Abstract
TASK2 is a member of the two-pore domain K+ channel family that plays a role in acid–base homeostasis; TASK2 knockout animals have plasma electrolyte patterns typical of the human clinical condition of renal tubular acidosis. It is expressed preferentially in epithelia, including the proximal tubules of the kidney. In common with the other TASK channels, TASK2 is sensitive to changes in extracellular pH, although the molecular mechanism of such pH sensing is not understood. We have examined the role of charged residues in the extracellular domains in pH sensing using a mutational approach. Mutant channels were expressed in CHO cells and studied by whole-cell and single-channel patch clamp. Neutralization of no single amino acid in isolation gave complete loss of pH sensitivity. However, the combined removal of five charged amino acids in the large extracellular loop linking the first transmembrane and pore domains, the M1–P1 loop, resulted in an essentially pH-insensitive channel, stabilized in the open state. Wild-type channels contain two such loops, but a concatemeric construct, comprised of one wild-type subunit and one containing the five mutations, was fully pH-sensitive, indicating that only one M1–P1 loop is required to yield a fully pH-sensitive channel, demonstrating a regulatory role of this distinctive structure in two-pore domain K+ channels. Thus, pH sensing in TASK2 channels is conferred by the combined action of several charged residues in the large extracellular M1–P1 loop.
Keywords: potassium channel, regulation, acid, proton, gating
Potassium channels are ubiquitous, being found throughout the plant and animal kingdoms (1). K+ conductances are generally dominant in cells and are important in the maintenance of fundamental cell properties, such as the membrane potential and cell volume. The membrane potential in turn affects ion and ion-coupled solute movements through ion channels and electrogenic transport mechanisms. Changes in pH are known to have multiple effects on sensitive tissues, and acid-sensitive channels, such as those of the TASK family, may represent a sensory mechanism by which these changes are initiated.
TASK2 is a member of the tandem-pore domain K+ channel (K2P channel) family and is located in various epithelial tissues including the pancreas, placenta, lung, small intestine, colon, and, especially, kidney (2). Recently, the importance of TASK2 in NaHCO3 absorption in the proximal tubule has been established, and TASK2-deficient animals have a metabolic acidosis and hypotension (3). TASK2 currents are noninactivating and show no voltage dependence (2). It has also been shown that intracellular sodium causes a concentration- and voltage-dependent block of TASK2 channels such that TASK2 demonstrates a weak inward rectification (4). TASK2 currents are inhibited by lidocaine and bupivacaine and potentiated by halothane and chloroform (2). TASK2 is also sensitive to changes in the osmotic potential of the external medium and participates in cell volume regulation (5).
As indicated by its name, the twin-pore domain acid-sensing K+ (TASK) channel family of K2P channels is sensitive to extracellular pH; currents are maximal at alkaline pH but are progressively inhibited as pH decreases. TASK2 displays 90% of its maximal current at pH 8.8 but only 10% at pH 6.5 (2). A pore-neighboring histidine residue at position 98 plays an important role in pH sensing in TASK1 and TASK3 (6–8). Mutation of this histidine to asparagine or aspartate largely abolishes the pH sensitivity of the channel. Because an uncharged asparagine residue is present in the equivalent position in TASK2, and substitution with histidine causes a paradoxical reduction in pH sensitivity, the mechanism of pH sensing must reside in other residues or domains (9). The following experiments were carried out to identify residues or domains that contribute to the pH sensitivity of TASK2.
Materials and Methods
Expression of Mouse TASK2 and Cell Culture. TASK2 (KCNK5, GenBank accession no. AF319542) was cloned from mouse kidney into the bicistronic mammalian expression vector pIRES-GFP (Clontech) in which the GFP coding sequence was replaced with the coding sequence for human CD8 antigen. CHO-K1 cells were transiently transfected by using FuGENE 6 transfection and identified by their ability to bind anti-CD8 immunomagnetic beads (Dynal, Oslo) as described (4).
