walter and Eliza Hall Institute, Melbourne, Australia) was used to produce culture supernatant, and the antibody was purified on a protein G column. Biotinylated anti-CD25 antibodies were detected with streptavidin Cy-Chrome (Pharmingen). Binding of anti-CD44 and anti-CD62L antibodies was detected by using fluorescein isothiocyanate-conjugated sheep anti-rat immunoglobulin G (Silenius, Melbourne, Australia) and then blocked with 1% rat serum-PBS, followed by staining with phycoerythrin-conjugated anti-CD4 (H129.19) or anti-CD8α (53-6.7). The cells were washed in PBS-0.1% bovine serum albumin-0.01% azide between staining steps. Negative control samples, which did not receive the primary antibody or were stained with a phycoerythrin-conjugated isotype control, were included for each mouse. The cells were either analyzed immediately or fixed in 1% paraformaldehyde-PBS and analyzed within 24 h. Data were acquired with a FACSort (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) and were analyzed with CellQuest software. Live cells were gated based on forward and side scatter. The number of cells was calculated by multiplying the number of spleen cells by the percentage of each population determined by flow cytometry.
In vivo proliferation with BrdU.
Mice were fed drinking water containing 1 mg of 5-bromo-2′-deoxyuridine (BrdU) (Sigma) per ml and 1% glucose, changed daily and protected from light, for 7 days prior to killing. Cells were prepared as described above, except that CD62L expression was detected by using biotinylated Mel-14 antibody and streptavidin Cy-Chrome. After surface staining for CD4 or CD8 and CD62L, the cells were stained for incorporated BrdU as described by Tough and Sprent (26). Briefly, spleen cell suspensions were dehydrated in ethanol, fixed and permeabilized in a 1% paraformaldehyde-0.01% Tween 20 solution, treated with 50 U of DNase (Sigma) per ml, and finally stained with fluorescein isothiocyanate-conjugated anti-BrdU antibody (Becton Dickinson Immunocytometry Systems). Samples that were not treated with DNase but were stained with anti-BrdU antibody were included as negative controls for each mouse. Samples from two or three mice that did not receive BrdU-containing water were stained as described above and were also used as negative controls.
ELISPOT assays.
White maxisorp plates (Nalgene Nunc International, Roskilde, Denmark) were coated with a solution containing 5 μg of anti-IL-2 (JES6-1A12) per ml or 5 μg of anti-IFN-γ (HB 170) (our laboratory) per ml in PBS overnight at 4°C. The next day the plates were blocked with Dulbecco modified Eagle medium (Life Technologies, Victoria, Australia) containing 10% fetal calf serum for at least 1 h prior to addition of cells. Twofold dilutions of spleen cells were added to triplicate wells to obtain concentrations of 2.0 × 105 to 2.5 × 104 cells/well. Irradiated (3,000 rads) spleen cells were added to act as antigen-presenting cells, which brought the final concentration of cells to 2.0 × 105 cells/well. Live M. avium, at a final concentration of 5 × 106 CFU/ml, was used as the recall antigen. After 24 and 72 h of incubation for IL-2 and IFN-γ, respectively, the plates were thoroughly washed with PBS-Tween 20 and distilled H2O. Bound cytokine was detected with biotinylated anti-IL-2 (JES6-5H4) or anti-IFN-γ (XMG1.2). After 1 h of incubation at room temperature, the plates were washed three times in PBS-Tween 20, and then 50 μl of a streptavidin alkaline phosphatase preparation (0.5 U/ml; Boehringer Mannheim GmbH, Mannheim, Germany) was added. After 30 min of incubation and three more washes, 50 μl of BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (Sigma) substrate was added, and the plates were incubated for 10 to 20 min at room temperature. Spots were counted by using a magnification of ×10. The results were expressed as the mean ± standard deviation number of cytokine-producing cells per 106 spleen cells based on the data for three individual mice per group.
Addition of IL-2 to IFN-γ recall cultures.
Spleen cells were prepared over Ficoll-Hypaque as described above. The cells were then diluted to obtain a concentration of 2 × 106 cells/ml and cultured in 2.0 ml of Dulbecco modified Eagle medium containing fetal calf serum (Life Technologies) with or without live M. avium cells at a final concentration of 5 × 106 CFU/ml and with (100 or 1 U/ml) or without exogenous IL-2. After 72 h, supernatants were collected and frozen at −20°C until the IFN-γ level was measured by determining inhibition of the growth of IFN-γ-sensitive WEHI 279 cells as described previously (22, 23). The specificity of the assay was confirmed for each positive sample by blocking with an IFN-γ-specific monoclonal antibody (HB170).
Statistical analysis.
Student’s t test was used to determine statistical significance. P values of <0.05 were considered to be significant.
RESULTS
Bacterial burden and decline of T cells during M. avium infection.
