Abstract
Porphyromonas gingivalis is an oral pathogen that has recently been associated with chronic inflammatory diseases such as atherosclerosis. The strength of the epidemiological associations of P. gingivalis with atherosclerosis can be increased by the demonstration that P. gingivalis can initiate and sustain growth in human vascular cells. We previously established that P. gingivalis can invade aortic, heart, and human umbilical vein endothelial cells (HUVEC), that fimbriae are required for invasion of endothelial cells, and that fimbrillin peptides can induce the expression of the chemokines interleukin 8 and monocyte chemotactic protein. In this study, we examined the expression of surface-associated cell adhesion molecules on endothelial cells in response to P. gingivalis infection by fluorescence-activated cell sorting FACS analysis and confocal microscopy. Coculture of HUVEC with P. gingivalis strain 381 or A7436 resulted in the induction in the expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and P- and E-selectins, which was maximal at 48 h postinfection. In contrast, we did not observe induction of ICAM-1, VCAM-1, or P- or E-selectin expression in HUVEC cultured with the noninvasive P. gingivalis fimA mutant DPG3 or when P. gingivalis was incubated with fimbrillin peptide-specific anti-sera prior to the addition to HUVEC. Furthermore, the addition of a peptide corresponding to the N-terminal domain of fimbrillin to HUVEC resulted in an increase in ICAM-1, VCAM-1, and P- and E-selectins, which was maximal at 48 h and similar to that observed for live P. gingivalis. Treatment of P. gingivalis-infected HUVEC with cytochalsin D, which prevented P. gingivalis invasion, also resulted in the inhibition of ICAM-1, VCAM-1, or P- and E-selectin expression. Taken together, these results indicate that active P. gingivalis invasion of HUVEC mediated via the major fimbriae stimulates surface-associated cell adhesion molecule expression. Stimulation of adhesion molecules involved in the recruitment of leukocytes to sites of inflammation by P. gingivalis may play a role in the pathogenesis of systemic inflammatory diseases associated with this microorganism, including atherosclerosis.
Porphyromonas gingivalis is an oral pathogen that causes a chronic local inflammatory disease (periodontal disease), which results in the destruction of the periodontal ligament and alveolar bone (17, 38). Destruction of host tissues results from the action of various toxic products released by P. gingivalis, as well as from the host responses elicited against this organism (5). The induction of this inflammatory response can lead to gingival ulceration and local vascular changes, which have the potential to increase the incidence and severity of transient bacteremias (12, 30). Based on the chronic nature of this disease, and the exuberant local and systemic host response to P. gingivalis infection (5, 30), recent studies have focused on the association of P. gingivalis-mediated periodontal infection and systemic diseases. Several reports support a definite relationship between periodontal infections and certain systemic conditions including atherosclerosis and cardiovascular disease (2, 3, 12, 22, 42).
Recent pathological studies have identified P. gingivalis in diseased atherosclerotic tissue by PCR (14). P. gingivalis infection of apoE mice has also been shown to increase the mean area and the extent of atherosclerotic lesions histologically relative to those in uninfected animals (4). The strength of the epidemiological and initial pathological associations of P. gingivalis with atherosclerosis can be increased by the demonstration that P. gingivalis can initiate and sustain growth in human vascular cells. We have previously demonstrated that P. gingivalis can invade and replicate in endothelial cells, indicating that this pathogen has the capability of localizing to the vascular wall (9, 10). Endothelial cells, among other vascular wall cells, may serve as reservoirs of P. gingivalis and P. gingivalis components and as contributors to immune stimulation during P. gingivalis infection. It has been proposed that P. gingivalis invasion of endothelial cells may induce alterations in the endothelial cell that could exhibit atherogenic properties (9). However, it is not clear how active invasion of endothelial cells by P. gingivalis modulates the inflammatory response of these cells.
A hallmark of atherosclerosis is the accumulation of blood-borne leukocytes in the inflamed tissues in response to antigenic stimulation (28). This process is initiated with the binding of leukocytes to the activated endothelium via the induced expression of cell adhesion molecules, including intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and P- and E-selectins (7, 21, 29, 36). Leukocyte chemotaxis and migration across the endothelium are modulated by several chemokines, including interleukin (IL)-8 and monocyte chemotactic protein (MCP)-1 which have specificites for neutrophils and monocytes, respectively (11, 13). Since the vascular endothelium is essential for the recruitment of leukocytes during atherogenesis, studies aimed at the inflammatory activation of endothelial cells by P. gingivalis may elucidate the role of this organism in atherosclerosis. We have recently demonstrated that P. gingivalis outer membrane components, including peptides corresponding to the N-terminal region of fimbrillin, can induce the expression of both IL-8 and MCP-1 in human endothelial cells (26). In this study, we examine the expression of the surface-associated cell adhesion molecules ICAM-1, VCAM-1, and E- and P-selectins following infection of endothelial cells with P. gingivalis. In addition, we define the role of P. gingivalis adherence mediated via fimbriae, in the induction of cell adhesion molecule expression in endothelial cells.
MATERIALS AND METHODS
Bacterial strains.
P. gingivalis A7436 was originally isolated from a patient with refractory periodontitis and was characterized in our laboratory (10). P. gingivalis wild-type strain 381 and the corresponding fimA mutant DPG3 have been described previously (24). P. gingivalis A7436 and 381 were grown on anaerobic blood agar plates (BBL media; Becton Dickinson Co., Cockeysville, Md.), and DPG3 was grown on anaerobic blood agar containing erythromycin (10 μg/ml) in an anaerobic chamber with 85% N2, 10% CO2, and 5% H2 for 3 to 5 days. The cultures were then inoculated into fresh Schaedler broth (Difco, Sparks, Md.) (supplemented with erythromycin for DPG3) and grown for 24 h or until the optical density at 660 nm reached 1.0.
Cell culture and infection of HUVEC with P. gingivalis.
First-passage human umbilical vein endothelial cells (HUVEC; Cascade Biologics Inc., Portland, Org.) cultures were maintained in media-200 supplemented with low-serum-growth supplement (20 μl/ml) (Cascade Biologics, Inc.) at 37°C in 5% CO2 in tissue culture flasks. For infection studies, cells were passaged into six-well dishes with 0.25% trypsin and EDTA (0.02%). For confocal microscopy, cells were grown on coverslips placed on the bottom of wells. Confluent fourth-passage cells were used in all experiments. HUVEC were plated at a concentration of 105 to 106 cells/ml, as determined by cell counting with a hemocytometer. The multiplicity of infection (MOI) was calculated based on the number of cells per well at confluence. P. gingivalis strains A7436, 381, and DPG3 grown to an optical density of 1.0 were centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in HUVEC growth media at a final concentration of 108 cells per ml (10). Bacterial suspensions (1 ml) were added to confluent HUVEC monolayers at an MOI of 1:100 and incubated at 37° in 5% CO2 for 2, 24, or 48 h. Control cultures were incubated with medium alone. Bacterial adherence and invasion were determined as described previously (10). At different times, HUVEC cultures were harvested and processed for fluorescence-activated cell sorting (FACS) analysis and confocal microscopy using fluorescein isothiocyanate (FITC)-labeled antibodies specific for ICAM-1, VCAM-1, and P- or E-selectin expression (see below). All assays were performed in triplicate. Viability of the endothelial cultures was monitored by either Trypan blue staining or with an Annexin V apoptosis detection kit (Vibrant Apoptosis; Molecular Probes, Eugene, Org.).