Generation of Mutants. Mutants of mouse TASK2 were generated by the QuikChange site-directed mutagenesis technique (Stratagene) and confirmed by sequencing (Lark Technologies, Cambridge, U.K.).The following single mutations were made: E28Q, H30N, K32N, E33Q, K35N, K36N, K42N, H44N, K47N, E48Q, E55Q, D58Q, K59N, D66Q, K107N, H220N, and R224N (Fig. 1). Amino acids are denoted by a single letter, followed by its position, followed by the residue to which it was mutated. Single mutations were also combined to produce an E28Q and K47N double mutant (2M-TASK2), a K32N, K35N, and K42N triple mutant (3M-TASK2), an E28Q, K32N, K35N, and K42N quadruple mutant (4M-TASK2), and an E28Q, K32N, K35N, K42N, and K47N quintuple mutant (5M-TASK2). By using PCR, two tandem constructs, WT-TASK2/WT-TASK2 and 5M-TASK2/WT-TASK2, were made by concatenating two TASK2 subunits (WT or 5M-TASK2). The introduction of restriction sites to facilitate this concatenation resulted in the inclusion of a single histidine residue between the final amino acids of the first TASK2 subunit (T502) and the start methionine (M1) of the second TASK2 domain.
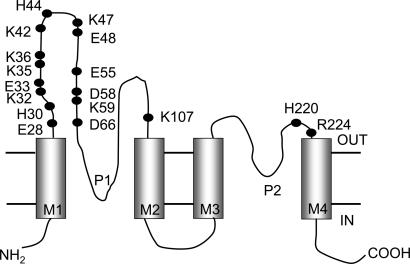
Fig. 1.
Topology of TASK2 and location of mutated residues. Diagrammatic representation of TASK2 structure showing four transmembrane domains (M1–M4) and two pore-domains (P1 and P2). OUT and IN refer to extracellular and intracellular compartments, respectively, and the cell membrane is delimited by the parallel, horizontal lines. Solid circles identify the charged residues in the extracellular domains, all of which were mutated in this study.
Electrophysiology and Solutions. Standard patch clamp techniques were used to measure channel activity in whole-cell and outside-out configurations (4, 10). All experiments were performed at room temperature. Bath solutions consisted of 145 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM Hepes, and 5 mM Pipes [piperazine-1,4-bis-(2-ethanesulfonic acid)] (pH 7.8). The pipette solution was 115 mM potassium gluconate, 20 mM KF, 10 mM KCl, 5 mM K2ATP, 5 mM EGTA, 5 mM MgCl2, 0.611 mM CaCl2, and 10 mM Hepes (pH 7.2, titrated with KOH); the calcium activity of this solution was 20 nM as determined by the react program (Godfrey Smith, Glasgow University, Glasgow, United Kingdom). Currents were recorded over the range -100 to +100 mV by using either a voltage pulse or voltage ramp protocol. All chemicals were from Sigma–Aldrich.
Analysis and Statistics. Results are given as means ± SEM, and statistical significance was assumed at the 5% level (P < 0.05).
Results
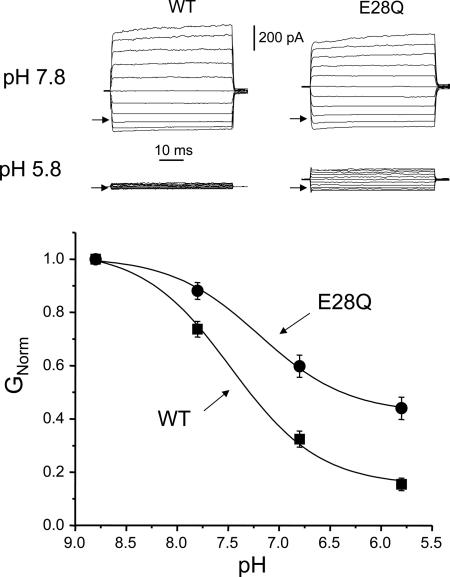
We expressed WT and mutant channels in CHO cells and examined the effect of varying the extracellular pH on channel currents (Fig. 2). As previously reported, WT-TASK2 currents are essentially time- and voltage-independent and are strongly inhibited at acid pH values, with a pK of 7.53 ± 0.06 (n = 14). We mutated each of the 17 potential titratable residues (D, E, K, and H) in the extracellular portion of the channel protein (Fig. 1) to a neutral residue (Q or N). All but one of these mutants (R224N) gave functional expression. Fig. 2 shows a typical recording from one of the mutants, E28Q, at pH 7.8 and pH 5.8. Substitution of the charged glutamate residue, E28, by the uncharged amino acid glutamine caused a reduction in pH sensitivity, as shown by a decrease in both the pK (7.12 ± 0.10, n = 8) and the degree of inhibition at pH 6.8 and pH 5.8. Remaining current at pH 6.8 and pH 5.8 was 32.4 ± 0.03% and 15.4 ± 0.02% for WT and 59.8 ± 0.04% and 44.0 ± 0.04% for E28Q (n = 8), respectively.