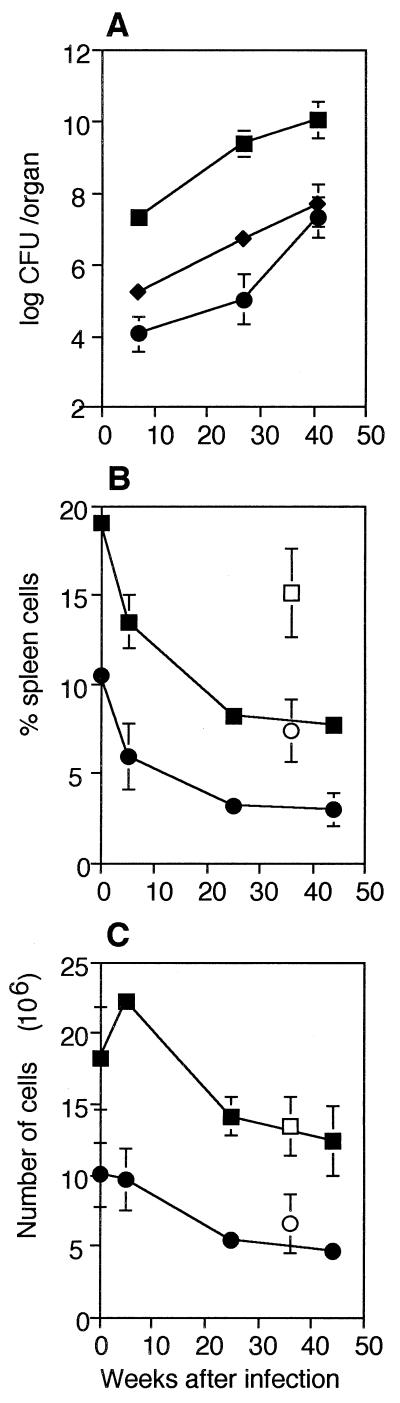
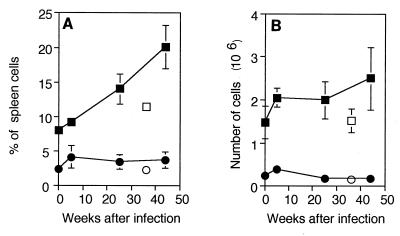
Six weeks after intranasal infection with 1 × 105 CFU of M. avium, there were 3.2 × 107 bacteria in the lungs and 1.9 × 104 bacteria in the liver of each mouse (Fig. 1A). The bacterial load increased steadily up to 30 weeks after infection, to 2.6 × 109 bacteria in the lungs and 5.6 × 106 bacteria in the liver of each infected mouse. As reported previously (10, 11), the percentages of CD4+ and CD8+ spleen cells in the spleens of infected mice declined after infection (Fig. 1B). In uninfected young mice approximately 20% of the spleen cells expressed CD4, while approximately 10% expressed CD8. Compared to these controls, by 6 weeks after infection there were significantly lower proportions of CD4+ (13.5%; P < 0.01) and CD8+ (6%; P < 0.02) spleen cells. Throughout infection the percentages of CD4+ and CD8+ cells continued to decline, although at a lower rate, to 8 and 4%, respectively, values which were significantly lower than the values obtained for the age-matched uninfected controls (P < 0.01 and P < 0.02, respectively).
FIG. 1.
Bacterial burden and CD4+ and CD8+ T-cell decline. (A) Numbers of bacteria in the lungs (▪), spleens (⧫), and livers (•) of mice at different times after intranasal infection with 1 × 105 CFU of M. avium per mouse. The means and standard deviations based on the data for five mice per group are shown. (B and C) Changes in the percentages (B) and numbers (C) of CD4+ and CD8+ T cells in the spleens of mice at different times after infection, assayed on the same day. The values are means and standard deviations based on the data for three mice per group. The results of one representative experiment of 10 similar experiments are shown. Symbols: ▪, CD4+, infected; □, CD4+, old uninfected; •, CD8+, infected; ○, CD8+, old uninfected.
When the values described above were converted to absolute numbers of T cells per spleen, it was apparent that the reductions in the percentages of CD4+ and CD8+ cells were the result of splenomegaly which accompanied infection. The number of CD4+ cells increased slightly 6 weeks after infection, from 18 × 106 to 22 × 106 cells/spleen (Fig. 1C). Later, the number of these cells declined until there were 12 × 106 CD4+ cells/spleen 45 weeks after infection. This value was not significantly different from the value obtained for the old normal control mice. Young normal mice had 10 × 106 CD8+ cells/spleen, and mice that had been infected for 6 weeks had very similar numbers of CD8+ cells (9.7 × 106 CD8+ cells/spleen). Again, the number of cells declined steadily until at 45 weeks after infection there were 4.6 × 106 CD8+ cells/spleen, a value which was not significantly different from the value obtained for the old normal control mice. Thus, after an early increase, the number of CD4+ cells returned to normal, while the number of CD8+ T cells was largely unaffected by infection.
Expression of activation markers on T cells during infection.
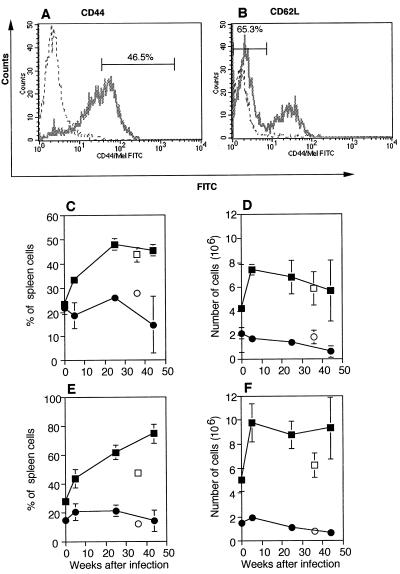
Flow cytometry was used to analyze expression of activation markers CD44 and CD62L on the surfaces of CD4+ and CD8+ cells during M. avium infection. Figures 2A and B show representative histograms of CD44 and CD62L staining gated on CD4+ T cells from mice infected 25 weeks earlier. There was a steady increase in the percentage of CD4+ cells displaying an activated phenotype (Fig. 2C and E). Six weeks after infection, the percentage of CD4+ T cells that were activated, CD44bright (Fig. 2A and C), or CD62L− (Fig. 2B and E) had increased compared to the percentage observed for age-matched normal control mice. In contrast, there was little change in the percentage of CD8+ T cells expressing the activation markers analyzed. At 43 weeks postinfection 45% of CD4+ T cells were CD44bright (Fig. 2C), a value which was not significantly greater than the value obtained for old normal control mice. At the same time there was a significant (P < 0.01) increase in the percentage of CD4+ CD62L− T cells (Fig. 2E) compared to the value obtained for old normal control mice. The changes in activation marker expression by CD8+ cells were not significantly different from the changes observed for the old normal control mice 43 weeks after infection.
