Abstract
Metastasis of primary tumors leads to a very poor prognosis for patients suffering from cancer. Although it is well established that not every tumor will eventually metastasize, it is less clear whether primary tumors acquire genetic alterations in a stochastic process at a late stage, which make them invasive, or whether genetic alterations acquired early in the process of tumor development drive primary tumor growth and determine whether this tumor is going to be metastatic. To address this issue, we tested genes identified in a large-scale comparative genomic hybridization analysis of primary tumor for their ability to confer metastatic properties on a cancer cell. We identified amplification of the ACK1 gene in primary tumors, which correlates with poor prognosis. We further show that overexpression of Ack1 in cancer cell lines can increase the invasive phenotype of these cells both in vitro and in vivo and leads to increased mortality in a mouse model of metastasis. Biochemical studies show that Ack1 is involved in extracellular matrix-induced integrin signaling, ultimately activating signaling processes like the activation of the small GTPase Rac. Taken together, this study supports a theory from Bernards and Weinberg [Bernards, R. & Weinberg, R. A. (2002) Nature 418, 823], which postulates that the tendency to metastasize is largely predetermined.
Keywords: cancer, metastasis
Despite increasing detailed molecular understanding of processes that underlie metastatic spread of cancers, such as increased cellular motility and invasiveness, the genetic alterations responsible for enabling human tumor metastasis are poorly understood. The prevailing view is that cancer cells acquire mutations late in tumor development that confer metastatic properties (1, 2), but experimental evidence of genes that are specifically mutated in human metastatic tumors is rare. In fact, comparative genomic hybridization (CGH) analysis of several different human tumor types has revealed a high degree of concordance between matched primary and metastatic tumors (3, 4). Both gene expression profiling and CGH analysis of primary tumors can predict metastasis (5, 6). A hypothesis has been proposed that metastasis is not caused by acquisition of new genetic abnormalities as previously thought (1, 2) but is predetermined by the specific genetic alterations that cancer cells acquire during primary tumor growth (7).
Here, we report the identification of an acquired genetic alteration in primary human tumors that fulfils this prediction. The gene encoding the non-receptor tyrosine kinase Ack1 (activated cdc42-associated kinase) is present within a small amplicon at 3q29 that can be found in human tumors of several tissue types. Copy number gain of the ACK1 gene as well as overexpression of Ack1 mRNA are most pronounced in advanced-stage primary tumors and metastatic tumors and rare in early-stage tumors, suggesting that primary tumors harboring genetic alterations like gene amplification that lead to Ack1 overexpression are predisposed to become metastatic.
Ack1 was originally identified as a cdc42-interacting protein, and it was suggested to be a cdc42 effector (8). An Ack isoform termed Ack2 was identified in a bovine cDNA library (9), but according to the literature and database searches, other species, including mouse and human, have only one Ack gene and protein (Ack1).
In a melanoma cell line, Ack1 was shown to be involved in chondroitin sulfate proteoglycan mediated cell spreading (10). Several reports studying overexpressed Ack1 in nontransformed cell lines in vitro present evidence for an important role of Ack1 in the transduction of Ras/cdc42 signals (11–14), and very recently it was suggested that Ack1 activity is required for the survival of v-Ras-transformed murine fibroblasts (15). Despite these findings, the in vivo consequences of Ack1 deregulation in the context of cancer remain unclear.
We were not able to identify a strong effect of Ack1 modulation on the growth of human cancer cell lines, but we found Ack1 to be involved in the process of metastasis in vitro and in vivo. In cancer cell lines of epithelial origin, its overexpression enhances cellular motility, invasiveness, and the ability to metastasize to the lung, resulting in increased mortality. We also show that ligand stimulation of α3β1-integrin, a signaling process that promotes metastasis, leads to activation of Ack1, and that Ack1 enhances p130Cas phosphorylation and activation of Rac, two other signaling molecules previously linked to metastasis.