Role of bacterial adherence and invasion in the induction of surface-associated cell adhesion molecule expression.
The role of bacterial adherence in P. gingivalis-mediated cell adhesion molecule expression was examined by using the P. gingivalis fimA mutant (DPG3) or by preincubating P. gingivalis with fimbrillin peptide-specific antisera. We have previously established that preincubation of P. gingivalis with fimbria-specific antisera inhibits P. gingivalis invasion of HUVEC (10). Likewise, Sojar et al. (39) have established that preincubation of P. gingivalis with specific anti-fimbrillin peptide sera inhibits P. gingivalis invasion of oral epithelial cells. Thus, to further define the role of fimbriae on the induction of cell adhesion expression, P. gingivalis was preincubated with rabbit polyclonal anti-fimbrillin peptide sera or a normal rabbit serum control (1/500 dilution) for 60 min prior to infection of HUVEC. We utilized polyclonal anti-fimbrillin peptide sera corresponding to amino acids 49 to 68 (VVMANTAGAMELVGKTLAEVK) and 69 to 90 (ALTTELTAENQEAAGLIMTAEP) of the mature fimbrillin protein as described previously (39). To examine the effects of invasion on cell adhesion molecule expression in response to P. gingivalis, we preincubated HUVEC with cytochalsin D (1 μg/ml in dimethylsulfoxide) for 30 min, followed by washing with PBS. Fresh medium was then added to the HUVEC together with P. gingivalis A7436. We previously determined that cytochalsin D treatment effectively abolishes P. gingivalis invasion of HUVEC (10). At designated times, cells were processed for FACS analysis and confocal microscopy using FITC-labeled antibodies specific for ICAM-1, VCAM-1 and P/E-selectin expression (see below).
Preparation of P. gingivalis and Escherichia coli LPS.
P. gingivalis and E. coli lipopolysaccharide (LPS) extraction was prepared by a hot phenol-water technique (41). LPS preparations were analyzed for protein contamination by electrophoresis by overloading a sodium dodecylsulfate-12.5% polyacrylamide gel stained with Coomassie blue and silver nitrate. LPS samples were also examined on commercially prepared 10 to 20% gradient gels. LPS was further analyzed for protein contamination by a bicinchoninic acid protein assay (Pierce, Rockford, Ill.). For HUVEC stimulation assays, LPS samples were diluted, sonicated in HUVEC culture media, and added to HUVEC cultures (see below).
Fimbrillin peptides.
Fimbrillin peptides based on the amino acid sequence of the native fimbrillin of P. gingivalis strain 381 and corresponding to amino acids 49 to 68 (VVMANTAGAMELVGKTLAEVK) and 171 to 185 (DANYLTGSLTTFNGA), as well as peptides corresponding to a scrambled version of each peptide to be tested, were commercially synthesized (BioSynthesis Inc., Lewisville, Tex.). All peptides were determined to be free of contaminating endotoxin by high-pressure liquid chromatography analysis as indicated by the manufacturer (BioSynthesis). Peptides were diluted to final concentrations of 2 and 10 μg/ml and added to HUVEC as described below. After the designated incubation time, HUVEC cultures were harvested and processed for FACS analysis and confocal microscopy.
FACS analysis.
For FACS analysis, cells were grown in triplicate in six-well dishes. Confluent fourth-passage HUVEC were incubated with P. gingivalis, P. gingivalis outer membrane components, or under the various conditions described above. Cells were then dissociated with trypsin and EDTA and processed for labeling with anti-ICAM-1, anti-VCAM-1, anti-E-selectin, and anti-P-selectin rabbit polyclonal antibodies (1:100 dilution; Serotec, Raleigh, N.C.) on ice as described by the manufacturer. FITC-conjugated isotope-specific immunoglobulin G (IgG; Serotec, Kidlington, Oxford, United Kingdom) was used as a negative control, and we did not observe reactivity with this IgG. Cells were labeled for 1 h with FITC-labeled primary antibodies (1:500), washed with PBS-1% bovine serum albumin, and fixed with 0.5% formaldehyde as described by the manufacturer. With a FACScan (Becton Dickinson, Sparks, Md.) flow cytometer, HUVEC were gated by forward- and side-scatter settings that were optimized with the use of autofluorescence of untreated HUVEC, and debris were excluded by a hardware gate on forward scatter. Only viable cells, typically 75 to 90% of the starting cultures as determined by Annexin V staining, were analyzed.
Confocal microscopy.
For immunofluorescence studies, HUVEC were grown on cover slips placed on the bottom of six well culture dishes. After incubation for designated times, the HUVEC monolayers were washed and fixed with ice-cold methanol-acetone (1:1) at −20°C. Fixed cells on coverslips were reacted with anti-ICAM-1, anti-VCAM-1 anti-E-selectin, and anti-P-selectin sera (1:100 dilution) for 45 min at room temperature, and washed three times with PBS. Coverslips were then inverted onto a slide containing 10 μl of Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, Calif.). Immunostaining was performed on ice, and slides were kept in the dark until analyzed by confocal microscopy with an Axiovert 100M Carl Zeiss Lazer Scanning Microscope (model LSM 500, version 2.5 S-12, Carl Zeiss Co., Heidelberg, Germany).
Statistical analysis.
To evaluate statistical significance of differences for adhesion molecules and selectins expression between experimental groups, analysis of variance with Fisher protected least significant difference and Bonferroni Dunn Post Hoc analysis were performed with the use of the StatView statistical program (SAS, Inc., Cary, N.C.) Statistical significance was assigned when the P value was <0.05 or < 0.001, as indicated in each figure.
RESULTS
P. gingivalis stimulates ICAM-1, VCAM-1, P-selectin, and E-selectin expression in endothelial cells.
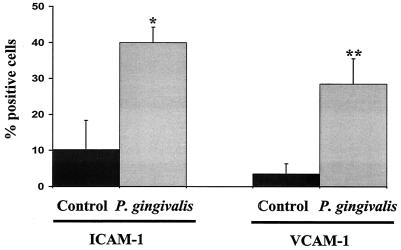
To determine whether infection of endothelial cells by P. gingivalis could stimulate the expression of surface-associated cell adhesion molecules, we incubated HUVEC with P. gingivalis strain A7436 and monitored cell adhesion molecule expression by FACS analysis and confocal microscopy. In preliminary experiments, we determined that the antisera used for the detection of ICAM-1, VCAM-1, P-selectin, and E-selectin did not react with P. gingivalis (data not shown). FACS analysis of P. gingivalis-infected HUVEC at 2 h postinfection demonstrated a 4- and 5.5-fold increase in ICAM-1 and VCAM-1 expression, respectively, compared to that in uninfected cultures (Fig. 1A). The stimulation of both ICAM-1 and VCAM-1 observed in P. gingivalis-infected cultures was statistically significant (P < 0.05 and P < 0.001, respectively) compared to that in uninfected HUVEC cultures. The expression of ICAM-1 and VCAM-1 in P. gingivalis-infected HUVEC was further confirmed by confocal microscopy. Following a 2-h incubation with P. gingivalis strain A7436, HUVEC cultures were found to exhibit extensive cell-associated staining by using either anti-ICAM-1 or anti-VCAM-1 serum (data not shown).