FIG. 2.
Changes in expression of activation markers after M. avium infection. (A and B) Representative staining of CD4+ T cells with CD44 (A) or CD62L (B) after 25 weeks of infection. The dotted lines show the results obtained for the negative controls. The bars show what was considered to be CD44bright or CD62L− and the percentage of CD4+ T cells that were CD44bright or CD62L− in each case. FITC, fluorescein isothiocyanate. (C to F) Percentages (C and E) and numbers (D and F) of CD4+ (▪) or CD8+ (•) cells that were CD44bright (C and D) or negative for CD62L (E and F). The number of cells was calculated by multiplying the percentage of activated T cells by the number of spleen cells recovered. All points represent means based on the data for three mice, and the error bars indicate standard deviations. Data for young normal control mice, age matched for 6 weeks after infection, are plotted at zero time. Data for the old normal control mice (CD4+ [□] and CD8+[○]) are plotted as if the mice were infected when they were 6 weeks old, the age when all mice were infected. The results of one representative experiment of three independent experiments are shown.
When the numbers of activated CD4+ and CD8+ cells were calculated, a similar pattern emerged. The numbers of CD44bright (Fig. 2D) and CD62L− (Fig. 2F) CD4+ cells increased sharply after 6 weeks and remained high until 43 weeks after infection. There were significant increases in the numbers of CD4+ CD44bright (P < 0.01) and CD4+ CD62L− (P < 0.02) T cells 6 weeks after infection compared to the values obtained for age-matched young normal control mice. Due to the decline in the percentage of CD4+ and CD8+ cells in the spleens, there were not significant differences in the numbers of activated (CD44bright or CD62L−) CD4+ T cells compared to the values obtained for the old normal control mice 43 weeks after infection. The number of CD8+ T cells that were CD44bright (Fig. 2D) or CD62L− (Fig. 2F) changed only slightly. At 6 weeks after infection there were not significant differences in the numbers of activated CD8+ T cells obtained from infected and age-matched control mice, and for the most part this was true after 43 weeks. Hence, the number of activated CD4+ T cells in the terminal stages of infection was high, although the number of activated CD8+ T cells changed very little during infection.
In vivo proliferation of T cells during M. avium infection.
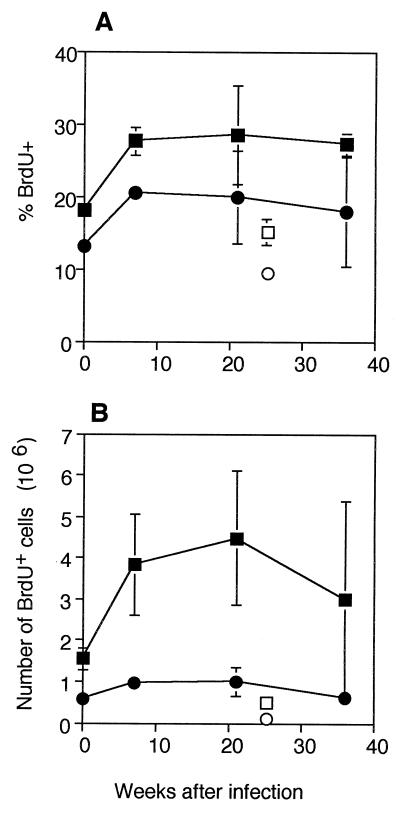
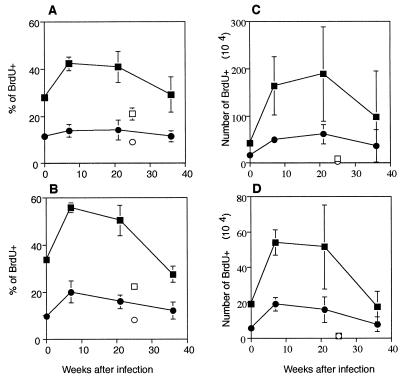
We next examined whether the increase in the number of T cells expressing the activated phenotype CD44bright CD62L− described above was followed by an increase in proliferation in vivo. BrdU incorporation over a 1-week period was used to assay in vivo proliferation of CD4+ and CD8+ T cells in M. avium-infected and uninfected mice. A significant increase in the percentage of proliferating CD4+ T cells was found when mice that were infected for 7 weeks were compared to uninfected control mice (Fig. 3A). In uninfected mice 18% of the CD4+ T cells had proliferated during the previous week, whereas 7 weeks after infection 28% of CD4+ T cells had proliferated (P < 0.002). A similar pattern was observed in the CD8+ T-cell population, although the percentage of BrdU+ cells was lower. In the normal mice, 13% of CD8+ T cells had proliferated, but by 7 weeks after infection the percentage had increased significantly (P < 0.001), to 21%. CD4+ and CD8+ T cells from mice that had been infected for more than 7 weeks did not show any further increase in proliferation. When the number of proliferating cells was calculated (Fig. 3B), there was a slight, although not significant, decline in proliferation in the final weeks of infection compared with the peak value. In all, there was a surprisingly constant number of proliferating T cells throughout the period of infection.
