Abstract
Mammalian corticogenesis occurs through a complex process that includes neurogenesis, in which neural progenitor cells proliferate, differentiate, and migrate. It has been reported recently that neurogenesis occurs in the subventricular zone (SVZ), a region previously thought to be the primary site of gliogenesis. It has been recognized that in the SVZ, intermediate progenitor cells, derived from radial glial cells that are multipotent neural stem cells, produce only neurons. However, the molecular mechanisms underlying the regulation of neural stem cells and intermediate progenitor cells as well as their contribution to overall corticogenesis remain unknown. The docking protein FRS2α is a major mediator of signaling by means of FGFs and neurotrophins. FRS2α mediates many of its pleiotropic cellular responses by recruiting the adaptor protein Grb2 and the protein tyrosine phosphatase Shp2 upon ligand stimulation. Here, we report that targeted disruption of Shp2-binding sites in FRS2α leads to severe impairment in cerebral cortex development in mutant mice. The defect in corticogenesis appears to be due at least in part to abnormalities in intermediate progenitor cells. Genetic evidence is provided that FRS2α plays critical roles in the maintenance of intermediate progenitor cells and in neurogenesis in the cerebral cortex. Moreover, FGF2-responsive neurospheres, which are cell aggregates derived from neural stem/progenitor cells (NSPCs), from FRS2α mutant mice were smaller than those of WT mice. However, mutant NSPCs were able to self-renew, demonstrating that Shp2-binding sites on FRS2α play an important role in NSPC proliferation but are dispensable for NSPC self-renewing capacity after FGF2 stimulation.
Keywords: cell signaling, docking proteins, neuronal development, stem cells, tyrosine phosphorylation
Development of the mammalian cerebral cortex involves complex processes, including neurogenesis, in which a variety of cells that were originally derived from neural stem cells proliferate, differentiate, and migrate in a precisely coordinated manner. There is a great therapeutic interest in neural stem cells, because these cells could potentially be applied for therapeutic regeneration of brain tissues that have been damaged by trauma, infarction, or neurodegenerative diseases (1).
It is thought that the most immature neuroepithelial cells lining the neural tube and the radial glial cells having radial fibers contacting with the pial surface of the developing brain correspond to neural stem cells (1–4). Many radial glial cells undergo asymmetrical division, generating one neuron and one self-renewing radial glial cell in the apical side of the ventricular zone (VZ), a pseudostratified neuroepithelial region that lines the lateral ventricles (4–7). It was previously believed that this type of cell division is a major source of cortical neurons. However, recent studies indicate that a major part of radial glial cells also undergo another type of asymmetrical division, generating one lineage-restricted intermediate progenitor cell and one self-renewing radial glial cell in the apical side of the VZ (8, 9). The previously unrecognized intermediate progenitor cells then migrate and generate two neurons in the subventricular zone (SVZ), which is adjacent to the VZ and was thought to be a primary region for gliogenesis and for generation of specific types of neurons (10, 11). The newly generated neurons migrate away from the VZ/SVZ and localize in the cortical plate (CP) to form the layers of the cerebral cortex (12).
FGFs and neurotrophins play important roles in many aspects of corticogenesis (13, 14); however, the intracellular signaling pathways activated by FGFs or neurotrophins during corticogenesis are not well understood. Cellular responses to FGFs and neurotrophins are mediated by receptor tyrosine kinases known as FGF receptors 1, 2, 3, and 4 or neurotrophic factor receptors TrkA/B/C (13, 15, 16). These activated receptors phosphorylate a variety of signaling proteins, including Shc, phospholipase Cγ, and the docking proteins FRS2α and FRS2β, resulting in a coordinated assembly of signaling complexes that activate multiple signaling pathways in the cells (17–22). During development of the central nervous system, Frs2α is expressed in the VZ/SVZ of the telencephalon, which gives rise to the cerebral cortex, whereas Frs2β is expressed in other areas, including the thalamus and hypothalamus. At late stages of corticogenesis, both genes are expressed in the CP (19).
The two members of the FRS2 family, FRS2α and FRS2β, contain N-terminal myristyl anchors and phosphotyrosine-binding domains followed by a long region that contains multiple tyrosine phosphorylation sites. Four tyrosine residues on FRS2α serve as binding sites for the adaptor protein Grb2, and two tyrosines serve as a binding site for the protein tyrosine phosphatase Shp2 (17, 20). Experiments with Frs2α-/- mouse embryonic fibroblasts transfected with vectors expressing tyrosine phosphorylation site mutants of FRS2α showed that the Shp2-binding sites play a primary role in FGF stimulation of the Ras/extracellular signal-regulated kinase (ERK) signaling cascade, whereas Grb2-binding sites on FRS2α are primarily responsible for recruitment of the docking protein Gab1 followed by stimulation of phosphatidylinositol 3-kinase and the anti-apoptotic protein kinase Akt (17, 22).
Disruption of the Frs2α gene results in early embryonic lethality due to multiple defects in FGF signaling, such as defects in trophoblast stem cells that self-renew in the presence of FGF4 and defects in cell movement through the primitive streak during gastrulation (23). To elucidate the signaling pathways emanating from the Shp2-binding sites and Grb2-binding sites of FRS2α,we generated mice carrying mutations of either the two tyrosine residues that act as the Shp2-binding sites (Frs2α2F) or the four tyrosine residues serving as the Grb2 binding sites (Frs2α4F). Frs2α2F/2F mice showed microphthalmia or anophthalmia, defects in early eye development (24) that are caused by defects in the interaction between the surface ectoderm and underlying neuroepithelium of the optic vesicle in the eye primordia, indicating that the Shp2-binding sites of FRS2α are essential for this process.