FIG. 1.
P. gingivalis infection stimulates ICAM-1 and VCAM-1 expression in HUVEC. P. gingivalis strain A7436 cultures were added to a HUVEC monolayer at an MOI of 100:1 and incubated at 37°C for 2 h. Cells were then collected and processed for FACS analysis for surface-associated ICAM-1 and VCAM-1. Values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC (control).
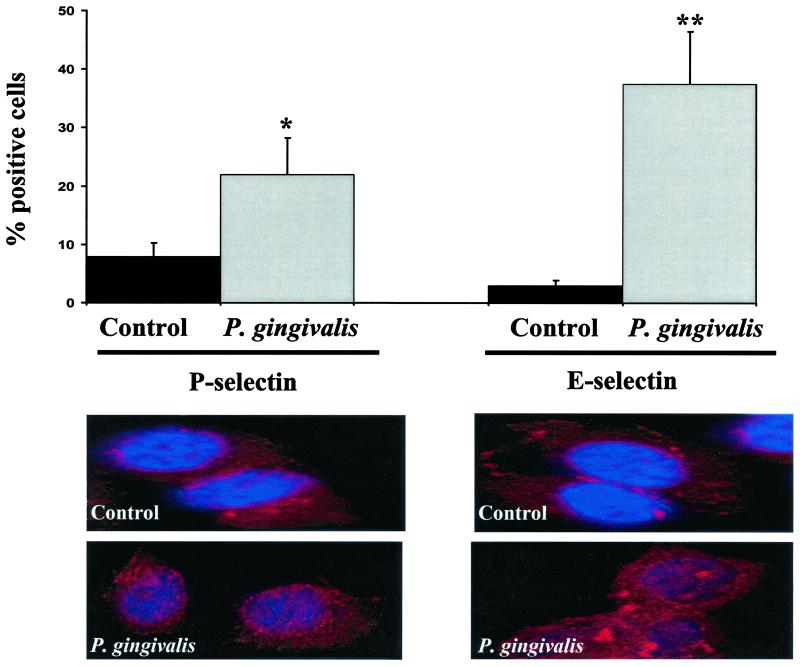
We also examined the expression of E- and P-selectins in P. gingivalis-infected HUVEC. In agreement with the results obtained for ICAM-1 and VCAM-1, we observed an increase in both cell surface-associated E- and P-selectin following the addition of P. gingivalis cultures to HUVEC detected at 2 h (Fig. 2A). This result was statistically significant for both P-selectin (P < 0.05) and E-selectin (P < 0.001). Expression of E- and P-selectin on the surface of P. gingivalis-infected HUVEC was confirmed by confocal microscopy. For these studies, P. gingivalis-infected HUVEC were also stained with TopRo3 as a nuclear counterstain. As shown in Fig. 2B, infection of HUVEC with P. gingivalis strain A7436 resulted in extensive cell-associated staining using anti-E- and P-selectin sera compared to that in uninfected HUVEC cultures. The upregulation of the cell adhesion molecules was most likely not mediated by tumor necrosis factor or IL-1β since these cytokines were not expressed in P. gingivalis-infected HUVEC culture supernatants (data not shown).
FIG. 2.
P. gingivalis infection stimulates E-selectin and P-selectin expression in HUVEC. P. gingivalis strain A7436 cultures were added to a HUVEC monolayer at an MOI 100:1 and incubated at 37°C for 2 h. (Top) Cells were collected and processed for FACS analysis for surface-associated E- and P-selectin. Values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC (control). (Bottom) Immunofluorescence staining of E- and P-selectin expression on HUVEC infected with P. gingivalis for 2 h. Fixed HUVEC on cover slips were incubated with R-phycoerythrin-conjugated P- and E-selectin-specific antisera and with TopRo3 as a nuclear counterstain.
The requirement for bacterial invasion of HUVEC for P. gingivalis-induced cell surface adhesion molecule expression was confirmed following treatment of HUVEC with cytochalsin D. We have previously established that pretreatment of HUVEC with cytochalsin D prevents uptake of P. gingivalis (10). Preincubation of HUVEC with cytochalsin D prior to the addition of P. gingivalis resulted in a 4-log decrease in the CFU associated with HUVEC compared to that in HUVEC without added cytochalsin D (Table 1). Expression of ICAM-1 and VCAM-1 was significantly decreased in P. gingivalis-infected HUVEC cultures which were exposed to cytochalsin D compared to expression in HUVEC without added cytochalsin D (data not shown). Taken together, these results demonstrate that infection of HUVEC by P. gingivalis induces the expression of cell surface-associated ICAM-1, VCAM-1, and E- and P-selectins.
TABLE 1.
Invasion of HUVEC by P. gingivalis
| Strain | Treatmenta | No. of bacteria (CFU)b |
|---|---|---|
| 381 | None | 3.2 × 106 ± 0.5 |
| 381 | NRS | 2.5 × 106 ± 0.4 |
| 381 | Anti-fimbrillin peptide sera | 1.5 × 102 ± 0.3c |
| 381 | Cytochalsin D | 1.1 × 102 ± 0.4d |
| DPG3 | None | 1.4 × 102 ± 0.2d |
P. gingivalis strain 381 was preincubated with distilled water (none), normal rabbit serum (NRS), or anti-fimbrillin peptide sera (corresponding to amino acid 69 to 90 of P. gingivalis fimbrillin), or pretreated with cytochalsin D prior to the addition of P. gingivalis as discussed in Materials and Methods.
Data are the total CFU associated with HUVEC and represent both bacteria adhering to and invading HUVEC at 48 h.
P <0.05 compared to P. gingivalis 381 treated with normal rabbit serum.
P <0.05 compared to P. gingivalis 381 without treatment.
Stimulation of cell adhesion molecule expression by P. gingivalis is dependent on fimbria-mediated adherence.