FIG. 3.
In vivo proliferation after infection: percentages (A) and numbers (B) of CD4+ or CD8+ BrdU+ T cells. Mice were fed BrdU in their drinking water for 7 days, and this was followed by staining of spleen cells for CD4 or CD8 and BrdU. The mice had been infected for different times, but their cells were assayed on the same day. The values are means based on the data obtained for three mice at different times after infection. The error bars indicate standard deviations. The results of one representative experiment of four similar experiments are shown. Symbols: ▪, CD4+, infected; □, CD4+, old uninfected; •, CD8+, infected; ○, CD8+, old uninfected.
Proliferation in activated CD4+ and CD8+ T-cell subpopulations.
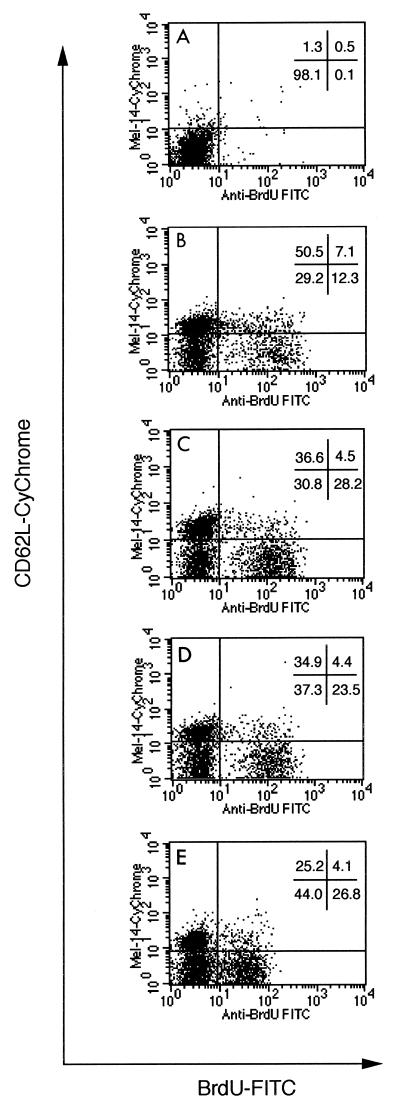
The increase in the percentage of cells expressing activation markers (Fig. 2), accompanied by the stagnant rate of proliferation (Fig. 3), suggested that as the infection progressed there was an accumulation of activated T cells that could not proliferate. Three-color staining for CD4 or CD8, BrdU, and CD62L was used to measure directly the numbers of activated cells which had proliferated. An example of the staining for CD62L and BrdU is shown in Fig. 4. The intensity of the staining for BrdU decreased 36 weeks after infection, although the BrdU+ population was still clearly detectable.
FIG. 4.
Scatter plots of BrdU and CD62L staining. Spleen cells were stained with monoclonal antibodies specific for CD4, CD62L, and BrdU. CD4+ cells were gated, and the results of staining for CD62L (Mel-14) (y axis) and BrdU (x axis) are shown. The percentage of cells falling in each quadrant is shown for each time point after infection. Similar results were obtained when CD8+ T cells were stained and gated in the same manner. (A) Negative control; (B) uninfected; (C) 7 weeks after infection; (D) 21 weeks after infection; (E) 36 weeks after infection. FITC, fluorescein isothiocyanate.
When proliferation of activated (CD62L−) and unactivated (CD62L+) CD4+ T cells was analyzed, it was clear that proliferation occurred preferentially in the activated, CD62L− T cells (Fig. 5). There was a significant (P < 0.002) increase in the percentage of CD4+ CD62L− T cells that were proliferating (BrdU+) after 7 weeks (42%) compared to the value obtained for the young normal control mice (28%) (Fig. 5A). At 21 weeks after infection 41% of the CD4+ CD62L− cells had proliferated, but by 36 weeks after infection only 29% of the CD4+ CD62L− cells had proliferated during the 7-day pulse period. This value was higher than the value obtained for the old normal control mice, but the difference was not significant. In contrast, the proportion of unactivated CD4+ CD62L+ cells that proliferated did not change significantly and was approximately 10% at all times after infection. Similar results were obtained when the CD8+ CD62L− cells were examined (Fig. 5B). In young normal control mice 34% of the CD8+ CD62L− cells were BrdU+. The proportion increased significantly (P < 0.001) to 56% after 7 weeks of infection and then dropped slightly to 50% after 21 weeks. After 36 weeks, 28% of the CD8+ CD62L− cells were proliferating in vivo, which represented a significant (P < 0.01) decline. The CD8+ CD62L+ T cells exhibited a small but significant (P < 0.05) increase in proliferation after 7 weeks of infection, and then the values returned to normal levels.
FIG. 5.