Materials and Methods
Materials. Human tumor samples (containing >50% tumor cells) were obtained from the Cooperative Human Tissue Network, Duke University, the University of Michigan, Asterand Co., and Ardais Co. Sequence-verified human cDNA clones (46,656) were purchased from Research Genetics (Huntsville, AL). Antibodies against the following proteins were used: Ack1 [rabbit polyclonal antibody (pAb) CVQL raised against Ack1 peptide (REEEKLKAEEIRIKGKYNISSEDYRQ)], phosphotyrosine [mAb 4G10 (PY); Upstate Biotechnology, Lake Placid, NY], integrin antibodies [mAbs, α3-(P1B5, ASC-1), β1-(21C8, 6S6); Chemicon], p130Cas, E- and N-cadherin, fibronectin, and α-, β-, and γ-catenin (mAbs; BD Transduction Laboratories), and α-smooth muscle actin (ascitic fluid; Sigma). ACK1 was PCR-amplified from placental cDNA (Invitrogen) by using primers designed from the published sequence, cloned into a retroviral vector, pLPC, and sequenced. Our ACK1 cDNA sequence is identical to the underlying genomic sequence in the human assembly but differs in a few nucleotides from the original cDNA sequence (8). The cell lines MDA-MB-231 and 4T1 were obtained from American Type Culture Collection, human mammary epithelial cells (HMEC) were obtained from Cambrex, and each was cultured according to the supplier's protocol. Extracellular matrix-coated culture dishes and tumor-invasion chambers were from BD Biosciences.
Western Immunoblotting, Adhesion, and Invasion Assays. Western analysis and invasion assays were performed as described in ref. 16. Briefly, cells were kept in suspension for 4 h and plated onto either laminin or collagen IV plates for 30 min. Cells were pretreated for 30 min with 20 μg/ml stimulatory-α3-(P1B5), β1-(21C8) or inhibitory-α3-(ASC-1), β1-(6S6)-integrin antibodies before plating. For adhesion assays, 2 × 104 cells were plated on laminin-precoated 96-well plates, incubated for 1 h, and washed three times with PBS. Adherent cells were quantified by using Cell-Titer-Glo Reagent (Promega) according to the supplier's protocol. For invasion assays, 5 × 104 (4T1) or 5 × 105 cells were plated on Matrigel-precoated FluoroBlok (BD Biosciences) invasion chambers. Conditioned NIH 3T3 medium was used as a chemoattractant. After 16 h (4T1) or 24 h (HMEC) of incubation, invaded cells were labeled with Calcein-AM, and fluorescence was read in a plate reader at 530/590 nm.
Viral Production and Infection of Target Cells. The production of amphotropic retroviruses and infection of target cells was described in ref. 16. The 4T1 cells were selected with 12 μg/ml puromycin. MDA-MB-231 and HMEC were selected in 2 μg/ml puromycin.
In Vivo Metastasis Assays, Moribundancy Study, and Isolation of Tumor Cells from Blood and Lung. The female BALB/c mice used for this study were between 6 and 8 weeks old and were obtained from Harlan. They were housed in microfilter cages. All cages, water, and food were autoclaved before use. The cages were maintained in an air-conditioned and light-controlled (12 h/day) room. Mice and mammary gland injection and isolation of tumor cells from blood and lung was performed as described in ref. 17. The experiment was performed twice with 10 and 30 mice per group, respectively.
For the moribundancy study (50 mice per group), the primary tumors were surgically removed when they reached a size between 1,000 and 1,500 mm3 according to established protocols (18). All animals were monitored until they were moribund, at which point they were killed. Animals that died within 24 h of the surgical procedure were removed from the analysis (six animals per group). All of the mice in this study were maintained in an animal facility accredited by the Institutional Animal Care and Use Committee of Amgen San Francisco.
Genomic Microarray Hybridizations. Genomic DNA microarray hybridizations were carried out essentially as described in ref. 19 except that instead of hybridizing in 3.4× SSC/0.3% SDS at 65°C, we hybridized in 25% formamide/5× SSC/0.1% SDS at 59°C. Microarrays were scanned with a GenePix 4000 scanner (Axon Instruments, Union City, CA) and analyzed with genepix pro 3.0 software. The chromosomal position of ≈98% of the cDNAs was determined by blat analysis (http://genome.ucsc.edu/cgi-bin/hgBlat). A perl program was used to assign chromosomal locations to the cDNA fluorescence ratios (corresponding to the amount of tumor DNA relative to normal DNA) that were then averaged over a window of five chromosomally contiguous cDNAs. The resultant data were converted into chromosomal plots by using splus (Insightful, Seattle).