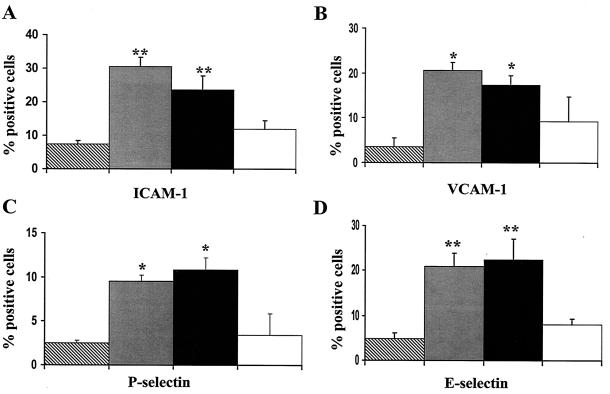
To determine whether adherence of P. gingivalis to HUVEC was required for the stimulation of cell adhesion molecule expression, we performed a series of experiments in which we blocked P. gingivalis adherence to HUVEC and monitored the cell adhesion molecule response of the HUVEC. For these studies, we utilized antisera raised against the N-terminal region of P. gingivalis fimbrillin (corresponding to two separate regions and encomposing amino acids 49 to 68 or 69 to 90); both antiserum preparations have been shown to inhibit the binding of fimbriae and of P. gingivalis to epithelial cells (39). The anti-fimbrillin peptide sera was also shown to react with P. gingivalis fimbriae as shown by immunogold labeling and immunoblot analysis indicating that these peptide domains are exposed on the surface of fimbrillin. Furthermore, we demonstrated that a peptide corresponding amino acids 48 to 69 stimulated cell adhesion molecule expression in HUVEC (see below). The ability of the anti-fimbrillin peptide sera to inhibit P. gingivalis strain 381 invasion of HUVEC was confirmed by culturing the P. gingivalis associated with HUVEC (Table 1). Preincubation of P. gingivalis with fimbrillin-specific antisera (corresponding to amino acids 69 to 90) prior to culture with HUVEC resulted in a 4-log decrease in the CFU associated with HUVEC. Similar results were obtained with the antisera raised against amino acid 49 to 68 of fimbrillin (data not shown). However, preincubation of P. gingivalis with normal rabbit sera did not inhibit the ability of P. gingivalis to invade HUVEC (Table 1). As shown in Fig. 3, when P. gingivalis was preincubated with fimbrillin peptide-specific antisera (corresponding to amino acids 69 to 90) prior to the addition to HUVEC, we detected a significant decrease in the expression of ICAM-1 and E-selectin in HUVEC compared to that in HUVEC infected with P. gingivalis that was preincubated with normal rabbit sera. A similar decrease in the expression of VCAM-1 and P-selectin was observed in HUVEC infected with P. gingivalis that was preincubated with fimbrillin peptide-specific antisera compared to that in HUVEC infected with P. gingivalis that was preincubated with normal rabbit sera.
FIG. 3.
P. gingivalis fimbrillin peptide-specific antisera inhibits ICAM-1, VCAM-1, and E- and P-selectin expression in P. gingivalis-infected HUVEC. P. gingivalis strain 381 cultures were preincubated with P. gingivalis fimbrillin-specific antisera for 2 h under anaerobic conditions prior to the addition to HUVEC. Following a 48-h incubation, HUVEC were harvested and processed for FACS analysis for detection of ICAM-1 (A), VCAM-1 (B), P-selectin (C), and E-selectin (D). In uninfected HUVEC (diagonal bars), HUVEC infected with P. gingivalis (gray bars), P. gingivalis incubated with normal rabbit serum (black bars), and HUVEC infected with P. gingivalis preincubated with fimbrillin-specific antisera (open bars), values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC.
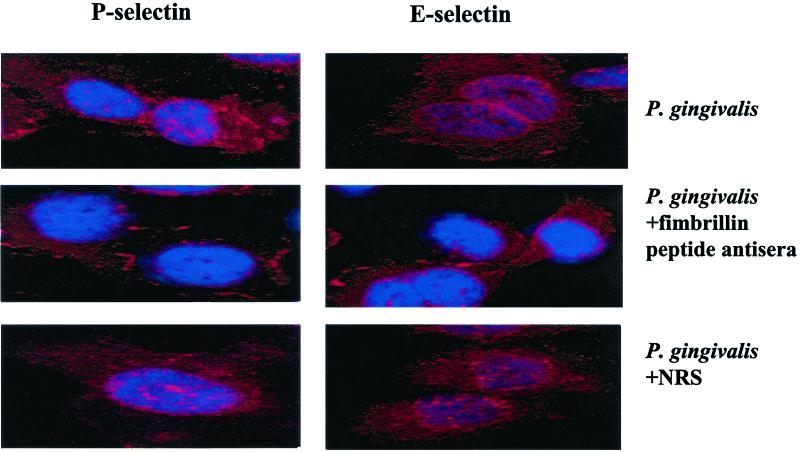
The expression of surface-associated cell adhesion molecules on HUVEC infected with P. gingivalis cultures that were preincubated with anti-fimbrillin peptide sera was further confirmed by confocal microscopy. Coculture of HUVEC with P. gingivalis strain 381 that was preincubated with normal rabbit serum resulted in extensive cell-associated staining using anti-ICAM-1, anti-VCAM-1, anti-E-selectin, and anti-P-selectin sera detected at 2 h postinfection (Fig. 4; data not shown). In contrast, we observed minimal staining for these cell adhesion molecules in HUVEC cocultured with P. gingivalis that was preincubated with fimbrillin peptide-specific antisera (Fig. 4; data not shown). These results indicate that blocking adherence of P. gingivalis mediated via the major fimbriae can effectively inhibit the expression of cell surface adhesion molecules seen in P. gingivalis-infected endothelial cells.
FIG. 4.
P. gingivalis fimbrillin peptide-specific antisera inhibits P- and E-selectin expression as detected by confocal microscopy. HUVEC were infected with P. gingivalis 381 for 2 h and processed for confocal microscopy for P- and E-selectin expression. HUVEC were stained with TopRo3 as a nuclear counterstain. HUVEC were infected with P. gingivalis, with P. gingivalis preincubated with fimbrillin peptide-specific antisera, or with P. gingivalis preincubated with normal rabbit serum (NRS) as indicated.