In the present study, we explored the role of the Shp2-binding sites of FRS2α in development of the cerebral cortex and cultured multipotent neural stem/progenitor cells (NSPCs). We found that the development of cerebral cortices of Frs2α2F/2F mice was severely impaired, probably because the lineage-restricted intermediate progenitor cells were not fully functional. Thus, Shp2-binding sites of FRS2α appear to play a critical role in maintaining these cells. Moreover, Shp2-binding sites of FRS2α are required for efficient proliferation of cultured NSPCs but are dispensable for self-renewal of NSPCs in response to FGF2 stimulation.
Materials and Methods
Animals and Paraffin Histology. A colony of Frs2α2F/+ mice was maintained in a Swiss–Webster background (Taconic). Mice were genotyped by PCR as described (24). Paraffin sections were prepared for hematoxylin/eosin or Nissl staining by using standard methods (24).
In Vivo BrdUrd Incorporation. Pregnant females were i.p. injected with BrdUrd (50 mg/kg of body weight, Sigma) dissolved in PBS at embryonic day (E) 10.5 and E12.5. The embryos were dissected after 1 h, and their fixed brains were frozen in OCT compound and coronally sectioned.
Immunofluorescence Microscopy. The following antibodies were used for immunostaining of frozen coronal sections: anti-phosphohistone H3 (rabbit polyclonal, 1:400 dilution, Upstate Biotechnology, Lake Placid, NY), anti-PCNA (proliferating cell nuclear antigen) (rabbit polyclonal, 1:500 dilution, Oncogene Science), anti-Musashi-1 (25, 26), and anti-Tbr2 (27). TUNEL assay was performed according to the manufacturer's instructions (Roche Applied Science, Indianapolis).
Immunoblotting. Embryo brains were lysed, and the lysates were subjected for immunoblotting as described in ref. 24. Anti-pERK, anti-ERK, anti-pAkt, and anti-Akt antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Neurosphere Assays. Neurosphere formation assay was performed as described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, and ref. 25.
Results
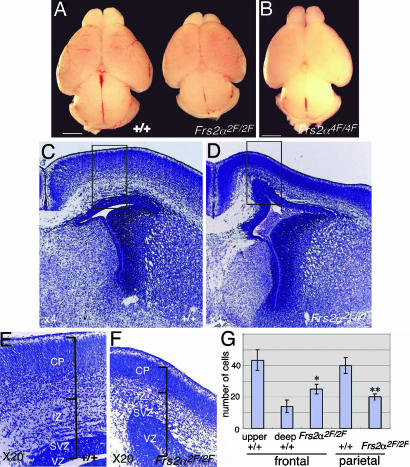
Frs2α2F/2F Mutant Mice Exhibit a Profound Defect in Corticogenesis. Examination of the gross morphology of mutant embryos revealed that all Frs2α2F/2F embryos had smaller heads than those of WT embryos, which was first recognized at E15.5. In contrast, Frs2α4F/4F heads were of normal size. To examine defects in brain development, we dissected out the brains at E18.5, because the Frs2α2F/2F genotype is lethal (the embryos did not survive beyond this stage of development) (24). Although the brains of WT and Frs2α4F/4F mice have a similar size (Fig. 1 A and B), the brains of Frs2α2F/2F mice are ≈10% smaller than those of WT or Frs2α4F/4F mice. Examination of gross morphology did not reveal significant patterning defects in Frs2α2F/2F brains (Fig. 1 A). Histologic analysis of serially sectioned brains revealed that cerebral cortices of Frs2α2F/2F mice were thinner than those of WT or Frs2α2F/+ mice (Fig. 1 C–F and black bars in E and F). At this stage of development, the cerebral cortex is composed of several layers, including the VZ, SVZ, and the intermediate zone (IZ), through which neurons and glial cells migrate, as well as the CP (28). In the developing cerebral cortex of Frs2α2F/2F mice, the thickness of the CP was less than half the thickness in WT or Frs2α2F/+ mice in all regions of the cerebral cortex (Fig. 1 C–F and data not shown). Although the CP of WT brain in the frontal region could be clearly divided on the basis of cell density into upper and deep layers (29), the CP of mutant brain showed no stratification (Fig. 1 E and F). Moreover, the thickness of the CP of Frs2α2F/2F brain was ≈70% of the upper layer of the CP of WT brain and had a lower cell density than that of WT brain in all regions of the cerebral cortex (Fig. 1 E–G and data not shown). These results suggest that the CP of mutant brain contained much fewer cells than that of WT brain.
Fig. 1.
Profound defects in cerebral cortex development of Frs2α2F/2F mice. (A and B) Gross morphology of WT, Frs2α2F/2F, and Frs2α4F/4F brains at E18.5. (Scale bar, 1 mm.) (C–F) Nissl-stained coronal sections of cerebral cortex of the indicated genotypes at E18.5. E and F are magnified images of the areas indicated in the squares in C and D, respectively. (G) Cell densities in the CP. Cells in 15 randomly selected areas in CP of coronal sections were counted for each genotype. Cells in upper and deep layers of WT CP were counted separately. *, P < 0.05; **, P < 0.01 vs. WT upper layer.