Fig. 2.
pH sensitivity of WT and mutant E28Q TASK2 channels. (Upper) Raw current–voltage (I–V) traces of WT and E28Q TASK2 at pH 7.8 and pH 5.8. Voltage was held at 0 mV, then clamped from -100 mV to +100 mV in 20-mV steps. Arrows indicate zero current level. (Lower) pH dose–response curves of WT (n = 14) and E28Q mutant. Solid curves are best fits to the Langmuir equation. GNorm = conductance normalized to that at pH 8.8.
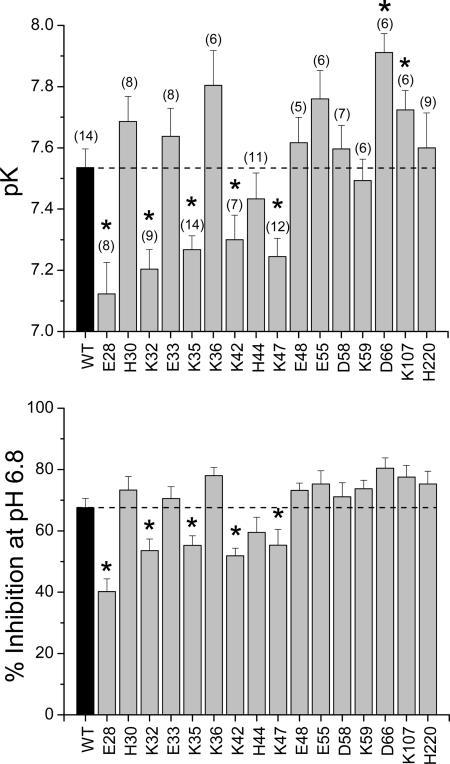
pK values and degree of inhibition at pH 6.8 were determined for each of 16 single-point mutants that showed expression, and the mean data are given in Fig. 3. Comparing the whole-cell conductances showed that there were no significant differences in the level of expression of WT and mutant channels (ANOVA, P > 0.05). Seven mutants exhibited statistically significant shifts in their pK values compared with WT. Five of these mutants, E28Q, K32N, K35N, K42N, and K47N, had a reduced pK, becoming less sensitive to pH, which was also reflected in a reduction in the degree of inhibition at pH 6.8 (Fig. 3). In contrast, two of the mutants, D66Q and K107N, had a significantly elevated pK but were without effect on the degree of inhibition at pH 6.8 and were not studied further (Fig. 3).
Fig. 3.
pH sensitivity of WT and mutant channels. pK (Upper) and percentage inhibition (Lower) at pH 6.8 relative to pH 8.8 for WT and mutant channels. Numbers on abscissa correspond to amino acid number. The number of determinations for each channel is given in brackets. *, Statistical significance compared with WT (P < 0.05).
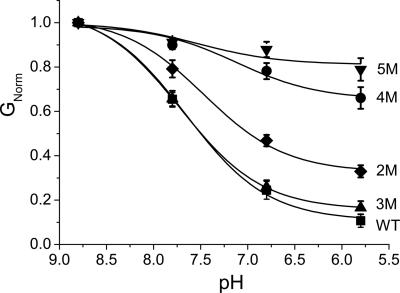
Because none of the single-point mutants had very marked reduction in pH sensitivity, we decided to test whether pH sensing depended on the combined contribution of more than one residue. Combined mutation of all five amino acids (5M-TASK2) markedly reduced the pH sensitivity of the resulting channel such that, at pH 5.8, 78.9 ± 0.05% (n = 8) of current remained (Fig. 4). Combined mutation of less than five of these amino acids resulted in channels of intermediate pH sensitivity between WT and 5M-TASK2.