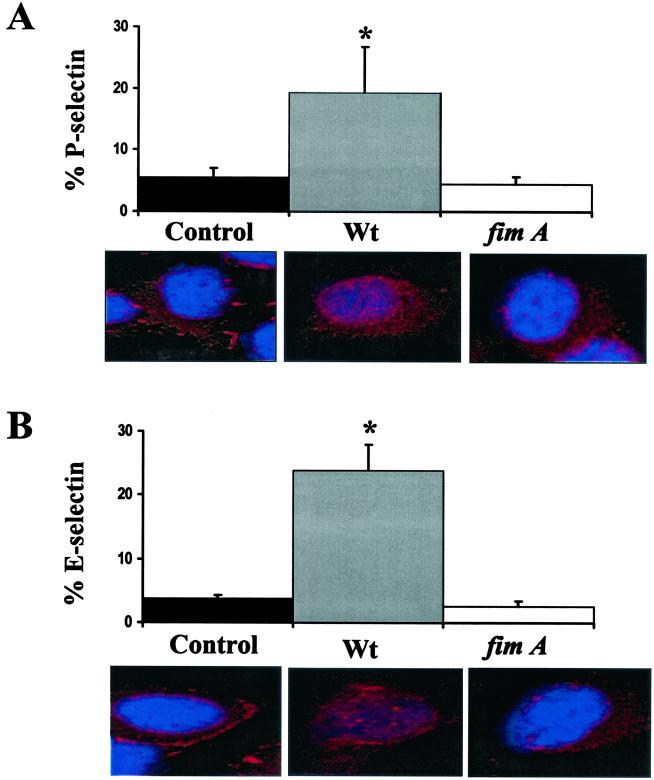
We further confirmed the role of P. gingivalis adherence in the stimulation of cell surface adhesion molecule expression following infection with a nonadherent, noninvasive P. gingivalis fimA mutant (DPG3). The nonadherent, noninvasive phenotype of P. gingivalis DPG3 was confirmed by culturing P. gingivalis associated with HUVEC (Table 1). P. gingivalis DPG3 exhibited a 4-log decrease in the CFU associated with HUVEC compared to that in wild-type strain 381. Likewise, we did not observe a significant increase in cell-associated E- or P-selectin expression in HUVEC infected with P. gingivalis strain DPG3 following a 2-h infection (Fig. 5A and C). As expected, we observed a significant increase (P < 0.05), in E- or P-selectin expression in the corresponding P. gingivalis wild-type strain 381 (Fig. 5A and C). These results were further confirmed by confocal microscopy. Infection of HUVEC with P. gingivalis wild-type strain 381 resulted in extensive cell-associated staining using anti- E- and P-selectin sera compared to that in uninfected HUVEC cultures. In contrast, we observed minimal staining for E- and P-selectin in P. gingivalis DPG3-infected HUVEC cultures (Fig. 5B and D).
FIG. 5.
Infection of HUVEC with a P. gingivalis fimA mutant does not stimulate P-selectin (A) and E-selectin (B) expression. HUVEC were infected with P. gingivalis strains 381 (wildtype [wt]) and DPG3 (fimA). Cells harvested at 2 h postinfection and processed for FACS analysis (upper panels) or for confocal microscopy (lower panels). Values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) compared to uninfected HUVEC.
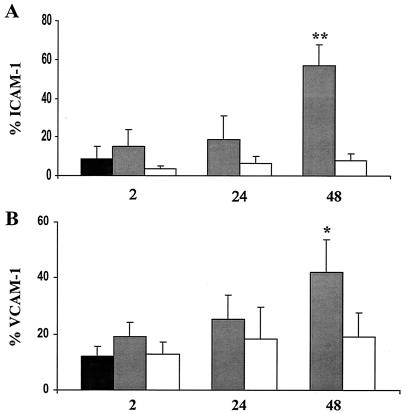
We observed similar results when we examined cell surface-associated VCAM-1 and ICAM-1 expression in HUVEC infected with P. gingivalis DPG3. We did not observe a significant increase in cell-associated ICAM-1 or VCAM-1 expression in HUVEC infected with P. gingivalis strain DPG3 even following a 48-h infection (Fig. 6). As expected, we observed a significant increase in VCAM-1 and ICAM expression in the corresponding P. gingivalis wild-type strain 381 (Fig. 6). These results were also confirmed by confocal microscopy (data not shown). Taken together, these results indicate that adherence of P. gingivalis to HUVEC mediate via fimbriae is required for the observed stimulation of cell adhesion molecules in P. gingivalis-infected HUVEC.
FIG. 6.
Infection of HUVEC with a P. gingivalis fimA mutant does not stimulate ICAM-1 and VCAM-1 expression. HUVEC were infected with P. gingivalis strains 381 and DPG3 (fimA); cells harvested at 2, 24, and 48 h postinfection and processed for FACS analysis for ICAM-1 (A) and VCAM-1 (B). For uninfected HUVEC (black bars), HUVEC infected with P. gingivalis strain 381 (gray bars), and HUVEC infected with P. gingivalis strain DPG3 (open bars), values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC.
P. gingivalis fimbrillin peptides stimulate ICAM-1, VCAM-1, and E- and P-selectin expression in HUVEC.
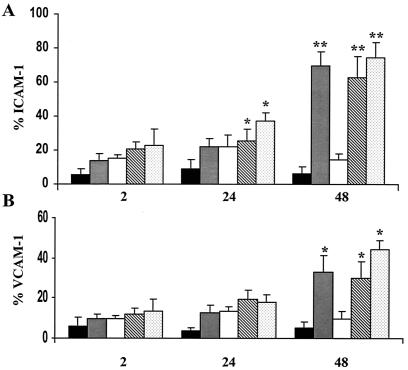
The results described above indicated that adherence of P. gingivalis to endothelial cells mediated via fimbriae could stimulate the production of surface-associated cell adhesion molecules. To determine whether fimbrillin itself could stimulate cell adhesion molecule expression in HUVEC, we utilized a peptide corresponding to the N-terminal region of the mature P. gingivalis fimbrillin protein. As shown above, uninfected HUVEC cultures were found to constitutively express low levels of surface-associated ICAM-1 and VCAM-1 (Fig. 7). The addition of a peptide corresponding to the N-terminal region of the mature P. gingivalis fimbrillin protein (amino acid 48 to 69) stimulated the expression of surface-associated ICAM-1 and VCAM-1, which was statistically significant at 48 h (Fig. 7). In contrast, HUVEC incubated with a scrambled peptide control expressed low levels of ICAM-1 and VCAM-1 (Fig. 7). The addition of P. gingivalis or E. coli LPS also stimulated the expression of surface-associated ICAM-1 and VCAM-1 (Fig. 7). Only a modest ICAM-1 and VCAM-1 response was observed with a peptide corresponding to amino acid 171 to 185 of the mature fimbrillin (data not shown).
FIG. 7.
P. gingivalis fimbrillin peptide stimulates ICAM-1 and VCAM-1 expression. HUVEC were incubated with media alone (black bars), a peptide corresponding to the N-terminal region of the P. gingivalis fimbrillin (amino acids 49 to 68; 10 μg/ml; gray bars), a scrambled peptide control (10 μg/ml; open bars), P. gingivalis LPS (10 μg/ml; diagonal bars), or E. coli LPS (0.1 μg/ml; stippled bars). Cells were harvested at 2, 24, and 48 h for FACS analysis for the detection of ICAM-1 (A) and VCAM-1 (B) expression. Values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC.