Quantitative Real-Time PCR (QPCR). Quantification of human cells in mice lungs is described in ref. 20. Fluorogenic TaqMan QPCR probes were designed by using the primer express software (Applied Biosystems). DNA copy number was quantified by using the Applied Biosystems 7700 or 7900 sequence detection system (Applied Biosystems). mRNA levels of ACK1 in tumors and in corresponding normal tissues (Clontech) were determined by performing quantitative PCR with fluorogenic TaqMan probes directly after reverse-transcriptase reactions. Absolute mRNA levels for Ack1 were within 50% for 3–10 different samples of each of the 15 normal tissue types examined. β-actin was used as a reference probe.
Results and Discussion
The ACK1 Gene Is Amplified and Ack1 mRNA Is Overexpressed in Human Tumors. Based on the observation that both gene expression profiling and CGH analysis of primary tumors can predict metastasis (5, 6), we initiated a search for specific, acquired genetic alterations and the resulting changes in gene expression in primary tumors that can confer metastatic properties. To identify highly localized regions of tumor-specific DNA amplification, and to overcome the low resolution associated with previous studies, at least 30 advanced-stage tumors from each of breast, esophageal, lung, ovarian, pancreatic, and prostate cancer types were surveyed by array CGH using the 46K set of sequence-verified cDNA clones.
We focused the analysis on chromosome 3, because previous work on chromosomal alterations in primary tumors found that 3q gain is associated with recurrence in prostate tumors and is a strong predictor of metastatic relapse in a node-negative breast cancer CGH study (6, 21). Although we detected relatively frequent amplification of broad regions of DNA on chromosome 3q, a few tumors showed a much tighter area of increased DNA copy number ≈2 Mb distal to the telomere at 3q (Fig. 1a). To determine the amplicon boundaries of this smaller region, we applied quantitative real-time PCR (QPCR) analysis to a panel of tumors harboring amplification to measure the DNA copy number at points distributed throughout the region. This analysis restricted the common area of amplification to a 135-kbp segment that contained only two RefSeq genes, a part of MUC4 (a single noncoding exon) and the complete coding sequence for ACK1 (Fig. 1b). Furthermore, this high-resolution analysis indicated that ACK1 was within a focal point of higher level amplification in tumors that by microarray analysis contained a broad region of 3q gain (data not shown).
Fig. 1.
ACK1 is amplified in chromosome 3, overexpressed, and associated with poor prognosis in human tumors. (a) Genomic microarray analysis of relative DNA copy number along chromosome (Chr.) 3 in several tumor types. (b) DNA copy number for eight tumors (two hormone-refractory prostate tumors, red and blue asterisks; two ovarian tumors, solid blue and green quadrilaterals; two esophageal tumors, blue and red dashes; a lung tumor, solid green circles; and a pancreatic tumor, open magenta circles). DNA copy number is plotted in megabase pairs against their nucleotide position in chromosome 3 (http://genome.ucsc.edu; April 2003 freeze). The data shown are from single DNA TaqMan assays [each with duplicate measurements with coefficient of variation (CV) <5%], and each assay was performed three times with an average CV ≅ 10%. (c) Corresponding ACK1 DNA and RNA values for lung, ovarian, and prostate tumors as determined by QPCR, with relative RNA levels on a log scale. Ack1 RNA was measured by quantitative QPCR in normal prostate tissues, primary prostate tumors, and hormone-refractory prostate tumors (d) (values >50 times are shown as 50); and normal lung tissues, stage 1 and 2 lung tumors, and stage 3 and 4 tumors (e). The values were normalized to the average value obtained with five corresponding normal tissue samples.
QPCR analysis of mRNA from tumor samples with Ack1-specific probe sets confirmed that ACK1 amplification resulted in elevated Ack1 RNA levels, and moreover a significant percentage of tumors overexpress ACK1 in the absence of gene amplification, showing that gene amplification is not the only way to overexpress and presumably activate Ack1 (Fig. 1c). The highest frequencies of ACK1 amplification in primary tumors (9% to 14%) were detected in ovarian and lung types, respectively (see Fig. 5a, which is published as supporting information on the PNAS web site); both of these tumor types have been previously reported to have frequent gain of 3q as judged by CGH (22).