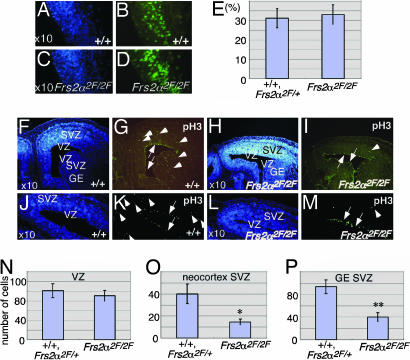
Because Frs2α is highly expressed in the neuroepithelium at the time when immature progenitors are proliferating (19), we examined proliferation of cortical progenitors at E10.5 and E12.5 by BrdUrd pulse-labeling experiments. The experiment presented in Fig. 2 A–E (and data not shown) demonstrates that the volume of VZ and the proportions of BrdUrd-labeled cells were similar in WT and mutant mice, indicating that the proliferation of neural progenitor cells was not strongly affected in mutant embryos at these stages of development.
Fig. 2.
Decreased cell division in SVZ of E14.5 Frs2α2F/2F mice. (A–E) BrdUrd pulse-labeled cells in telencephalon at E12.5 (B and D) and percentage of BrdUrd-labeled cells in 10 randomly selected areas of coronal sections (E) (values are mean ± SD). A and B and C and D are the same sections. Nuclei are labeled with Hoechst 33258 in A and C.(F–P) The number of phosphohistone H3-positive cells are decreased in SVZ but not in the apical side of VZ in Frs2α2F/2F mice. (G, I, K, and M) Coronal sections were immunostained with anti-phosphohistone H3 antibodies. Arrows indicate stained cells in the apical side of VZ. Arrowheads indicate stained cells in SVZ. F and G, H and I, J and K, and L and M are the same sections. (F, H, J, and L) Nuclei were labeled with Hoechst 33258. (N–P) Labeled cells were counted in 10 randomly selected areas of the coronal sections and are reported as mean ± SD per area unit. *, P < 0.05; **, P < 0.01 vs. WT or heterozygous.
Intermediate Progenitor Cells Are Not Fully Functional in Frs2α2F/2F Mice. Extensive histologic analysis revealed that the Frs2α2F/2F cortex failed to develop properly between E14.5 and E15.5. To examine the primary defects in Frs2α2F/2F cortex, we performed immunohistochemical analysis of E14.5 embryos. We first stained brain sections with anti-phosphohistone H3 (pH3), a marker of metaphase (30). Many dividing cells were observed at the apical side of the VZ (arrows) and in the SVZ (arrowheads) of cerebral cortex and in the lateral/medial ganglionic eminence (GE) in WT mice (Fig. 2 F, G, J, and K). In mutant cortex, the number of dividing cells was decreased significantly in the SVZ, whereas levels of mitosis in VZ were similar to those in WT mice (Fig. 2 H, I, L, and M). Quantitation of stained cells in VZ and SVZ of cerebral cortex and GE revealed that although there was no significant difference in the levels of mitotic activities in VZ, the number of mitotic cells was lower in mutant SVZ than in WT or heterozygous SVZ (Fig. 2 N–P). Thus, the intact mitotic activities at the apical VZ of the mutant embryos suggest that mutant radial glial cells are fully functional for mitosis.
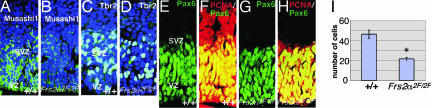
We next stained brain sections with antibodies against Musashi-1, a RNA-binding protein predominantly expressed in NSPCs (25, 26). Although the VZ/SVZ of WT embryos showed strong immunoreactivity with anti-Musashi-1 antibodies, reduced levels of staining were observed in mutant embryos (Fig. 3 A and B). We next stained brain sections with antibodies against Tbr2, a marker for intermediate progenitor cells (27). The number of Tbr2+ cells was significantly reduced in mutant embryos compared with those of WT embryos (Fig. 3 C, D, and I). We also stained brain sections with antibodies against PCNA, which is expressed mainly from G1 through S phase of the cell cycle, and Pax6, a transcription factor expressed in neuroepithelial cells, including radial glial cells, in the VZ. It is reported that many PCNA+ cells in the SVZ correspond to the intermediate progenitor cells (27). There was no significant difference in the number of Pax6+ cells in WT and mutant embryos (Fig. 3 E and G). In contrast, the expression of PCNA in the SVZ was reduced in mutant embryos compared with PCNA expression in the SVZ of WT embryos (Fig. 3 F and H). Last, we stained brain sections with anti-nestin, a marker for NSPCs, and found that expression levels of nestin were reduced in mutant brain (data not shown). We also examined apoptosis by using TUNEL assay; however, we did not observe significant differences in the number of apoptotic cells in the VZ/SVZ of dorsal telencephalon between WT or mutant embryos (data not shown). Therefore, it is likely that the intermediate progenitor cells that should be dividing in the SVZ failed to proliferate and produce neurons.
Fig. 3.
Decreased expression of Musashi-1, Tbr2, and PCNA in E14.5 Frs2α2F/2F brains. (A–H) Coronal sections were immunostained with anti-Musashi-1 (A and B), anti-Tbr2 (C and D), anti-Pax6 (E–H), and anti-PCNA (F and H) antibodies. Nuclei were labeled with TO-PRO-3 iodide (A–D). (I) Tbr2+ cells were counted in 10 randomly selected areas of the coronal sections and are reported as mean ± SD per area unit (n = 3 for WT, and n = 3 for Frs2α2F/2F; *, P < 0.01 vs. WT control).