In vivo proliferation of activated T cells after M. avium infection. Mice were fed BrdU in their drinking water for 7 days. Spleen cells were stained for CD4 or CD8, CD62L, and BrdU. Percentages (A) and numbers (C) of BrdU+ cells gated on CD4+ CD62L− or CD4+ CD62L+ T cells and percentages (B) and numbers (D) of BrdU+ gated on CD8+, CD62L−, or CD62L+ T cells are shown. The values are means and standard deviations based on the data for three mice per group. The mice had been infected for different times, but their cells were assayed on the same day. The results of one representative experiment of two similar experiments are shown. Symbols: ▪, infected, CD62L−; □, old uninfected, CD62L−; •, infected, CD62L+; ○, old uninfected, CD62L+.
The numbers of activated T cells that proliferated in vivo exhibited a very similar pattern. There were more activated, proliferating CD4+ cells than equivalent CD8+ cells, due to the higher percentage of CD4+ CD62L− cells. In both the CD4+ populations (Fig. 5C) and the CD8+ populations (Fig. 5D) there were significant (P < 0.05 and P < 0.02, respectively) increases in the numbers of activated (CD62L−) and proliferating (BrdU+) cells after 7 weeks of infection. At later times the numbers of activated and proliferating cells remained relatively constant before the values declined 36 weeks after infection. The number of proliferating and activated CD4+ T cells was not significantly different from the number obtained for old normal control mice after 36 weeks of infection. The number of unactivated (CD62L+) CD4+ and CD8+ T cells that proliferated increased slightly, but significantly (P < 0.01), after 7 weeks of infection. As expected, proliferation occurred preferentially among activated CD62L− CD4+ and CD8+ T cells in the early stages of infection. However, in the later stages of infection fewer of the activated T cells were able to proliferate in vivo.
Role of IL-2 in declining T-cell response.
IL-2 is a major driver of T-cell proliferation, and it has been suggested that an inability to respond to or produce IL-2 accounts for nonresponsiveness in tuberculosis (24). Therefore, expression of CD25 on T cells was assessed as a possible reason for failure of proliferation. However, as shown in Fig. 6, expression of CD25 on CD4+ T cells continued to increase with time after infection, as did expression of the other activation markers (Fig. 2). In the final stages of infection, CD25 could be detected on approximately 20% of CD4+ T cells. It is therefore unlikely that failure of CD25 accounted for the decline in proliferation of CD4+ T cells. Expression on CD8+ T cells was much lower at all times, which is consistent with the lower rate of proliferation of such cells. The numbers of CD4+ CD25+ and CD8+ CD25+ T cells increased, although not significantly, soon after infection and remained stable throughout the study. Hence, the increase in the percentage of CD25+ T cells was compensated for by the decrease in the numbers of CD4+ and CD8+ T cells.
FIG. 6.
Changes in expression of CD25 after M. avium infection. Expression of CD25 was determined by flow cytometry. The mean percentages (A) and numbers (B) of CD4+ CD25+ (▪) and CD8+ CD25+ (bull;) T cells in three mice are shown. Data for young normal control mice, age matched for 6 weeks after infection, were plotted at zero time. Data for old normal control mice (CD4+[□] and CD8+[○]) were plotted as if the mice were infected when they were 6 weeks old, the age when all mice were infected. The results of one representative experiment of three similar experiments are shown.
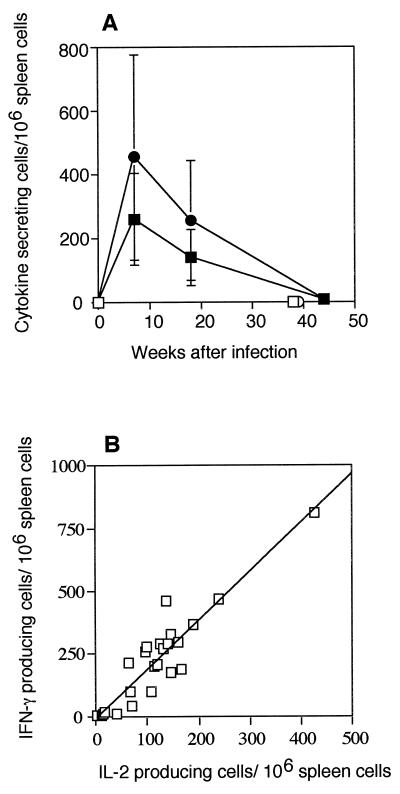
We have previously shown that IFN-γ production declines late in M. avium infection (11). Therefore, we speculated that the decline in the in vivo proliferation of activated T cells during infection could be due to a similar decline in IL-2 production. IL-2 was not detectable in the supernatants of spleen cells from M. avium-infected mice stimulated with live M. avium (data not shown), so an ELISPOT assay was used to determine the number of cells that secreted IFN-γ and IL-2 in response to M. avium. The results of this analysis (Fig. 7A) show that there were sharp increases in the numbers of IFN-γ- and IL-2-producing cells 7 weeks after infection, and then the numbers of cytokine-producing cells declined in parallel until there were fewer than 10 IFN-γ- or IL-2-producing cells per 106 spleen cells 44 weeks after infection. Pooled results from three independent experiments showed that there was a strong (r2 = 0.85) correlation between the numbers of IFN-γ- and IL-2-producing cells from individual mice (Fig. 7B). The deficit in IFN-γ production persisted once the decline in T cells had been taken into account (data not shown) and was far greater than the loss of CD4+ T cells.
FIG. 7.