Ack1 overexpression is frequent in aggressive lung tumors, with 20 of 47 later stage lung tumors exhibiting elevated ACK1 RNA levels (6- to 35-fold) (Fig. 1d), whereas only 2 of 33 early stage tumors displayed overexpression of ACK1 (6- to 10-fold). A similar association with tumor stage was observed in prostate cancer. Although we did not have access to late-stage primary tumors, Ack1 overexpression was frequently found in metastatic, hormone-refractory prostate tumors (10 of 13 samples displayed 5- to >100-fold overexpression) but rarely in early-stage, primary prostate tumors (1 of 53 samples; Fig. 1e). Three of 13 metastatic hormone-refractory prostate tumors harbored low-level ACK1 amplification, whereas none of 64 primary prostate tumors showed amplification, although the relatively low tumor cellularity of primary prostate tumor samples may have obscured low-level amplification. The pronounced overexpression of ACK1 in advanced lung and prostate tumors indicates an association with cancer progression and suggests that it could be a gene underlying the association of 3q gain with poor prognosis (21).
Ack1 Overexpression Can Lead to Increased Metastasis in Vivo. The results described above establish that ACK1 is genomically altered in aggressive tumors and a candidate for a genetic determinant of metastasis. To study the functional consequences of this genomic alteration, we forcibly overexpressed ACK1 in a human breast cancer cell line, MDA-MB-231, by retroviral infection. This cell line has been used extensively to model metastatic processes and has normal levels of endogenous ACK1 gene dosage and expression (data not shown). We injected pools of MDA-MB-231 cells overexpressing Ack1 (231-Ack1) or empty-vector (231-pLPC) intravenously into immunocompromised mice. After 60 days, lungs of the mice were harvested, and we measured the tumor burden in the lungs by QPCR detection of human-specific DNA sequences (20). When compared with the control group, we observed a dramatic increase of tumor cells in the lungs in the Ack1-overexpressing group, demonstrating that overexpression of Ack1 can enhance metastasis (Fig. 2a). To explore the generality of this finding, we repeated this analysis using an alternate model system, the 4T1 mouse mammary tumor model (17). Ack1-overexpressing (4T1-Ack1) and control (4T1-pLPC) cells were implanted into the mammary fat pad of syngeneic, female BALB/c mice. Tumor volume measurements over the course of the experiment showed that primary tumor growth was not altered by overexpression of Ack1 (Fig. 5b). After 28 days, we harvested the lungs and propagated the tumor cells in vitro by cultivating them in selective media that only allowed the transfected cells to survive. As shown in Fig. 2b, after 21 days, colony formation of 4T1-Ack1 cells was markedly elevated (84 ± 4 colonies), compared with 4T1-pLPC cells (43 ± 2 colonies). This result confirms that Ack1 overexpression is leading to a higher tumor burden in the lung.
Fig. 2.
Ack1 overexpression in tumor cells enhances metastasis and mortality in vivo.(a) Experimental metastasis assay with 231-pLPC and 231-Ack1 cells after 60 days. Human cells in mouse lungs were quantified by applying ALU-QPCR as described in ref. 20. Samples have been analyzed in quadruplicate and normalized against normal lung; each bar represents the results from an individual lung. (b and c) Colony formation of 4T1 cells isolated from lungs (b) or blood (c) of BALB/c mice 28 days after implantation. Tumor cells were cultivated for 21 days in vitro under selective conditions before colonies were fixed, stained, and counted. The experiment was performed twice with 10 and 30 mice per group (data shown are average of 30 individual samples). (d) Kaplan–Meier survival analysis of BALB/c mice inoculated with 4T1 cells overexpressing Ack1 or vector control. The primary tumor was removed when it reached a volume of 1,000–1,500 mm3, and the animals were monitored until they were moribund (44 animals per group). The experiment was stopped when all animals in the Ack1 group died and statistical significance was reached (P < 0.0001; paired t test).
We used the 4T1 system, which mimics human tumor metastasis in that the tumor cells need to escape from a primary site, to explore which step in the in vivo process of metastasis was being affected by Ack1 overexpression. To detect the presence of circulating cancer cells in vivo, blood samples from mice carrying primary 4T1-pLPC and 4T1-Ack1 tumors were collected and propagated in the presence of puromycin. Both groups showed similar colony formation, indicating that Ack1 has no effect on escape from the primary tumor, entry into the blood system, or survival of the cells in the blood system (Fig. 2c). This finding implies that Ack1 affects the ability of circulating tumor cells to escape the blood system and/or invade and colonize the lung.
Finally, we studied the effect of the Ack1-dependent, increased metastasis on survival. Like in the previous studies, Ack1-overexpressing (4T1-Ack1) and control (4T1-pLPC) cells were implanted into the mammary fat pad of syngeneic, female BALB/c mice, but in this study the primary tumors were surgically removed when they reached a size between 1,000 and 1,500 mm3. Animals that died within 24 h of the surgical procedure were removed from the analysis. All animals were monitored until they were moribund, at which point they were killed (44 mice per group). After 50 days, all animals in the Ack1 group were dead, whereas in the control group 29% of the animals were still alive (Fig. 2d).