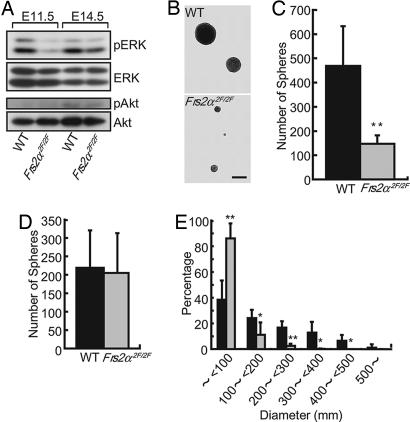
Taken together, these findings suggest that Shp2-binding sites of FRS2α are required for maintenance of the intermediate progenitor cells but not stem cells. In addition, we examined activation of ERK and Akt in brain tissues by using anti-phospho-ERK and anti-phospho-Akt, antibodies that recognize specifically the activated forms of ERK and Akt, respectively. The experiment depicted in Fig. 4A shows that although ERK activation was reduced in mutant brains at E11.5 and E14.5, no difference was detected in Akt activation in WT or mutant brains (Fig. 4A).
Fig. 4.
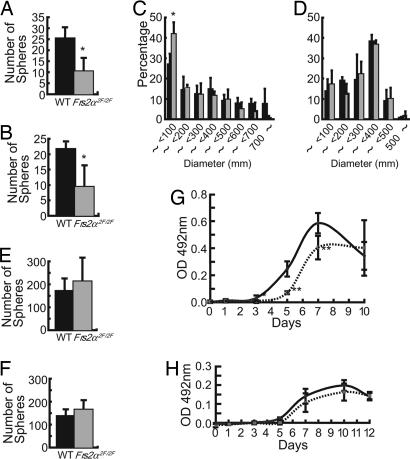
Decreased activation of ERK in brain tissues of Frs2α2F/2F embryos and decreased proliferation of FGF2-responsive NSPCs derived from E11.5 Frs2α2F/2F brains. (A) Total cell lysates from brain tissues at the indicated stages were analyzed by immunoblotting with anti-phospho-ERK, anti-phospho-Akt, anti-ERK, and anti-Akt antibodies. (B) Secondary neurospheres of indicated genotypes cultured in the presence of FGF2 (20 ng/ml) for 12 days. (Scale bar, 500 μm.) (C) Number of primary neurospheres cultured in the presence of FGF2 (20 ng/ml) (n = 3 for WT, and n = 3 for Frs2α2F/2F; **, P < 0.05 vs. WT control). (D) Number of secondary neurospheres was counted and indicated as mean ± SD after the primary neurospheres were dissociated and 5 × 102 cells per well were plated and cultured in FGF2 (20 ng/ml) for 12–13 days (n = 3 for WT, and n = 3 for Frs2α2F/2F). (E) Diameters of the secondary neurospheres of the indicated genotypes (n = 3 for WT, and n = 3 for Frs2α2F/2F). WT is represented by black bars and Frs2α2F/2F is represented by gray bars in C–E.
Shp2-Binding Sites of FRS2α Are Important for FGF2 Stimulation of Cultured NSPC Proliferation. It has been shown that multipotent NSPCs located in telencephalic germinal zone/VZ can be stimulated to proliferate in response to FGF2 or EGF stimulation and form clonally derived spherical cell clusters called neurospheres in vitro (1, 3, 31, 32). FGF2-responsive NSPCs recognized by the neurosphere formation assay can be isolated as early as E8.5, but EGF-responsive NSPCs appear later at ≈E13.5. Emerging evidence indicates that neurosphere cells are composed of heterogeneous cell populations, including stem cells and multipotent progenitor cells that are derived from the former and are not yet identified in vivo (33, 34). To gain further insight into the role of Shp2-binding sites of FRS2α in the biology of NSPCs, we took advantage of this system and further characterized NSPCs isolated from mutant or WT mice.
Cells derived from E11.5 brains were cultured in the presence of FGF2, and the number of primary neurospheres was counted. This experiment demonstrated that the cultures derived from the brains of mutant mice gave fewer neurospheres compared with those derived from the brains of WT mice (Fig. 4C). To examine the self-renewing ability of these neural stem cells, the primary neurospheres were dissociated and cultured again in the presence of FGF2. Because self-renewing neural stem cells are capable of reforming secondary neurospheres and each neurosphere is derived from a single neural stem cell, the frequency of secondary neurosphere formation corresponds to the self-renewing capacity of the neural stem cells grown in the primary neurospheres (25). There was no significant difference in frequency of secondary neurosphere formation from cells derived from WT or mutant mice (Fig. 4D), indicating that mutant neural stem cells grown in the primary neurospheres had intact self-renewing capacity in response to FGF2 stimulation. The WT cells formed variable sizes of neurospheres from <100 μm to ≥500 μm in diameter (mean value of 173 ± 52 μm, n = 5), whereas the mutant cells formed only small neurospheres, with >90% of mutant neurospheres <100 μm in diameter (mean value of 58 ± 15 μm, n = 5) (Fig. 4 B and E). These results indicate that mutant neural stem cells grew less efficiently than those derived from WT mice.