Changes in the numbers of cytokine-producing cells during M. avium infection. (A) ELISPOT assays were used to determine the numbers of IL-2- and IFN-γ-producing cells after stimulation in vitro with M. avium. Cells cultured without M. avium had ≤5 cytokine-producing cells/106 spleen cells (data not shown). The values are means and standard deviations based on the data for groups of three mice. The results of one representative experiment of three independent experiments are shown. Symbols: ▪, IL-2-producing cells; □, IL-2 normal cells; •, IFN-γ-producing cells; ○, IFN-γ normal cells. (B) Scatter plot of the number of IFN-γ- and IL-2-producing cells from the same mouse at different times after M. avium infection. Data pooled from three independent experiments are shown.
Since the decline in IL-2 production occurred in parallel with the decline in IFN-γ production, the ability of exogenous recombinant IL-2 to rescue the loss of IFN-γ production late in infection was tested. The CD4+ T cells in spleens were enumerated by fluorescence-activated cell sorting before culture. IL-2 was added to cultures of spleen cells obtained from mice at different times after infection. Spleen cells were cultured with M. avium as the antigen in the presence of 100 or 1 U of recombinant human IL-2 per ml or no recombinant human IL-2. The results (Table 1) were expressed as units of IFN-γ produced per 1 × 105 CD4+ T cells present in the spleen cell cultures to correct for changes in the number of CD4+ T cells in the spleens during infection. Mice infected with M. avium for 8 weeks exhibited an IFN-γ response even without added IL-2, while mice infected for 39 weeks or uninfected mice exhibited very little IFN-γ response, even when the low numbers of CD4+ cells were corrected for. Addition of IL-2 (either 1 or 100 U/ml) substantially increased the IFN-γ response of mice infected for 8 weeks and even the response of uninfected mice, provided that M. avium was present in the cultures. In contrast, cells from mice that were infected for 39 weeks showed only a minor increase in the IFN-γ response even in the presence of 100 U of IL-2/ml. Significantly (P < 0.02) less IFN-γ was produced per CD4+ T cell from mice that had been infected for 39 weeks than was produced per CD4+ cell from age-matched uninfected control mice or mice that had been infected for 8 weeks, when preparations were supplemented with equivalent amounts of IL-2. The small amounts of IFN-γ produced by mice that were infected for 39 weeks were presumed to be due to the carryover of bacteria from the heavily infected animals. The inability of cells from mice infected 39 weeks earlier to produce IFN-γ was emphasized by the high levels of IFN-γ produced by normal spleen cells in response to the combination of IL-2 and M. avium. These cells did not produce detectable IFN-γ in response to IL-2 or M. avium alone. NK cells were a likely source of the high levels of IFN-γ in these cultures. Hence, the decline in IFN-γ production was due neither to a decline in the number of CD4+ T cells nor to the deficit in the supply of IL-2, since exogenous IL-2 was not able to restore the IFN-γ response in cells from mice that had been infected for a long time.
TABLE 1.
Effect of IL-2 supplementation on IFN-γ production in response to M. avium in vitro
| IL-2 added (IU/ml) | IFN-γ production (IFN-γ units/105 CD4+ T cells)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mice infected for 8 weeks
|
Mice infected for 39 weeks
|
Young normal mice
|
Old normal mice
|
|||||
| With M. avium | Without M. avium | With M. avium | Without M. avium | With M. avium | Without M. avium | With M. avium | Without M. aviumb | |
| 0 | 160 ± 108 | <1 | 11 ± 14c | <1 | <1 | <1 | <1 | <1 |
| 1 | 632 ± 218 | <1 | 36 ± 47d | 10 ± 9 | 192 ± 169 | <1 | 220 ± 105 | <1 |
| 100 | 1,406 ± 694 | <1 | 73 ± 109c | 45 ± 21 | 468 ± 317 | <1 | 1,397 ± 802 | <1 |
The values are means ± standard deviations based on the data for individual cultures from groups of five mice.
The P value was <0.01 for all comparisons with old uninfected mice.
Significantly different (P < 0.02) from the value obtained for mice infected for 8 weeks.
Significantly different (P < 0.001) from the value obtained for mice infected for 8 weeks.
DISCUSSION
We investigated the changes in three facets of T-cell activation: activation marker expression, in vivo proliferation, and cytokine production during chronic M. avium infection. Our results show that progressive M. avium infection leads to an increase in the percentage of T cells that express the activated phenotype markers CD44bright, CD62L− and CD25+. Infection resulted in an initial increase in T-cell proliferation in vivo, but little further change was seen as the infection progressed. More detailed analysis of CD62L expression and in vivo proliferation revealed that activated (CD62L−) T cells proliferated less in mice that were infected for more than 30 weeks than in mice that were infected for less time. As expected, there was greater activation and proliferation in CD4+ cells than in CD8+ cells, in keeping with the relative roles of these cells in infection (23). The number of cells producing IL-2 declined in parallel with the loss of IFN-γ production, but replacement of IL-2 in cultures of spleen cells obtained from mice late in infection did not restore the ability of the cells to produce IFN-γ.