Ack1 Overexpression Induces Metastatic Phenotypes in Vitro. To further evaluate the proposed metastatic role for Ack1, we examined its effects on in vitro correlates of metastasis: adhesion, motility, epithelial-mesenchymal transition, and invasion. Ack1 overexpression did not affect the adhesiveness of MDA-MB-231 cells to either laminin- or collagen IV-coated plates or TNF-α-activated human umbilical vein endothelial cells significantly (Fig. 5c and d). However, we did observe an effect of Ack1 overexpression on motility as determined by wound-healing assays in 4T1 cells. Compared with the control cells 4T1-pLPC, 4T1-Ack1 cells had significantly enhanced motility as judged by this assay (Fig. 5e). Because increased motility of epithelial cells often results from a cellular transition termed the epithelial-mesenchymal transition (EMT) (23), we examined whether Ack1 overexpression could induce EMT in primary HMEC. Ack1 overexpression changed the cobblestone-like appearance and extensive cell–cell contacts of HMEC with a spindle-like, fibroblastic morphology with far fewer cell–cell contacts (Fig. 3a). We observed a large increase in the mesenchymal markers fibronectin and α-smooth-muscle actin but no dramatic decrease in the epithelial-marker proteins (Fig. 3b). Although Ack1 overexpression alone does not induce all of the stereotypical changes associated with EMT (23), it might contribute to this transformation. We then examined effects of Ack1 overexpression on invasiveness in both HMEC and 4T1 cells and in both cases observed a 2-fold increase in the Matrigel-invasion assays (Fig. 3c).
Fig. 3.
Ack1 overexpression enhances invasiveness in vitro, phenotypical changes, and enhanced motility in HMEC and 4T1 cells. (a) HMEC-pLPC or HMEC-Ack1 cells were plated at low-density and photographed after 3 days. A representative photograph is shown. (b) Expression pattern of epithelial-marker (Left), mesenchymal-marker proteins (Right), and Ack1 expression in HMEC-pLPC and HMEC-Ack1 cells. (c) Matrigel-invasion assay with HMEC-pLPC or HMEC-Ack1 and 4T1-pLPC or 4T1-Ack1 cells after 24 h. The experiment was performed twice in octaduplicates (P < 0.001; Student's t test). Invasiveness has been quantified as described in the legend of Fig. 5.
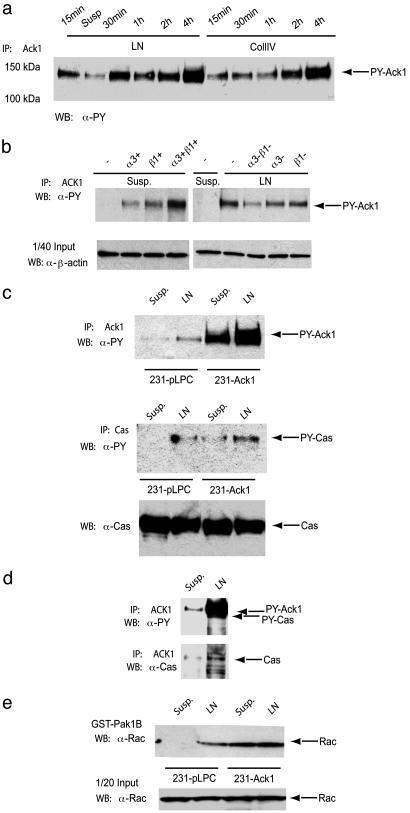
Ack1 Is Involved in Integrin Signaling. To elucidate the molecular mechanism by which Ack1 contributes to the process of metastasis, we examined whether Ack1 affected the function of proteins that have clearly established roles in cellular processes that underlie the transition from primary to metastatic cancer. In this context, integrins are essential signaling molecules for cell attachment, cell-cycle progression, apoptosis, and cell migration in normal and transformed cells (24), and integrin signals play a crucial role in the progression from tumor growth to metastasis (25, 26). In the tumor microenvironment, laminin and collagen IV are the most common extracellular matrix (ECM) proteins, representing over 70% of all possible integrin ligations (25). Therefore, we investigated the activation of Ack1 through these particular ECM proteins. We plated MDA-MB-231 on either laminin- or collagen IV-coated plates and measured tyrosine phosphorylation of Ack1 (Fig. 4a). Compared with cells in suspension (or cells plated on uncoated tissue culture vessels; data not shown), Ack1 tyrosine phosphorylation was dramatically increased when cells were plated on either ECM substrate, suggesting that integrin ligation is involved in its activation. To confirm this and rule out other mechanisms activated in the attachment process, we stimulated the suspension breast cancer cell line DU-4475 with soluble laminin and observed again an increase in tyrosine-phosphorylated Ack1 (Fig. 5f). To determine which integrins were involved, we used integrin antibodies that were directed to the α3- and β1-subunits of the major laminin