After EGF-responsive NSPCs arise during development, there are at least two types of NSPCs (3, 31, 32). One type is FGF2-dependent but not EGF-responsive, and the other type of NSPC is responsive to both EGF and FGF2. Either growth factor is required for self-renewal. We next dissected telencephalon from E14.5 embryos and cultured the cells in the presence of EGF alone or FGF2 together with EGF. We counted the number of primary neurospheres and found that the mutant telencephalon gave decreased numbers of neurospheres in both culture conditions, compared with those derived from WT mice (Fig. 5 A and B). We also examined secondary neurosphere formation. There was no significant difference in the frequency of the secondary neurosphere formation between WT and mutant cells either in the presence of EGF alone or when EGF was added with FGF2 (Fig. 5 E and F). In contrast, in the presence of both EGF and FGF2, WT cells formed larger secondary neurospheres (mean diameter of 300 ± 15 μm, n = 3) than cells derived from mutant mice (mean diameter of 210 ± 31 μm, n = 3; P < 0.05) (Fig. 5C). In contrast, in the presence of EGF alone, there was no significant difference in size of neurospheres between WT (mean diameter of 254 ± 17 μm, n = 3) and mutant (mean diameter of 263 ± 24 μm, n = 3) mice (Fig. 5D). The MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxyme-thoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] assay showed that WT cells in the secondary neurospheres grew faster than mutant cells in the presence of EGF when added with FGF2 (Fig. 5G). There was no significant difference in the proliferation rate between WT or mutant cells when the cells were stimulated with EGF alone (Fig. 5H).
Fig. 5.
Decreased proliferation of NSPCs derived from E14.5 Frs2α2F/2F telencephalon is seen in response to FGF2 plus EGF but not in response to EGF alone. (A and B) Number of primary neurospheres cultured in the presence of FGF2 (20 ng/ml) plus EGF (20 ng/ml) (A) and EGF (20 ng/ml) alone (B) (n = 3 for WT, and n = 3 for Frs2α2F/2F; *, P < 0.05 vs. WT control). (C and D) Diameters of the secondary neurospheres of the indicated genotypes cultured in the presence of FGF2 plus EGF (C) and EGF alone (D)(n = 3 for WT, and n = 3 for Frs2α2F/2F; *, P < 0.05 vs. WT control). (E and F) Number of secondary neurospheres was counted and indicated as mean ± SD after the primary neurospheres were dissociated and 5 × 102 cells per well were plated and cultured in the same growth factors, FGF2 (20 ng/ml) plus EGF (20 ng/ml) (E) or EGF (20 ng/ml) alone (F) for 12–13 days (n = 3 for WT, and n = 3 for Frs2α2F/2F). (G and H) MTS assay during secondary neurosphere formation in the presence of FGF2 (20 ng/ml) plus EGF (20 ng/ml) (G) or EGF (20 ng/ml) alone (H)(*, P < 0.05; **, P < 0.005 vs. WT control). WT is represented by black bars and Frs2α2F/2F is represented by gray bars in A–F. WT is represented by black lines and Frs2α2F/2F is represented by dashed lines in G and H.
Finally, we used a neurosphere differentiation assay to analyze multipotency of neurosphere cells cultured on plates that were precoated with poly-d-lysine in the absence of growth factors. Under these conditions, cells differentiate into both neuronal and glial cell lineages (25). After 7 days, these cells were immunostained with TuJ1 (a neuron-specific isoform of β-tubulin), anti-O4 (oligodendrocyte marker), or anti-GFAP (glial fibrillary acidic protein, an astrocyte marker) antibodies. As reported previously, cells in >90% of WT neurospheres differentiated into various lineages, including neurons, astrocytes, and oligodendrocytes (25). A similar percentage of mutant neurospheres also contained cells that had differentiated into the various lineages (97.4 ± 4.4%). These results clearly demonstrate that the Shp2-binding sites of FRS2α are specifically required for efficient proliferation of cultured NSPCs in response to FGF2 but not EGF and are not essential for self-renewing activity of these cells. Taken together, these experiments demonstrate that activation of the ERK signaling pathway mediated by Shp2-binding sites on FRS2α is likely to be essential for the proliferation of the intermediate progenitor cells in vivo and cultured NSPCs but not for the self-renewal of neural stem cells.
Discussion
In the present study, we demonstrate that the Shp2-binding sites of FRS2α play a critical role in mammalian corticogenesis.
The Shp2-Binding Sites of FRS2α Are Essential for Maintenance of Intermediate Progenitor Cells in the SVZ. On the basis of our present results, it is likely that neural stem cells proliferate normally in the brains of Frs2α2F/2F mice. At E11.5, most cells in the neuroepithelium are immature neural progenitor cells that divide symmetrically. By E12.5, radial glial cells, which are also neural stem cells, arise and become the major cell population in the VZ (4, 6). The BrdUrd pulse-labeling experiments revealed that mutant cells proliferated at a rate similar to that of WT cells at E10.5–12.5 (Fig. 2 A–E). At E14.5, there was no significant difference between WT and mutant in the number of mitotic cells stained by anti-phosphohistone H3 antibodies in the apical side of the VZ, where neural stem cells divide. Because neural stem cells proliferate extensively during this period, it is likely that their proliferative activity remains normal (Fig. 2 F–N).
In contrast, at E14.5, in mutant brains, the number of mitotic cells in the SVZ, where the intermediate progenitor cells divide (7–9), was significantly lower than that of WT brains (Fig. 2 F–P). In mutant brains, the number of Tbr2+ cells was reduced, and expression levels of PCNA in cells expressing low levels of Pax6 in the SVZ was also reduced (Fig. 3 C–I). These results suggest that the intermediate progenitor cells in Frs2α2F/2F brain are not fully functional and are not able to enter the S phase efficiently. Therefore, a reasonable possibility is that intermediate progenitor cells depend on signaling mediated through the Shp2-binding sites of FRS2α to maintain their function as neuronal progenitors.