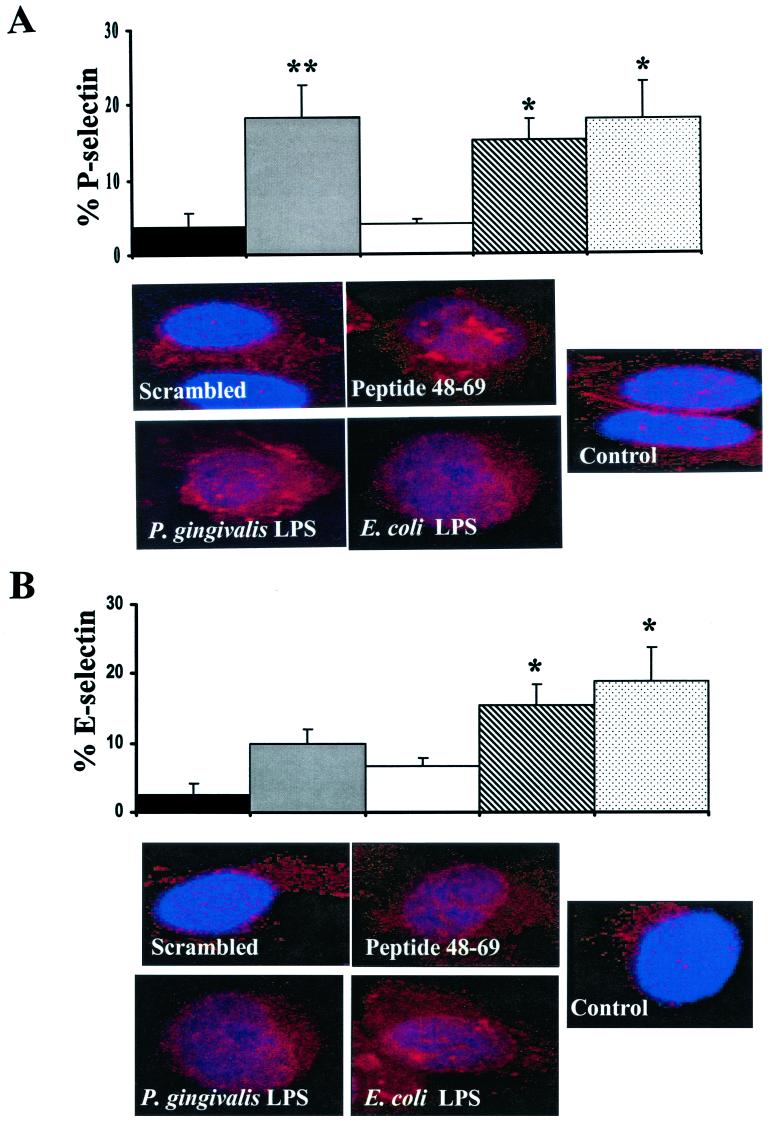
We observed a similar increase in both E- and P-selectin following stimulation with the fimbrillin-specific peptide. Uninfected HUVEC cultures were found to constitutively express low levels of surface-associated E- and P-selectin (Fig. 7). The addition of a peptide corresponding to the N-terminal region of the mature P. gingivalis fimbrillin protein (amino acid 48 to 69) stimulated the expression of surface-associated E- and P-selectin, which was statistically significant at 2 h (Fig. 8). HUVEC incubated with a scrambled peptide control expressed low levels of E- and P-selectin (Fig. 8). P. gingivalis or E. coli LPS was also found to stimulate the expression of surface-associated E- and P-selectin in HUVEC (Fig. 8). Confocal microscopy further confirmed the expression of surface-associated E- and P-selectin localized to the HUVEC cell membrane in cultures treated with the fimbrillin peptide and P. gingivalis or E. coli LPS (Fig. 8). Taken together, these results indicate that both P. gingivalis LPS and a peptide corresponding to the N-terminal region of fimbrillin can mimic the surface-associated cell adhesion molecule response observed in P. gingivalis-infected endothelial cells.
FIG. 8.
P. gingivalis fimbrillin peptide stimulate P- and E-selectin expression. HUVEC were incubated with media alone (black bars), a peptide corresponding to the N-terminal region of the P. gingivalis fimbrillin (amino acids 49 to 68; 10 μg/ml; gray bars), a scrambled peptide control (10 μg/ml; open bars), P. gingivalis LPS (10 μg/ml; diagonal bars), or E. coli LPS (0.1 μg/ml; stippled bars). Cells were harvested at 2 h for FACS analysis (upper panels) or confocal microscopy (lower panels) for the detection of P-selectin (A) and E-selectin (B) expression. Values are the means ± standard errors of the means for three independent experiments. Statistical significance was assigned when the P value was <0.05 (*) or <0.001 (**) compared to uninfected HUVEC.
DISCUSSION
Recent studies point to the importance of bidirectional signaling between bacteria and host cells and to the relevance of this activity in bacterial virulence (1, 15, 16). Contact between bacterial fimbriae and their complementary receptors on host cells is a dynamic event that can trigger distinct responses in host cells as well as in bacteria (1, 8). In the present study we have established that adherence of P. gingivalis to endothelial cells mediated via the major fimbriae stimulates the expression of cell surface-associated ICAM-1, VCAM-1, and E- and P-selectin. The requirement for bacterial adherence was confirmed by the use of the nonadherent, noninvasive P. gingivalis fimA mutant and by preincubation of P. gingivalis with anti-fimbrillin peptide-specific sera prior to infection of HUVEC. We also found that treatment of HUVEC with cytochalsin D, which prevented P. gingivalis uptake into HUVEC, also prevented the expression of ICAM-1, VCAM-1, and E- and P-selectin. These results indicate that both adherence and uptake of P. gingivalis are required for efficient stimulation of these cell adhesion molecules in HUVEC.
Initial binding of P. gingivalis to host cells is mediated primarily via the major P. gingivalis fimbriae. In vitro, P. gingivalis fimbriae have been demonstrated to bind to various host cells, including human epithelial cells, erythrocytes, and gingival fibroblasts (5, 25, 39, 41). A P. gingivalis fimA mutant (DPG3) has also been demonstrated to be impaired in its ability to adhere to and invade epithelial and endothelial cells (10, 27). Furthermore, the ability of P. gingivalis to invade primary gingival epithelial cells has been correlated with fimA expression (43). P. gingivalis fimbriae have also been reported to induce the expression of inflammatory cytokines in human gingival fibroblasts and mouse peritoneal macrophages and to induce monocyte adhesion to HUVEC (18, 19, 31–35). A recent study has demonstrated that P. gingivalis fimbriae use molecules of the β-2 integrin family (CD18) on mouse macrophages as cellular receptors and that CD18 may play a functional role in signaling for the fimbria-induced expression of IL-1β and tumor necrosis factor from these cells (40). Specific domains of the fimbrillin protein have been shown to be important in triggering signaling in host cells (31–35, 39). In particular, the amino-terminal domain corresponding to amino acid residues 49 to 90 of the fimbrillin protein has been demonstrated to function as a major epithelial cell binding domain of P. gingivalis fimbriae (39). Our results indicate that this amino-terminal domain also mediates adherence of P. gingivalis to endothelial cells and that this peptide domain is sufficient for the stimulation of ICAM-1, VCAM-1, and E- and P-selectins in endothelial cells.