receptor. We either incubated the cells with stimulatory α3 and β1 antibodies in suspension or preincubated the cells with inhibitory α3- and β1-integrin antibodies before plating onto laminin-coated plates (Fig. 4b). The α3- and β1-integrin stimulatory antibodies enhanced tyrosine phosphorylation of Ack1, whereas inhibitory α3- and β1-integrin antibodies reduced laminin-mediated activation of Ack1. This establishes that α3- and β1-integrin are at least partially responsible for Ack1 activation. Both laminin and collagen IV share β1-integrin as a major heterodimerization partner for various α-integrins, and although our data show that α3- and β1-integrins can activate Ack1 in this experimental setting, the involvement of other α- or β-integrins cannot be excluded. Interestingly, recent studies have demonstrated that α3β1-integrin facilitates lung metastases (25) and together with our data suggest that α3β1-induced Ack1 tyrosine kinase signaling contributes to the metastatic process.
Fig. 4.
Ack1 is tyrosine-phosphorylated upon α3β1-ligation, associates with p130Cas, and enhances GTP loading of Rac in the breast cancer cell line MDA-MB-231. (a) Tyrosine phosphorylation of Ack1 displays different kinetics when plated onto laminin (LN) or collagen IV (CollIV) plates in MDA-MB-231 cells. Please note that in Fig. 1a, lane 2 corresponds to cells kept in suspension. (b) Tyrosine phosphorylation of Ack1 depends on α3β1-integrin ligation. MDA-MB-231 cells were pretreated for 30 min with either stimulatory (+)- or inhibitory (-)-integrin antibodies before plating onto LN or plastic (PL). (c) Forced expression of Ack1 increases tyrosine phosphorylation of p130Cas upon LN stimulation in 231-Ack1 cells. (d) Ack1 associates with p130Cas upon LN stimulation in parental MDA-MB-231 cells. (e) GST-Pak1B pull down with 231-pLPC and 231-Ack1 cells after laminin stimulation. Cell lysates were subjected to immunoprecipitation (IP) with anti-Ack1 (Ack1) (a–d topmost panel) or p130Cas (c Middle). Tyrosine phosphorylation was analyzed by Western blotting (WB) with monoclonal antiphosphotyrosine antibody (α-PY). Loading of proteins and coprecipitation of p130Cas was controlled by reblotting with α-Ack1 and α-p130Cas. GTP-loaded Rac was analyzed by Western blotting with anti-Rac antibody (α-Rac). Arrows indicate the detected proteins.
It was previously reported that the invasion of melanoma cells can be induced by α4β1-ligation and subsequent tyrosylphosphorylated p130Cas, an important component of the integrin network, involved in cytoskeletal regulation and cell adhesion (10, 27). Thus, we tested whether upon laminin stimulation Ack1 interacted with and enhanced tyrosine phosphorylation of p130Cas. 231-Ack1 and 231-pLPC cells were plated onto laminin plates, and Ack1 or p130Cas was immunoprecipitated (Fig. 4c). As expected, tyrosine phosphorylation of Ack1 and p130Cas was enhanced upon plating the cells onto laminin, but the phosphorylation of p130Cas was greater in cells overexpressing Ack1. This finding suggests that Ack1 can mediate tyrosine phosphorylation of p130Cas. To substantiate our finding, immunoprecipitates of Ack1 were analyzed by Western blotting with anti-p130Cas. And indeed, upon laminin stimulation Ack1 interacts with p130Cas, which coincides with increased tyrosine phosphorylation of p130Cas (Fig. 4d). Another important step in integrin signaling is the activation of Rac (27). Because Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness and Rac is highly expressed in aggressive breast cancer (28), we next studied the Ack1-dependent activation of Rac in MDA-MB-231 cells. To discriminate between inactive-GDP- and active-GTP-bound Rac, pull-down assays with the Cdc42/Rac interacting domain of Pak1B (GST-Pak1B) were performed. Rac was constitutively bound to GTP in 231-Ack1 cells, whereas in 231-pLPC cells activation of Rac was inducible upon laminin stimulation (Fig. 4e).