More than a half of cortical neurons are derived from the dorsal telencephalon through radial migration routes. In addition, cortical interneurons arise from the medial GE, which is derived from the ventral telencephalon through tangential migration routes (11). We found that cell division in the SVZ of the medial GE was decreased in mutant brain (Fig. 2 G, I, and P). The decreased cell division in the SVZ of the medial GE may also be a reason for the reduction in cortical cell numbers in the mutant brain (Fig. 1G), although the existence of intermediate progenitor cells in the GE has not yet been confirmed. Furthermore, there was no stratification in the CP of Frs2α2F/2F cortex at E18.5 (Fig. 1 D and F). It is possible that neurons from mutant mice are not able to normally migrate, as reported for TrkB mutant mice (see discussion below). It is also possible that the differentiation of specific neuronal subtypes in the cortex of Frs2α2F/2F mice is compromised, resulting in impaired cortical stratification. Our results suggest that even with intact proliferation of neural stem cells in the VZ, the failure of intermediate progenitor cell division in the SVZ leads to profound defects in corticogenesis.
Potential Upstream Regulators and Downstream Effectors of FRS2α-Dependent Corticogenesis. Several FGFs, FGF receptors, neurotrophins, and neurotrophin receptors are expressed in the VZ/SVZ (13, 35, 36). A few of the mice carrying targeted mutations of these genes are reported to have defects in the early stages of development of the cerebral cortex. FGF2-/- mice have fewer cortical neurons but normal cortical stratification with no difference in the thickness of the cerebral cortex (37, 38). This phenotype is due to reduced proliferation of neural stem cells in the VZ by E11.5. Embryos carrying a conditional deletion of the TrkB allele using nestin enhancer-driven Cre recombinase or point mutations on phospholipase C-γ and Shc/FRS2-binding sites of TrkB showed defects in migration of newly born cortical neurons, resulting in mild reductions in the thickness of the cerebral cortex (39). These phenotypes are both milder than that of Frs2α2F/2F mice. It is noteworthy that in addition to FGF2, FGF11 is expressed in the VZ. In addition, the expression of TrkC in the developing cerebral cortex overlaps the expression pattern of TrkB in this region, suggesting that products of these genes may compensate for each other's loss (13, 36).
We previously reported the expression patterns of Frs2α and Frs2β during brain development by using in situ hybridization with specific probes (19). Frs2α is expressed diffusely in neuroepithelium as early as E9.5, whereas Frs2β expression is first detectable at ≈E11.5. At E11.5, Frs2α but not Frs2β is expressed at high levels in the VZ of any region of the nervous system. A similar pattern of expression is observed at E14.5; Frs2α but not Frs2β is expressed strongly in the VZ and SVZ of telencephalon. At E18.5, both genes are abundantly expressed in the developing CP, and Frs2β is strongly expressed in basal ganglia, thalamus, and hypothalamus. Therefore, it is unlikely that FRS2β compensates for the loss of FRS2α function in VZ/SVZ, because FRS2β is expressed at relatively low levels in this region (19).
It was reported that FGF2-/- mice had mild defects in proliferation of neural stem cells at early stages (38, 40). The differences between the phenotypes of FGF2-/- mice and Frs2α2F/2F mice suggest that activation of different pathways from those mediated through Shp2-binding sites of FRS2α in FGF2 signaling is sufficient for proliferation of neural stem cells during normal development. However, the intermediate progenitor cells are strongly dependent on pathways emanating from the Shp2-binding site but not from Grb2-binding sites of FRS2α. Given that FGF2 and FGF11 are expressed in the VZ, where neural stem cells and the intermediate progenitor cells are localized, a reasonable assumption is that FGF2 and FGF11 are likely to induce tyrosine phosphorylation of FRS2α in both cells. Moreover, if there is a defect in migration of neurons for cortical stratification in Frs2α2F/2F mice, it may be due, at least in part, to impaired TrkB signaling. It is possible that FRS2α functions downstream of isoforms of FGFRs that are activated by FGF2 and FGF11 as well as downstream of TrkB and TrkC during the process of corticogenesis.
Consistent with our previous finding that the Shp2-binding sites of FRS2α are primarily responsible for activation of ERK (24), Frs2α2F/2F brains showed reduced ERK activation (Fig. 4A). In addition, ERK activation in mutant brains was higher at E14.5 than at E11.5 (Fig. 4A), probably because at E14.5, ERK activation is mediated in part by FRS2α-independent mechanisms. Indeed, EGF-responsive NSPCs arise by this stage. Thus, activation of ERK through FRS2α may regulate expression of Musashi-1 and Tbr2 and proliferation of intermediate progenitor cells in VZ/SVZ.
Shp2-Binding Sites of FRS2α Play Important Roles in Cultured NSPCs Proliferation. We found that the number of primary neurospheres derived from Frs2α2F/2F brain were reduced as compared with those derived from WT mice that were stimulated with FGF2 alone, EGF alone or in the presence of both FGF2 and EGF (Figs. 4C and 5 A and B). However, there were no significant differences in the frequency of secondary neurosphere formation induced by the same stimuli (Figs. 4D and 5 E and F). Furthermore, although neural stem cells in Frs2α2F/2F brain appeared to be fully competent in cell proliferation during development in vivo, the Frs2α2F/2F neurosphere cells showed defects in proliferation in response to FGF2 but not EGF (Figs. 4E and 5 C, D, G, and H).