Fig. 4.
Effect of combined mutations on TASK2 pH sensitivity. pH dose–response curves of WT (▪, n = 14), 2M (E28Q and K47N; ♦, n = 8), 3M (K32N, K35N, and K42N triple mutant; ▴, n = 8), 4M (E28Q, K32N, K35N, and K42N quadruple mutant; •, n = 7), and 5M (E28Q, K32N, K35N, K42N, and K47N quintuple mutant; ▾, n = 8). Solid curves are best fits to the Langmuir equation. GNorm = conductance normalized to that at pH 8.8.
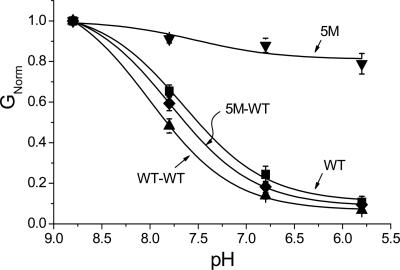
pH Sensitivity of TASK2 Concatemers. Both concatemeric channels, WT-TASK2/WT-TASK2 and 5M-TASK2/WT-TASK2, displayed levels of expression and outwardly rectifying currents not different from homodimeric WT-TASK2. Both channels were fully pH-sensitive (Fig. 5). It should be noted that concatemerization itself does not alter pH sensing because the WT–WT tandem dimer behaves similarly to WT-TASK2.
Fig. 5.
pH sensitivity of TASK2 concatemers. pH dose–response curves of the WT-WT concatemer (▴, n = 8) and 5M-WT concatemer (♦, n = 8). WT (▪, n = 14) and 5M (▾, n = 8) monomer results are shown for comparison. Solid curves are best fits to the Langmuir equation. GNorm = conductance normalized to that at pH 8.8.
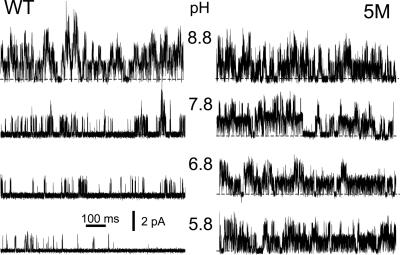
Single-Channel Data. In outside-out patches with a sodium-rich pipette solution and a low-sodium bath solution at pH 8.8, the mean probability of opening (N.Po) was 0.61 ± 0.19 (n = 6). When bath pH was reduced from pH 8.8 to pH 5.8, N.Po fell significantly, to 0.07 ± 0.02 (n = 6) (Fig. 6). In the 5M mutant, N.Po at pH 8.8 was 1.29 ± 0.17 (n = 5) and was unchanged at pH 5.8, N.Po = 1.30 ± 0.40 (n = 6). The single-channel currents were not significantly different between WT and the 5M mutant channels (at pH 8.8 and a holding potential of 0 mV, WT currents were 1.24 ± 0.07 pA, n = 6; M5 currents were 1.16 ± 0.13 pA, n = 6). Thus, in agreement with the whole-cell studies, the 5M mutant was pH-insensitive.
Fig. 6.
pH sensitivity of WT and 5M-TASK2 channels in outside-out patches. Recordings were made at 0 mV at the indicated bath pH values, with an outwardly directed K+ gradient, from patches obtained from cells expressing either WT (Left) or 5M mutant (Right) channels. Closed channel current level is indicated by the dotted lines, and openings are upward deflections.
Discussion
TASK2 is a member of the K2P channel family that is sensitive to extracellular pH (pHo). In an attempt to understand the molecular mechanism by which TASK2 is able to sense changes in the pHo, we have mutated each of the 17 potential titratable residues (D, E, K, and H) in the extracellular portion of the channel protein (Fig. 1) to a neutral residue (Q or N). We expressed the mutant channels in CHO cells and examined the effect of varying the extracellular pH on channel currents, measured at 0 mV. All but one of these mutants (R224N) gave functional expression.