Previous work suggests that laminin-5-activated α3β1-integrins can facilitate lung colonization of tumor cells, by promoting their adhesion to laminin-5 on the vasculature (25, 29). Our data indicate that α3β1-ligation activates Ack1, leading to increased Rac activity and cell motility. These results establish that, upon overexpression of Ack1, tumor cells can acquire a more invasive phenotype. In vivo studies suggest that enhanced Ack1 activity contributes significantly to the extravasation process, thereby efficiently completing the metastatic cascade and increasing the mortality rate.
Surprisingly, we did not observe an effect of Ack1 overexpression on the growth of the primary tumor in the 4T1 model. Using standard transformation assays like growth in soft agar or growth or survival in low serum, we did not identify a pronounced effect of Ack1 overexpression in a set of human cancer cell lines (data not shown). Nevertheless, we suspect that there is a role for Ack1 in biological aspects of cancer progression that underlies the selection for its amplification in premetastatic lesions. This role may be as a modifier of the oncogenic activity of other genetic events that occur in premetastatic lesions. In fact, a recent report provides evidence that Ack1 activity supports the survival of Ras transformed murine fibroblasts (15).
Taken together, these findings confirm the prediction that certain primary tumors have genetic alterations that directly influence metastasis (7). Although early work with rodent fibroblasts suggested that activating mutations in Ras oncogenes were a metastatic determinant, subsequent analysis of different cell types including human cells failed to substantiate this link (30). Our data suggest that ACK1 might be one of the first reported examples of an amplified gene as a metastatic determinant in primary tumors, and the properties described here for Ack1 make it a potential target for developing anti-metastatic cancer therapeutics.
Supplementary Material
Acknowledgments
We thank Martin Broome for many helpful discussions.
Author contributions: E.H.v.d.H., A. Strelow, H.W., and S.P. designed research; E.H.v.d.H., Y.Y.D., A. Strelow, A. Slavin, L.C., J.O., M.R., S.L., L.-H.S., K.Q.N., and H.W. performed research; H.W. contributed new reagents/analytic tools; T.H. and H.W. analyzed data; and E.H.v.d.H., H.W., and S.P. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CGH, comparative genomic hybridization; HMEC, human mammary epithelial cells; QPCR, quantitative real-time PCR.
References
- 1.Fidler, I. J. & Kripke, M. L. (1977) Science 197, 893-895. [DOI] [PubMed] [Google Scholar]
- 2.Poste, G. & Fidler, I. J. (1980) Nature 283, 139-146. [DOI] [PubMed] [Google Scholar]
- 3.Israeli, O., Gotlieb, W. H., Friedman, E., Korach, J., Goldman, B., Zeltser, A., Ben-Baruch, G., Rienstein, S. & Aviram-Goldring, A. (2004) Cancer Genet. Cytogenet. 154, 16-21. [DOI] [PubMed] [Google Scholar]
- 4.Patmore, H. S., Ashman, J. N., Cawkwell, L., MacDonald, A., Stafford, N. D. & Greenman, J. (2004) Br. J. Cancer 90, 1976-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van 't Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Bernards, R. & Friend, S. H. (2003) Breast Cancer Res. 5, 57-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen, E. A., Baak, J. P., Guervos, M. A., van Diest, P. J., Jiwa, M. & Hermsen, M. A. (2003) J. Pathol. 201, 555-561. [DOI] [PubMed] [Google Scholar]
- 7.Bernards, R. & Weinberg, R. A. (2002) Nature 418, 823. [DOI] [PubMed] [Google Scholar]