The earliest change in the immunological status of M. avium-infected mice was a decline in the percentage of CD4+ and CD8+ T cells in the spleens. This decline was consistently observed ∼6 weeks after infection. When the number of cells was calculated, there was no decrease in the number of cells until more than 20 weeks after infection. This implies that the decline in the percentage of CD4+ and CD8+ T cells was due to dilution of these cells by other cell populations rather than to specific deletion of CD4+ or CD8+ T cells. Recent studies in our laboratory have shown that there is a concomitant increase in the percentage of B220+ B cells in the spleens of M. avium-infected mice, but no changes in the percentages of NK1.1+ NK or γδ TCR+ cells were seen at any time after infection. NK1.1+ cells were present, but their abundance did not change during infection. Low percentages (<1%) of γδ T cells were detected, but again the values did not change during infection (B. Gilbertson and C. Cheers, unpublished data). Thus, CD4+ and CD8+ T cells are diluted by B cells, not by NK, NKT, or γδ T cells.
In one of the few studies that have investigated T-cell activation following primary mycobacterial infection, the intensity of expression of CD44 on splenic T cells was reported to increase during the 3 weeks following experimental infection of mice with Mycobacterium tuberculosis (14). This CD44bright population was further subdivided into CD45Rbhi, CD45Rblo, and CD45Rbneg subsets, which increased in sequence following infection. Recently, a significant increase in the number of CD4+ and CD8+ pulmonary cells with an activated phenotype 8 weeks after infection of mice with M. tuberculosis was reported (9). To our knowledge, this is the first study of changes in T-cell activation for longer periods of time after infection with M. avium.
In our model of M. avium infection, mice were infected with a relatively low dose (1 × 105 CFU/mouse) by the more physiologically relevant intranasal route, and the course of infection was monitored until the mice reached a terminal decline, about 40 weeks after infection. This allowed observation of changes in T-cell function and phenotype during the complete course of infection, relatively free from potential artifacts caused by intravenous delivery of very large numbers (∼108) of viable bacteria.
We examined the activation markers CD62L (L-selectin) and CD25 (IL-2Rα chain), as well as CD44 (pgp-1), whose expression has been associated with immunological memory. While the association of these molecules with immunological activation and memory is controversial (12, 26), it does seem that CD62L is downregulated upon activation of T cells, while the intensity of CD44 expression is increased. The numbers of cells expressing these characteristics increased during infection, remaining high even in the terminal stages. Interestingly, expression of CD44 was not significantly different from expression in normal age-matched control mice, but there were more cells expressing low levels of CD62L in infected mice than in uninfected mice.
When in vivo proliferation of activated (CD62L−) T cells was examined, it was found that the capacity of these cells to proliferate in vivo increased 6 to 8 weeks after infection and then remained stable until ∼35 weeks after infection, when it declined sharply. Thus, as the infection progressed, there was an accumulation of activated T cells which were not able to proliferate, a situation which is known to be a prelude to apoptosis (18). One trigger for this outcome may be the loss of signaling by IL-2, either because of failure of production or downregulation of CD25 (18).
The IL-2 receptor is made up of three components, the high-affinity IL-2-binding α chain (CD25), the β chain, and the common γ chain. The role of the IL-2/IL-2R complex signaling pathway in the decline in immunological function seen in mycobacterial infections has been the subject of investigations for a long time. In tuberculosis patients, the decline in purified protein derivative-specific proliferation in vitro was associated with a decline in CD25 (25), which made the patients unable to respond to IL-2. This was clearly not the case in this study, in which CD25 expression increased towards the terminal stages of infection. However, it may be that one of the other components of the IL-2R complex was lost. A deficit in the supply of IL-2 has also been proposed as a mechanism for the decline in immunological function seen in experimental Mycobacterium bovis BCG infections (21, 25). Orme et al. (21) proposed that IL-2 was rapidly consumed by the large number of activated T cells that emerged after infection of mice with large doses of BCG. They showed that exogenous IL-2, added in vitro in the form of IL-2-containing supernatants, was able to reverse the decline in proliferation of murine T cells in response to concanavalin A and purified protein derivative. Furthermore, in vivo administration of IL-2-containing supernatants restored the delayed-type hypersensitivity response after BCG infection (7).
The number of cells producing IL-2 certainly declined in our model, but it declined in parallel with IFN-γ production by cultured T cells. Addition of IL-2 to cultures increased IFN-γ production by cells from mice infected for 8 weeks and, interestingly, by cells from young or old normal mice. This occurred only in the presence of M. avium as an antigen and is reminiscent of the conditions required for activation of NK cells to produce IFN-γ (2). It was striking that addition of IL-2 to cells from mice that had been infected for 39 weeks was not able to restore the ability of the cells to produce IFN-γ despite the fact they were still expressing CD25. Activated macrophages or other cell populations do not inhibit production of IFN-γ in vivo, as previous work in our lab showed that purified T cells supplemented with antigen-presenting cells from uninfected mice were still not able to produce IFN-γ (Gilbertson and Cheers, unpublished data).
It is worth noting that one difference between this study and some other studies which have looked at the role of IL-2 in the poor immune response to mycobacteria is that in the other studies the researchers examined the response much earlier in infection, when the IFN-γ deficiency was only relative. At 8 weeks after infection, we also found that addition of IL-2 increased IFN-γ production. At this early stage it is desirable to exert feedback control on the immune response, lest the inflammatory activity do excessive damage to the tissues. In contrast, we found that late in infection, when there is an almost total loss of responsiveness, addition of IL-2 had no effect. It is not known whether an in vivo supply of excess IL-2 during infection at the times when other researchers found it to be a useful supplement would have delayed the onset of the final decline in the immune response that we observed (19).