Although it has been shown that some progenitor cells in early postnatal and adult brain, behave like stem cells in vitro, generating neurospheres and differentiating into neurons and glia, the existence of such cells in fetal neurosphere cells and brain has not yet been confirmed (33, 34). However, from our findings it is reasonable to raise a hypothesis that such multipotent progenitor cells but not neural stem cells require signals mediated by the Shp2-binding sites of FRS2α for the maintenance of their stem cell potential. Indeed, cells in the VZ are composed of heterogeneous cell populations as evidenced by expression of several transcription factors, supporting the idea that multipotent progenitor cells reside in the VZ in addition to the neural stem cells and intermediate progenitor cells (7, 27). If so, multipotent progenitor cells in addition to the lineage-restricted intermediate progenitor cells may not be fully functional in mutant brain, resulting in reduction of primary neurosphere formation. Reduced expression of Musashi-1 and nestin that was detected in the VZ/SVZ of mutant brains (Fig. 3 A and B and data not shown) is in accord with this hypothesis, because loss of function of both Musashi-1 and -2 in vitro resulted in reduction of neurosphere formation and cell proliferation (41). During the course of differentiation from radial glial cells and neural stem cells, multipotent progenitor cells to intermediate progenitor cells, signaling mediated through the Shp2-binding sites of FRS2α may play important roles in the function of the latter two cell types. Alternatively, small defects in proliferating activity and/or survival of the mutant stem and/or multipotent progenitor cells in response to FGF2 may exert a significant influence only on the primary initiation of neurosphere formation in the presence of FGF2 or FGF2 plus EGF. In fact, the Frs2α2F/2F neurosphere cells retain their self-renewing capacity, indicating that the mechanism underlying self-renewal of cultured neural stem cells does not depend on the integrity of Shp2-binding sites on FRS2α, a notion that is consistent with the in vivo results as well.
Supplementary Material
Acknowledgments
We thank R. Nishinakamura for the protocol of the neurosphere assay. This work was supported by Grant-in-Aid for Scientific Research 1458069 (to N.G.); Grant-in-Aid Special Project Research on Cancer-Bioscience 12215024 (to M.S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant from the program “Research for the Future” of the Japan Society for Promotion of Science (to M.S.); National Institutes of Health Grants R01 AR051448 and R01 AR051886; and funds from the Ludwig Institute for Cancer Research (to J.S.).
Author contributions: S.Y., J.S., and N.G. designed research; S.Y., I.Y., T.S., M.M., R.F.H., I.L., H.O., M.S., and N.G. performed research; S.Y. analyzed data; and S.Y., J.S., and N.G. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: VZ, ventricular zone; SVZ, subventricular zone; CP, cortical plate; En, embryonic day n; NSPC, neural stem/progenitor cell; ERK, extracellular signal-regulated kinase; PCNA, proliferating cell nuclear antigen; GE, ganglionic eminence.
References
- 1.Okano, H. (2002) J. Neurosci. Res. 69, 698-707. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla, A., Garcia-Verdugo, J. M. & Tramontin, A. D. (2001) Nat. Rev. Neurosci. 2, 287-293. [DOI] [PubMed] [Google Scholar]
- 3.Rao, M. S. (1999) Anat. Rec. 257, 137-148. [DOI] [PubMed] [Google Scholar]
- 4.Malatesta, P., Hack, M. A., Hartfuss, E., Kettenmann, H., Klinkert, W., Kirchhoff, F. & Gotz, M. (2003) Neuron 37, 751-764. [DOI] [PubMed] [Google Scholar]
- 5.Fishell, G. & Kriegstein, A. R. (2003) Curr. Opin. Neurobiol. 13, 34-41. [DOI] [PubMed] [Google Scholar]
- 6.Anthony, T. E., Klein, C., Fishell, G. & Heintz, N. (2004) Neuron 41, 881-890. [DOI] [PubMed] [Google Scholar]
- 7.Miyata, T., Kawaguchi, A., Saito, K., Kawano, M., Muto, T. & Ogawa, M. (2004) Development (Cambridge, U.K.) 131, 3133-3145. [DOI] [PubMed] [Google Scholar]
- 8.Noctor, S. C., Martinez-Cerdeno, V., Ivic, L. & Kriegstein, A. R. (2004) Nat. Neurosci. 7, 136-144. [DOI] [PubMed] [Google Scholar]
- 9.Haubensak, W., Attardo, A., Denk, W. & Huttner, W. B. (2004) Proc. Natl. Acad. Sci. USA 101, 3196-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luskin, M. B. (1993) Neuron 11, 173-189. [DOI] [PubMed] [Google Scholar]
- 11.Letinic, K., Zoncu, R. & Rakic, P. (2002) Nature 417, 645-649. [DOI] [PubMed] [Google Scholar]
- 12.Hatten, M. E. (2002) Science 297, 1660-1663. [DOI] [PubMed] [Google Scholar]