- 8.Manser, E., Leung, T., Salihuddin, H., Tan, L. & Lim, L. (1993) Nature 363, 364-367. [DOI] [PubMed] [Google Scholar]
- 9.Yang, W. & Cerione, R. A. (1997) J. Biol. Chem. 272, 24819-24824. [DOI] [PubMed] [Google Scholar]
- 10.Eisenmann, K. M., McCarthy, J. B., Simpson, M. A., Keely, P. J., Guan, J. L., Tachibana, K., Lim, L., Manser, E., Furcht, L. T. & Iida, J. (1999) Nat. Cell Biol. 1, 507-513. [DOI] [PubMed] [Google Scholar]
- 11.Nur, E. K. M. S., Kamal, J. M., Qureshi, M. M. & Maruta, H. (1999) Oncogene 18, 7787-7793. [DOI] [PubMed] [Google Scholar]
- 12.Kato, J., Kaziro, Y. & Satoh, T. (2000) Biochem. Biophys. Res. Commun. 268, 141-147. [DOI] [PubMed] [Google Scholar]
- 13.Kato-Stankiewicz, J., Ueda, S., Kataoka, T., Kaziro, Y. & Satoh, T. (2001) Biochem. Biophys. Res. Commun. 284, 470-477. [DOI] [PubMed] [Google Scholar]
- 14.Teo, M., Tan, L., Lim, L. & Manser, E. (2001) J. Biol. Chem. 276, 18392-18398. [DOI] [PubMed] [Google Scholar]
- 15.Nur, E. K. A., Zhang, A., Keenan, S. M., Wang, X. I., Seraj, J., Satoh, T., Meiners, S. & Welsh, W. J. (2005) Mol. Cancer Res. 3, 297-305. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst, E. H., Weber, I. & Ullrich, A. (2005) Int. J. Cancer 113, 689-698. [DOI] [PubMed] [Google Scholar]
- 17.Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., Savagner, P., Gitelman, I., Richardson, A. & Weinberg, R. A. (2004) Cell 117, 927-939. [DOI] [PubMed] [Google Scholar]
- 18.Pulaski, B. A. & Ostrand-Rosenberg, S. (2000) in Current Protocols in Immunology, eds. Coligan, J. E., Kruisbeek, A. M., Margulies, D. H., Shevach, E. M. & Strober, W. (Wiley, New York), pp. 20.2.1-20.2.16.
- 19.Pollack, J. R., Perou, C. M., Alizadeh, A. A., Eisen, M. B., Pergamenschikov, A., Williams, C. F., Jeffrey, S. S., Botstein, D. & Brown, P. O. (1999) Nat. Genet. 23, 41-46. [DOI] [PubMed] [Google Scholar]
- 20.van der Horst, E. H., Leupold, J. H., Schubbert, R., Ullrich, A. & Allgayer, H. (2004) BioTechniques 37, 940-942, 944, 946. [DOI] [PubMed] [Google Scholar]
- 21.van Dekken, H., Alers, J. C., Damen, I. A., Vissers, K. J., Krijtenburg, P. J., Hoedemaeker, R. F., Wildhagen, M. F., Hop, W. C., van der Kwast, T. H., Tanke, H. J. & Schroder, F. H. (2003) Lab. Invest. 83, 789-796. [DOI] [PubMed] [Google Scholar]
- 22.Struski, S., Doco-Fenzy, M. & Cornillet-Lefebvre, P. (2002) Cancer Genet. Cytogenet. 135, 63-90. [DOI] [PubMed] [Google Scholar]
- 23.Thiery, J. P. (2002) Nat. Rev. Cancer 2, 442-454. [DOI] [PubMed] [Google Scholar]
- 24.Brakebusch, C., Bouvard, D., Stanchi, F., Sakai, T. & Fassler, R. (2002) J. Clin. Invest. 109, 999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo, W. & Giancotti, F. G. (2004) Nat. Rev. Mol. Cell Biol. 5, 816-826. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio, A. M., Bachelder, R. E., Chung, J., O'Connor, K. L., Rabinovitz, I., Shaw, L. M. & Tani, T. (2001) J. Mammary Gland Biol. Neoplasia 6, 299-309. [DOI] [PubMed] [Google Scholar]
- 27.Bouton, A. H., Riggins, R. B. & Bruce-Staskal, P. J. (2001) Oncogene 20, 6448-6458. [DOI] [PubMed] [Google Scholar]
- 28.Lozano, E., Betson, M. & Braga, V. M. (2003) BioEssays 25, 452-463. [DOI] [PubMed] [Google Scholar]
- 29.Wang, H., Fu, W., Im, J. H., Zhou, Z., Santoro, S. A., Iyer, V., DiPersio, C. M., Yu, Q. C., Quaranta, V., Al-Mehdi, A. & Muschel, R. J. (2004) J. Cell Biol. 164, 935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muschel, R. J., Williams, J. E., Lowy, D. R. & Liotta, L. A. (1985) Am. J. Pathol. 121, 1-8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.