Lysine and Aspartic Acid Residues, but Not Histidines, in the M1–P1 Loop Contribute to pH Sensing by TASK2. Of the 16 single mutations tested, seven caused significant shifts in their affinity for H+ ions (Fig. 3); five, including E28Q, K32N, K35N, K42N, and K47N mutations, caused a decrease in the affinity for H+ (alkaline shift), thereby making the channel less sensitive to pHo. This reduced pH sensitivity, apparent in the reduction in pK, was also reflected in the degree of blockade, where there was a significant decrease in the degree of inhibition at pH 6.8 (Fig. 3). Interestingly, mutation of neither of the two histidines had any significant effect: in TASK1 and TASK3 a pore-neighboring histidine residue is involved in sensing pHo (6, 8). This histidine is located immediately distal to the selectivity filter of the first pore-forming domain, and, when mutated to a neutral, nontitratable residue, pH sensitivity is reduced. However, TASK2 does not possess a histidine residue at the equivalent position (amino acid 103 is an asparagine), and substitution of a histidine at this position did not increase the channel's sensitivity to extracellular pH, as might be expected, but decreased it (9). Thus, pH sensing in TASK2 is different from that in TASK1 and TASK3.
E28 Plays a Central Role in pH Sensing. Another distinction from other TASK channels, and TASK3 in particular, is that pH sensitivity does not seem to be determined by a single residue: neutralization of none of the charged residues was able to abolish the pH sensitivity (Figs. 2 and 3). Therefore, we surmised that several of these residues may collaborate to confer pH sensitivity, as in the case of ROMK1 (21). However, when we combined three of the four K-to-N mutations, each of which individually reduced the pH sensitivity significantly, we had a surprising result: contrary to the expected cumulative effect, leading to almost complete removal of pH sensitivity, the resultant channel (3M) was as sensitive to pH as the WT channel (Fig. 4). Thus, the mechanism seemed to be not as simple as predicted by the studies of ROMK1 (21). When we neutralized the negatively charged residue E28 in the 3M (K32N; K35N; K42N) background, the resultant mutant channel (4M) showed a marked reduction in its pH sensitivity (≈70% loss at pH 5.8; see Fig. 4). Finally, when K47 was also neutralized (5M; see Fig. 4), pH sensitivity was essentially lost, there being no significant difference in conductance between pH 8.8 and pH 5.8. These data suggest that the critical residue in pH sensing by TASK2 is E28 and that pH sensing by E28 is modulated by the positive charges associated with the four lysine residues. A negatively charged residue (E) has been shown to play a role in sensing changes in the intracellular pH of Kir 4.1 (11).
Network of Charged Residues in the M1–P1 Loop of a Single Subunit Is Enough to Confer pH Sensitivity. K2P channel subunits dimerize through SID domains in the M1–P1 loop to form functional channels (6, 12, 13). Therefore, each channel will have two extracellular M1–P1 loops that may be covalently linked by a disulfide bridge from conserved cysteines (C51) in each loop (and, likewise, the 5M mutant will therefore have two mutated loops in the functional dimeric protein). This raises the possibility that the two M1–P1 loops may interact to bring about channel gating, perhaps through changes in electrostatic interactions. To test this possibility, we made a 5M-WT M1–P1 loop concatemer that contained one mutated and one WT M1–P1 loop. This construct was as pH-sensitive as WT-TASK2 (Fig. 5), indicating, first, that only one M1–P1 loop is necessary for pH sensing and, second, that pH sensing does not rely on interaction between the two loops. The second point is reinforced by previous work concerning disulfide bridge formation between C51 residues in neighboring TASK2 subunits, where chemical reduction of the disulfide bond by DTT caused dissociation of channel subunits, yet the pH sensitivity was unaltered (13). The redundancy of having two, rather than one, pH-sensing loops is somewhat akin to that of N-type inactivation in voltage-gated channels, in which inactivation requires the presence of only one ball domain, whereas WT channels contain four such domains (14).