- 13.Reuss, B. & von Bohlen und Halbach, O. (2003) Cell Tissue Res. 313, 139-157. [DOI] [PubMed] [Google Scholar]
- 14.Cameron, H. A., Hazel, T. G. & McKay, R. D. (1998) J. Neurobiol. 36, 287-306. [PubMed] [Google Scholar]
- 15.Henderson, C. E. (1996) Curr. Opin. Neurobiol. 6, 64-70. [DOI] [PubMed] [Google Scholar]
- 16.Schlessinger, J. (2000) Cell 103, 211-225. [DOI] [PubMed] [Google Scholar]
- 17.Hadari, Y. R., Gotoh, N., Kouhara, H., Lax, I. & Schlessinger, J. (2001) Proc. Natl. Acad. Sci. USA 98, 8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lax, I., Wong, A., Lamothe, B., Lee, A., Frost, A., Hawes, J. & Schlessinger, J. (2002) Mol. Cell 10, 709-719. [DOI] [PubMed] [Google Scholar]
- 19.Gotoh, N., Laks, S., Nakashima, M., Lax, I. & Schlessinger, J. (2004) FEBS Lett. 564, 14-18. [DOI] [PubMed] [Google Scholar]
- 20.Kouhara, H., Hadari, Y. R., Spivak-Kroizman, T., Schilling, J., Bar-Sagi, D., Lax, I. & Schlessinger, J. (1997) Cell 89, 693-702. [DOI] [PubMed] [Google Scholar]
- 21.Huang, L., Gotoh, N., Zhang, S., Shibuya, M., Yamamoto, T. & Tsuchida, N. (2004) Biochem. Biophys. Res. Commun. 324, 1011-1017. [DOI] [PubMed] [Google Scholar]
- 22.Ong, S. H., Hadari, Y. R., Gotoh, N., Guy, G. R., Schlessinger, J. & Lax, I. (2001) Proc. Natl. Acad. Sci. USA 98, 6074-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotoh, N., Manova, K., Tanaka, S., Murohashi, M., Hadari, Y., Lee, A., Hamada, Y., Hiroe, T., Ito, M., Kurihara, T., et al. (2005) Mol. Cell. Biol. 25, 4105-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotoh, N., Ito, M., Yamamoto, S., Yoshino, I., Song, N., Wang, Y., Lax, I., Schlessinger, J., Shibuya, M. & Lang, R. A. (2004) Proc. Natl. Acad. Sci. USA 101, 17144-17149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura, Y., Sakakibara, S., Miyata, T., Ogawa, M., Shimazaki, T., Weiss, S., Kageyama, R. & Okano, H. (2000) J. Neurosci. 20, 283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko, Y., Sakakibara, S., Imai, T., Suzuki, A., Nakamura, Y., Sawamoto, K., Ogawa, Y., Toyama, Y., Miyata, T. & Okano, H. (2000) Dev. Neurosci. 22, 139-153. [DOI] [PubMed] [Google Scholar]
- 27.Englund, C., Fink, A., Lau, C., Pham, D., Daza, R. A., Bulfone, A., Kowalczyk, T. & Hevner, R. F. (2005) J. Neurosci. 25, 247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnavelas, J. G. (2000) Trends Neurosci. 23, 126-131. [DOI] [PubMed] [Google Scholar]
- 29.Tarabykin, V., Stoykova, A., Usman, N. & Gruss, P. (2001) Development (Cambridge, U.K.) 128, 1983-1993. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer, C., Tiveron, M. C., Bodmer, R. & Cremer, H. (2004) Cereb. Cortex 14, 1408-1420. [DOI] [PubMed] [Google Scholar]
- 31.Tropepe, V., Sibilia, M., Ciruna, B. G., Rossant, J., Wagner, E. F. & van der Kooy, D. (1999) Dev. Biol. 208, 166-188. [DOI] [PubMed] [Google Scholar]
- 32.Martens, D. J., Tropepe, V. & van Der Kooy, D. (2000) J. Neurosci. 20, 1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belachew, S., Chittajallu, R., Aguirre, A. A., Yuan, X., Kirby, M., Anderson, S. & Gallo, V. (2003) J. Cell Biol. 161, 169-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, S. J. (2001) Curr. Opin. Cell Biol. 13, 666-672. [DOI] [PubMed] [Google Scholar]
- 35.Peters, K. G., Werner, S., Chen, G. & Williams, L. T. (1992) Develpment (Cambridge, U.K.) 114, 233-243. [DOI] [PubMed] [Google Scholar]
- 36.Tessarollo, L., Tsoulfas, P., Martin-Zanca, D., Gilbert, D. J., Jenkins, N. A., Copeland, N. G. & Parada, L. F. (1993) Development (Cambridge, U.K.) 118, 463-475. [DOI] [PubMed] [Google Scholar]
- 37.Ortega, S., Ittmann, M., Tsang, S. H., Ehrlich, M. & Basilico, C. (1998) Proc. Natl. Acad. Sci. USA 95, 5672-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccarino, F. M., Schwartz, M. L., Raballo, R., Nilsen, J., Rhee, J., Zhou, M., Doetschman, T., Coffin, J. D., Wyland, J. J. & Hung, Y. T. (1999) Nat. Neurosci. 2, 246-253. [DOI] [PubMed] [Google Scholar]
- 39.Medina, D. L., Sciarretta, C., Calella, A. M., von Bohlen und Halbach, O., Unsicker, K. & Minichiello, L. (2004) EMBO J. 23, 3803-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raballo, R., Rhee, J., Lyn-Cook, R., Leckman, J. F., Schwartz, M. L. & Vaccarino, F. M. (2000) J. Neurosci. 20, 5012-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakakibara, S., Nakamura, Y., Yoshida, T., Shibata, S., Koike, M., Takano, H., Ueda, S., Uchiyama, Y., Noda, T. & Okano, H. (2002) Proc. Natl. Acad. Sci. USA 99, 15194-15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.