What causes the decline in T-cell function in advanced M. avium infection? The mycobacterium-specific T cells may be depleted or exhausted. T-cell exhaustion has been shown to play a role in the decline in T-cell function in chronic viral infections (28). Our results are similar to the results obtained for a T-cell population that emerged after infection with a mixture of retroviruses referred to as BM5 (20). In this model a population of activated (CD44bright CD62L−) CD4+ T cells emerged. This was accompanied by a loss of production of several cytokines, including IL-2 and IFN-γ. Overproduction of cytokines or toxic mediators may also inhibit the function of T cells in advanced infections. Many mediators have been suggested to play a role in the functional impairment of T cells; these mediators include tumor necrosis factor (17), NO (6, 13), transforming growth facter β (5, 15), and IFN-γ (8). Products secreted from the bacteria themselves may also play a role (4). The high concentration of antigen may also have an impact on T-cell function. At this time the mechanisms of T-cell functional decline are not known. Defining the functional changes associated with advanced M. avium infection should allow investigation of the mechanisms of the decline in T-cell function in advanced mycobacterial infections.
Acknowledgments
This work was supported by grant 980639 from the Australian National Health and Medical Research Council.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Appelberg, R. 1996. Immune response to atypical mycobacteria. Res. Immunol. 147: 560–564. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft, G. J. 1993. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 5: 503–510. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, W. 1968. An extension of the 51Cr release assay for the estimation of mouse cytotoxins. Transplantation 6: 761–764. [DOI] [PubMed] [Google Scholar]
- 4.Brozna, J. P., M. Horan, J. M. Rademacher, K. M. Pabst, and M. J. Pabst. 1991. Monocyte responses to sulfatide from Mycobacterium tuberculosis: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha, and altered protein phosphorylation. Infect. Immun. 59: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champsi, J., L. S. Young, and L. E. Bermudez. 1995. Production of TNF-alpha, IL-6 and TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology 84: 549–554. [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colizzi, V. 1984. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect. Immun. 45: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, C. G., A. G. D. Bean, H. Hooi, H. Briscoe, and W. J. Britton. 1999. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 67: 3242–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florido, M., A. S. Goncalves, R. A. Silva, S. Ehlers, A. M. Cooper, and R. Appelberg. 1999. Resistance of virulent Mycobacterium avium to gamma interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect. Immun. 67: 3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbertson, B., J. Zhong, and C. Cheers. 1999. Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with Mycobacterium avium. J. Immunol. 163: 2073–2080. [PubMed] [Google Scholar]
- 12.Goldrath, A. W., L. Y. Bogatzki, and M. J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes, M. S., M. Florido, T. F. Pais, and R. Appelberg. 1999. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J. Immunol. 162: 6734–6739. [PubMed] [Google Scholar]
- 14.Griffin, J. P., and I. M. Orme. 1994. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect. Immun. 62: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, C. S., J. J. Ellner, R. Blinkhorn, and Z. Toossi. 1997. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc. Natl. Acad. Sci. USA 94: 3926–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden, M., M. R. Dubin, and P. H. Diamond. 1971. Frequency of negative intermediate-strength tuberculin sensitivity in patients with active tuberculosis. N. Engl. J. Med. 285: 1506–1509. [DOI] [PubMed] [Google Scholar]
- 17.Kremer, L., J. Estaquier, I. Wolowczuk, F. Biet, J.-C. Ameisen, and C. Locht. 2000. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect. Immun. 68: 4264–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17: 221–253. [DOI] [PubMed] [Google Scholar]
- 19.Maeurer, M. J., P. Trinder, G. Hommel, W. Walter, K. Freitag, D. Atkins, and S. Storkel. 2000. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 68: 2962–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muralidhar, G., S. Koch, M. Haas, and S. L. Swain. 1992. CD4 T cells in murine acquired immunodeficiency syndrome: polyclonal progression to anergy. J. Exp. Med. 175: 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme, I. M., M. J. H. Ratcliffe, and F. M. Collins. 1984. Acquired immunity to heavy infection with Mycobacterium bovis bacillus Calmette Guerin and its relationship to the development of non-specific unresponsiveness in vitro. Cell. Immunol. 88: 285–296. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds, D. S., W. H. Boom, and A. K. Abbas. 1987. Inhibition of B lymphocyte activation by interferon gamma. J. Immunol. 139: 767–773. [PubMed] [Google Scholar]
- 23.Saunders, B. M., and C. Cheers. 1995. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T-cell subsets and gamma interferon. Infect. Immun. 63: 2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toossi, Z., and J. J. Ellner. 1996. Mechanisms of anergy in tuberculosis. Curr. Top. Microbiol. Immunol. 215: 221–238. [DOI] [PubMed] [Google Scholar]
- 25.Toossi, Z., M. E. Kleinhenz, and J. J. Ellner. 1986. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J. Exp. Med. 163: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tough, D. F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanham, G., Z. Toossi, C. S. Hirsch, R. S. Wallis, S. K. Schwander, E. A. Rich, and J. J. Ellner. 1997. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tuber. Lung Dis. 78: 145–158. [DOI] [PubMed] [Google Scholar]
- 28.Zinkernagel, R. M., D. Moskophidis, T. Kundig, S. Oehen, H. Pircher, and H. Hengartner. 1993. Effector T-cell induction and the T cell memory versus peripheral deletion of T cells. Immunol. Rev. 133: 199–223. [DOI] [PubMed] [Google Scholar]