Reports vary on whether P. gingivalis infection or P. gingivalis components can stimulate cell adhesion molecule expression in various host cells. In our study, we established that P. gingivalis outer membrane components, as well as live organisms stimulated the expression of surface-associated VCAM-1, ICAM-1, and E- and P-selectins. Our results are in contrast to those of studies which have reported that P. gingivalis LPS does not stimulate the expression of soluble E-selectin in HUVEC or that live P. gingivalis does not stimulate neutrophil adhesion to these cells (6). Another study reported that P. gingivalis cultures can attenuate the expression of soluble ICAM-1 from epithelial cells cocultured with human polymorphonuclear leukocytes (23). A third study recently reported that P. gingivalis inhibits soluble ICAM-1 expression in gingival epithelial cells (20). Intricate differences in the endothelial cells used in our study may account for the different results observed for epithelial cells. In addition, the differences reported by previously published studies and our studies may be due to differences in the P. gingivalis strains used and to growth of the bacteria used for the infection assays, as well as the time that cells were exposed to P. gingivalis cultures or outer membrane components. Indeed, we did observe some differences in the ability of P. gingivalis strains 381 and A7436 to stimulate cell adhesion molecules. For the studies described herein, P. gingivalis cultures were grown to the logarithmic phase in liquid broth. In contrast, in studies described by Darveau et al. (6) and Madianos et al. (23), bacteria were grown on agar plates and presumably the majority of bacteria were in the stationary phase of growth. P. gingivalis adherence to endothelial cells is maximal during logarithmic growth (C. A. Genco, unpublished data). It has also been reported that the fimA promoter activity decreases by 50% upon culture of P. gingivalis on solid agar medium (43). Since our studies indicate that P. gingivalis fimbria-mediated adherence is required for the stimulation of cell adhesion molecule expression, organisms isolated from stationary-phase cultures may not be as effective in adherence and subsequent activation of cell adhesion molecule expression. Furthermore, our studies were intended to mimic a chronic infection in which P. gingivalis was present throughout the incubation period. We demonstrated that cell surface-associated adhesion molecule expression was maximal at 48 h postinfection. the ability of P. gingivalis to stimulate cell adhesion molecules at later times may well explain the differences in previous studies (6, 20, 23, 37) and the results presented in this study.
Our recent studies presented in an accompanying manuscript (26) indicate that the initial response following attachment of P. gingivalis to the endothelial cell mediated via fimbriae includes the expression of IL-8 and MCP-1. Stimulation of these chemokines, which function in the recruitment of neutrophils and monocytes to the endothelial cell, together with the stimulation of adhesion molecules involved in the recruitment of leukocytes to sites of inflammation by P. gingivalis may play a role in the pathogenesis of systemic inflammatory diseases associated with this microorganism, including atherosclerosis. Recruitment and adhesion of circulating monocytes and leukocytes to endothelial cells represent the first steps in an inflammatory reaction that is regulated by a complex communication between the cell types involved. It is well-established that leukocyte accumulation is an important feature of inflammatory diseases, and the infiltration of circulating leukocytes to foci of infection can be instrumental in the resolution of an infection. However, promoting the infiltration of leukocytes into atherosclerotic plaque may contribute to an exacerbation of these lesions and possibly to the initiation of cardiovascular events. Leukocyte chemotaxis and migration across the endothelium are modulated by several chemokines, including IL-8 and MCP-1. Circulating leukocytes adhere to the endothelial cells of the vessel wall via cell adhesion molecules including selectins, ICAM-1, and VCAM-1. The expression of surface adhesion molecules can be modulated by host-derived inflammatory mediators, as well as by microbes or microbial products. Stimulation of endothelial cells results in both transient and sustained increases in cell adhesion molecule expression on endothelial cell surfaces and facilitates adhesiveness for neutrophils. Transient increases in endothelial adhesion are largely attributable to P-selectin, whereas sustained increases are effected by E-selectin and ICAM-1. ICAM-1 is thought to facilitate leukocyte attachment and transendothelial migration at inflammatory sites, whereas E- and P-selectins mediate neutrophil and monocyte rolling, which is the end result of tight binding of these cells to endothelial cells (21). E-selectin, ICAM-1, and VCAM-1 are instrumental in the recruitment of monocytes and have been detected in human atheromatous lesions (7, 29).
In summary, our studies indicate that P. gingivalis can stimulate cell surface-associated adhesion molecules in endothelial cells, which is mediated in part by fimbria-mediated adherence. The ability of P. gingivalis to stimulate cell surface-associated adhesion molecules in endothelial cells may have important consequences in the pathogenesis of systemic inflammatory diseases associated with this organism, including atherosclerosis. Studies to define the role of cell adhesion molecule stimulation in a P. gingivalis in vivo infection model system are currently in progress.
Acknowledgments
This study was supported by Public Health Service grant PO1DE13191 from the National Institute of Dental and Craniofacial Research to C.A.G.
We acknowledge Dana Graves, Thomas Van Dyke, and Salomon Amar for stimulating discussions and scientific advice. We also thank Hakim Sojar for antifimbrillin peptide-specific antisera.