However, subunit interaction is important in determining other K2P channel characteristics. Pore-lining residues of neighboring subunits of TASK1 channels interact with one another to affect ion selectivity, conductance, and gating (15). Furthermore, full pH sensitivity required that both subunits contained H at position 98; when only one of the subunits contained H98, with H98N on the other, the resulting tandem channels displayed a pH sensitivity intermediate to that of the WT and mutant (6). By contrast, in the current study, concatemerizing pH-sensitive WT-TASK2 and pH-insensitive 5M-TASK2 subunits yielded a fully pH-sensitive channel, and not one of intermediate sensitivity. Thus, pH sensing requires the presence of only one WT M1–P1 domain and is presumably independent of interaction between opposing M1–P1 domains.
Possible Mechanism. Two principal types of gate have been identified: intracellular (inner helix bundle crossing) and extracellular (C-type inactivation) (16). In K2P channels, gating is thought to be controlled via a gate situated in the external vestibule of the pore, which resembles the C-type inactivation gate of Kv channels. In both Kv and TASK2 channels, the critical residue contributing to extracellular gating is a negatively charged glutamate (E418 in Shaker channel and E28 in KCNK0, equivalent to E27 in TASK2) situated at the top of the outer helix (S5 in Shaker and M1 in TASK2) of the pore domain; removal of the negative charge from this position in Shaker and KCNK0 leads to rapid closure of the gate (17, 18). This glutamate residue is conserved throughout the K2P channel family (http://receptors.ucsf.edu/KCN/seq/003/003.MSF.mview.html; see ref. 19) and is presumably involved in gating in all family members. Owing to its conservation, and the importance of E27 in the proposed mechanism (see below), we made an E27Q mutant, but this failed to express. Nevertheless, in the current study we have shown that neutralization of the adjacent E residue (E28 in TASK2), immediately external to the conserved glutamate and exclusive to TASK2, reduces channel sensitivity to pH. Conceivably, in WT channels, protonation of E28 will influence the ionization state by reducing the ability of E27 to release its proton, leading to pH-dependent channel gating. In KcsA, the carboxyl group of the corresponding residue (E51) forms hydrogen bonds with the backbone amide nitrogen atoms of V84 and T85 (K107 and T108 in TASK2) and with the side-chain hydroxyl of T85 (T108 in TASK2) of the pore helix, helping to keep the gate in the open conformation. When the carboxyl group is neutralized, these hydrogen bonds are lost and the gate closes more readily (17, 18). It is possible that, in TASK2, protonation of E28 weakens or removes the hydrogen bonds between E27 and K107/T108, leading to the closure of the gate.
The pKa of glutamate in a polypeptide chain is estimated to be 4.3. This means that at pH 5.8 this residue would be expected to be almost fully ionized and to support the open conformation of the gate. However, the pK for the H+ block of the WT-TASK2 channel is ≈7.5. This means that the pKa of E28 is likely to be ≈3 pH units higher than the theoretical value. The pKa value of an acidic group is affected by the charge on its neighbors, being raised by an adjacent anion and lowered by an adjacent cation (20). Thus, the proximity of two glutamate residues could raise the pK of E28 (and/or E27). However, a number of other charged residues in the M1–P1 loop seem to influence pH sensitivity, but in the absence of three-dimensional structural information it is hard to predict how they would influence the pKa of E28. Nonetheless, pH sensitivity was not fully lost until four additional positively charged lysine residues in the M1–P1 loop were neutralized. A change in the effective pK of an amino acid by the combined effect of other amino acids within the parent protein has been reported for another K+ channel; in Kir1.1 (ROMK1) pH sensing occurs via a lysine residue (pKa ≈ 10.5) whose pKa is lowered into the neutral range by electrostatic interactions with arginine residues in the amino and carboxyl termini (21). A number of mutations within this R-K-R triad contribute to antenatal Bartter's syndrome by shifting the channel's pK, causing channel inactivation under physiological condition (21). Thus, pH sensing may depend on the net charge of several residues, rather than a single residue.
In summary, pH sensing in TASK2 depends on charged residues in the large extracellular loop between the first transmembrane and pore domains. Furthermore, the presence of only one such domain is sufficient to confer full pH sensitivity on TASK2.
Acknowledgments
This work was supported by the Wellcome Trust. A.A. was supported by a grant from the Saudi Arabian Government.
Author contributions: M.J.M., A.S., and M.H. designed research; M.J.M. and A.A. performed research; M.J.M., A.A., and M.H. analyzed data; and M.J.M., A.S., and M.H. wrote the paper.
Abbreviation: K2P channel, tandem-pore domain K+ channel.
References
- 1.Hille, B. (2001) Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA).
- 2.Reyes, R., Duprat, F., Lesage, F., Fink, M., Salinas, M., Farman, N. & Lazdunski, M. (1998) J. Biol. Chem. 273, 30863-30869. [DOI] [PubMed] [Google Scholar]
- 3.Warth, R., Barriere, H., Meneton, P., Bloch, M., Thomas, J., Tauc, M., Heitzmann, D., Romeo, E., Verrey, F., Mengual, R., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8215-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton, M. J., Chipperfield, S., Abohamed, A., Sivaprasadarao, A. & Hunter, M. (2004) Am. J. Physiol. 288, F162-F169. [DOI] [PubMed] [Google Scholar]
- 5.Niemeyer, M. I., Cid, L. P., Barros, L. F. & Sepulveda, F. V. (2001) J. Biol. Chem. 276, 43166-43174. [DOI] [PubMed] [Google Scholar]
- 6.Lopes, C. M., Zilberberg, N. & Goldstein, S. A. (2001) J. Biol. Chem. 276, 24449-24452. [DOI] [PubMed] [Google Scholar]
- 7.Kim, Y., Bang, H. & Kim, D. (2000) J. Biol. Chem. 275, 9340-9347. [DOI] [PubMed] [Google Scholar]
- 8.Rajan, S., Wischmeyer, E., Xin, L. G., Preisig-Muller, R., Daut, J., Karschin, A. & Derst, C. (2000) J. Biol. Chem. 275, 16650-16657. [DOI] [PubMed] [Google Scholar]
- 9.Morton, M. J., O'Connell, A. D., Sivaprasadarao, A. & Hunter, M. (2003) Pflügers Arch. 445, 577-583. [DOI] [PubMed] [Google Scholar]
- 10.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100. [DOI] [PubMed] [Google Scholar]
- 11.Xu, H., Yang, Z., Cui, N., Chanchevalap, S., Valesky, W. W. & Jiang, C. (2000) J. Physiol. 528, 267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesage, F., Reyes, R., Fink, M., Duprat, F., Guillemare, E. & Lazdunski, M. (1996) EMBO J. 15, 6400-6407. [PMC free article] [PubMed] [Google Scholar]
- 13.Niemeyer, M. I., Cid, L. P., Valenzuela, X., Paeile, V. & Sepulveda, F. V. (2003) Mol. Membr. Biol. 20, 185-191. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon, R., Aldrich, R. W. & Lee, A. W. (1993) Science 262, 757-759. [DOI] [PubMed] [Google Scholar]
- 15.Chapman, M. L., Krovetz, H. S. & VanDongen, A. M. (2001) J. Physiol. 530, 21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yellen, G. (2002) Nature 419, 35-42. [DOI] [PubMed] [Google Scholar]
- 17.Zilberberg, N., Ilan, N. & Goldstein, S. A. (2001) Neuron 32, 635-648. [DOI] [PubMed] [Google Scholar]
- 18.Larsson, H. P. & Elinder, F. (2000) Neuron 27, 573-583. [DOI] [PubMed] [Google Scholar]
- 19.Horn, F., Vriend, G. & Cohen, F. E. (2001) Nucleic Acids Res. 29, 346-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyte, J. (1995) Structure in Protein Chemistry (Garland, New York).
- 21.Schulte, U., Hahn, H., Konrad, M., Jeck, N., Derst, C., Wild, K., Weidemann, S., Ruppersberg, J. P., Fakler, B. & Ludwig, J. (1999) Proc. Natl. Acad. Sci. USA 96, 15298-15303. [DOI] [PMC free article] [PubMed] [Google Scholar]