Editor: E. I. Tuomanen
REFERENCES
- 1.Abraham, S. N., A. B. Jonsson, and S. Normark. 1998. Fimbriae-mediated host-pathogen cross-talk. Curr. Opin. Microbiol. 1: 75–81. [DOI] [PubMed] [Google Scholar]
- 2.Arbes, S. J., Jr., G. D. Slade, and J. D. Beck. 1999. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J. Dent. Res. 78: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 3.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 67: 1123–1137. [DOI] [PubMed] [Google Scholar]
- 4.Chung, H. J., C. M. E. Champagne, J. H. Southerland, S. Geva, Y. Liu, D. W. Paquette, R. N. Madianos, J. D. Beck, and S. Offenbacher. 2000. Effect of Porphyromonas gingivalis infection on atheroma formation in ApoE (+/−) mice. J. Dent. Res. 79: 313. [Google Scholar]
- 5.Cutler, C. W., J. R. Kalmar, and C. A. Genco. 1995. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 3: 45–51. [DOI] [PubMed] [Google Scholar]
- 6.Darveau, R. P., M. D. Cunningham, T. Bailey, C. Seachord, K. Ratcliffe, B. Bainbridge, M. Dietsch, R. C. Page, and A. Aruffo. 1995. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect. Immun. 63: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, M. J., A. J. Gordon, A. J. H. Gearing, R. Pigott, N. Woolf, D. Katz, and A. Kyriakopoulos. 1993. The expression of adhesion molecules ICAM-1, PECAM, and E-selectin in human atherosclerosis. J. Pathol. 171: 223–229. [DOI] [PubMed] [Google Scholar]
- 8.Deghmane, A. E., S. Petit, A. Topilko, Y. Pereira, D. Giorgini, M. Larribe, and M. K. Taha. 2000. Intimate adhesion of Neisseria meningitides to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 19: 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande, R. G., M. Khan, and C. A. Genco. 1998. Invasion strategies of the oral pathogen Porphyromonas gingivalis: implications for cardiovascular disease. Invasion Metastasis 18: 57–69. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande, R. G., M. B. Khan, and C. A. Genco. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 66: 5337–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerard, C., and B. J. Rollins. 2001. Chemokines and disease. Nat. Immunol. 2: 108–115. [DOI] [PubMed] [Google Scholar]
- 12.Genco, R. J. 1998. Periodontal disease and risk for myocardial infection and cardiovascular disease. Cardiovas. Rev. Rep. 19: 34–40. [Google Scholar]
- 13.Graves, D. T., and Y. Jiang. 1995. Chemokines, a family of chemotactic cytokines. Crit. Rev. Oral Biol. Med. 6: 109–118. [DOI] [PubMed] [Google Scholar]
- 14.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71: 1554–1560. [DOI] [PubMed] [Google Scholar]
- 15.Hedlund, M., C. Wachtler, E. Johansson, L. Hang, J. E. Somerville, R. P. Darveau, and C. Svanborg. 1999. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol. Microbiol. 33: 693–703. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39: 542–552. [DOI] [PubMed] [Google Scholar]
- 17.Holt, S. C., J. Ebersole, J. Felton, M. Brunsvold, and K. S. Kornman. 1988. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239: 55–57. [DOI] [PubMed] [Google Scholar]
- 18.Hirose, K., E. Isogai, H. Mizugai, H. Miura, and I. Ueda. 1996. Inductive effect of Porphyromonas gingivalis fimbriae on differentiation of human monocytic tumor cell line U 937. Oral Microbiol. Immunol. 11: 62–64. [DOI] [PubMed] [Google Scholar]
- 19.Hirose, K., I. Isogai, and I. Ueda. 2000. Porphyromonas gingivalis fimbriae induce adhesion of monocytic cell line U937 to endothelial cells. Microbiol. Immunol. 44: 17–22. [DOI] [PubMed] [Google Scholar]
- 20.Huang, G. T., D. Kim, J. K. Lee, H. K. Kuramitsu, and S. K. Haake. 2001. Interleukin-8 and intercellular adhesion molecule 1 regulation in epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect. Immun. 69: 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr, J. R. 1999. Cell adhesion molecules in the pathogenesis of and host defense against microbial infection. J. Clin. Pathol. Mol. Pathol. 52: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loos, B. G., J. Craandijk, F. J. Hoek, P. M. Wertheim-van Dillen, and U. van der Velden. 2000. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J. Periodontol. 71: 1528–1534. [DOI] [PubMed] [Google Scholar]
- 23.Madianos, P. N., P. N. Papapanou, and J. Sandros. 1997. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect. Immun. 65: 3983–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malek, R., J. G. Fisher, A. Caleca, M. Stinson, C. J. van Oss, J. Y. Lee, M. I. Cho, R. J. Genco, R. T. Evans, and D. W. Dyer. 1994. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J. Bacteriol. 176: 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura, T., A. Amano, I. Nakagawa, and S. Hamada. 1999. Specific interactions between Porphyromonas gingivalis fimbriae and human extracellular matrix proteins. FEMS Microbiol. Lett. 175: 267–272. [DOI] [PubMed] [Google Scholar]
- 26.Nassar, H., H-H. Chou, M. Khlgatian, F. C. Gibson III, T. E. Van Dyke, and C. A. Genco. 2001. Role for fimbriae and lysine-specific cysteine proteinase gingipain K in expression of interleukin-8 and monocyte chemoattractant protein expression in Porphyromonas gingivalis infected endothelial cells. Infect. Immun. 70: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Njoroge, T., R. J. Genco, H. T. Sojar, N. Hamada, and C. A. Genco. 1997. A role of fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect. Immun. 65: 1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noll, G. 1998. Pathogenesis of atherosclerosis: a possible relation to infection. Atherosclerosis 140(Suppl): S3–S9. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien, K. D., M. D. Allen, O. T. McDonald, A. Chait, J. M. Harlan, D. Fishbein, J. McCarty, M. Ferguson, K. Hudkins, C. D. Benjamin, R. Lobb, and C. Alpers. 1993. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plagues. Clin. Investig. 92: 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Offenbacher, S., P. N. Madianos, C. M. Champagne, J. H. Southerland, D. W. Paquette, R. C. Williams, G. Slade, and J. D. Beck. 1999. Periodontitis-atherosclerosis syndrome: an expanded model of pathogenesis. J. Periodontal Res. 34: 346–352. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa, T., and S. Hamada. 1994. Hemagglutinating and chemotactic properties of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Infect. Immun. 62: 3305–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa, T. 1994. The potential protective immune responses to synthetic peptides containing conserved epitopes of Porphyromonas gingivalis fimbrial protein. J. Med. Microbiol. 41: 349–358. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa, T., Y. Kusumoto, H. Uchida, S. Nagashima, H. Ogo, and S. Hamada. 1991. Immunological activities of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 180: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa, T., H. Ogo, H. Uchida, and S. Hamada. 1994. Humoral and cellular immune responses to the fimbriae of Porphyromonas gingivalis and their synthetic peptides. J. Med. Microbiol. 40: 397–402. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa, T., H. Uchida, and S. Hamada. 1994. Porphyromonas gingivalis fimbriae and their synthetic peptides induce proinflammatory cytokines in human peripheral blood monocyte cultures. FEMS Microbiol. Lett. 116: 237–242. [DOI] [PubMed] [Google Scholar]
- 36.Penberthy, T. W., Y. Jiang, and D. T. Graves. 1997. Leukocyte Adhesion Molecules 8: 380–388. [DOI] [PubMed] [Google Scholar]
- 37.Sandros, J., C. Karlsson, D. F. Lappin, P. N. Madianos, D. F. Kinane, and P. N. Papapanou. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79: 1808–1814. [DOI] [PubMed] [Google Scholar]
- 38.Socransky, S. S., and A. D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63: 322–331. [DOI] [PubMed] [Google Scholar]
- 39.Sojar, H. T., Y. Han, N. Hamada, A. Sharma, and R. J. Genco. 1999. Role of the amino-terminal region of Porphyromonas gingivalis fimbriae in adherence to epithelial cells. Infect. Immun. 67: 6173–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeshita, A. Y. Murakami, Y. Yamashita, M. Ishida, S. Fujisawa, S. Kitano, and S. Hanazawa. 1998. Porphyromonas gingivalis fimbriae β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signaling. Infect. Immun. 66: 4056–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide extraction with phenol water and further applications of the procedure. Methods Carbohydr. Chem. 5: 83–91. [Google Scholar]
- 42.Wu, T., M. Trevisan, R. J. Genco, K. L. Falkner, J. P. Dorn, and C. T. Sempos. 2000. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high-density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am. J. Epidemiol. 151: 273–282. [DOI] [PubMed] [Google Scholar]
- 43.Xie, H., O. Chung, Y. Park, and R. J. Lamont. 2000. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 65: 2265–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]