Abstract
TNF-α has been linked to the development of type 1 diabetes (T1D). We previously reported that neonatal treatment of nonobese diabetic (NOD) mice with TNF-α accelerated the onset of T1D, whereas TNF-α blockade in the same time period resulted in a complete absence of diabetes. The mechanisms by which TNF-α modulates development of T1D in NOD mice remain unclear. Here we tested the effects of TNF-α on the maturation of dendritic cells (DCs) in the NOD mouse. We found that neonatal treatment with TNF-α caused an increase in expression of maturation markers on CD11c+CD11b+ DC subpopulations, whereas treatment with anti-TNF-α resulted in a decrease in expression of maturation markers in the CD11c+CD11b+ subset. Moreover, neonatal treatment with TNF-α resulted in skewed development of a CD8α+CD11b-CD11c+ DC subset such that TNF-α decreases the CD8α+CD11c+ DC subset, increases the CD11c+CD11b+ subset, and causes an increase in the expression of CD40 and CD54 on mature DCs capable of inducing immunity. Anti-TNF-α-treated mice had an increase in the CD8α+CD11c+ DCs. Notably, adoptively transferred naïve CD4+ T cells from BDC2.5 T cell receptor transgenic mice proliferated in the pancreatic lymph nodes in TNF-α-treated NOD mice but not in anti-TNF-α-treated mice. Finally, we show that anti-TNF-α-treated mice showed immunological tolerance to islet cell proteins. We conclude that TNF-α plays an important role in the initiation of T1D in the NOD mouse by regulating the maturation of DCs and, thus, the activation of islet-specific pancreatic lymph node T cells.
Keywords: immune tolerance, autoreactive T cells, pancreatic lymph nodes
Type 1 diabetes (T1D) in humans and nonobese diabetic (NOD) mice is a complex, multifactorial autoimmune disease in which an islet cell-specific T cell immune response destroys insulin-producing β cells in the islets of Langerhans (1, 2). Genetic susceptibility is determined by multiple genes, and inheritance of susceptibility is a complex polygenic trait. The principal effector mechanism appears to be the action of CD4+ and CD8+ T cells, both T cell subsets being required for transfer of disease in NOD mice (2). In humans and NOD mice, MHC class II genes, the major genetic factor determining susceptibility, are a necessary but not sufficient requirement for full genetic predisposition to T1D (3, 4). The NOD mouse develops spontaneous T1D that shares many characteristics with the human disease (2, 5, 6). Much of our knowledge of the course and pathogenesis of this disease comes from studies in the NOD mouse (2), including several of the principal islet cell autoantigens, the kinetics of development of insulitis and overt diabetes, and the peptide-binding characteristics of the NOD I-Ag7 class II MHC molecule (7).
TNF-α plays multiple roles in the development and function of the immune system. Manipulation of TNF-α and its receptors revealed numerous aspects of their functions in autoimmune disease. TNF-α promotes up-regulation of adhesion molecules and activation of macrophages and is necessary for the development of T1D in NOD mice (8, 9). We have previously studied the effects of neonatal administration of TNF-α or anti-TNF-α in NOD mice, and we identified an abrupt transition (or checkpoint) at 3 weeks of age in NOD mice receiving these treatments (9). Briefly, systemic administration of nontoxic doses of TNF-α i.p. from birth to days 21–24 resulted in an increased incidence of diabetes in NOD females, with an earlier onset. In contrast, administration of anti-TNF-α in the same time period completely prevented diabetes during the 1-year study period. These effects of TNF-α and anti-TNF-α showed an abrupt transition, or checkpoint, at 3–4 weeks of age. Strikingly, administration of TNF-α at 4 weeks of age or later, for a period of 3 weeks, resulted in a decrease in the diabetes incidence and a significant delay in disease onset, whereas administration of anti-TNF-α at 4 weeks of age or later resulted in either no change in diabetes incidence or an increase in incidence with an earlier onset of diabetes (9). Taken together, these results indicate that TNF-α can positively or negatively regulate the peripheral tolerance of T cells to β islet antigens depending on the age of NOD mice at the time of exposure/expression of TNF-α; however, the mechanism underlying the paradoxical effects observed in the neonatal period has remained unclear.
A “3-week checkpoint” was also identified by Katz et al. (10) in BDC2.5 T cell receptor (TCR) transgenic (tg) mice. Analysis of BDC2.5 tg mice showed that ≈90% of the T cells expressed the tg BDC2.5 TCR. Insulitis was not detectable in these animals until ≈19–21 days of age, at which time an explosive insulitis developed, with extensive infiltration of the islets with lymphocytes (10). Although the mechanisms underlying the abrupt onset of severe insulitis in BDC2.5 tg mice at 19–21 days of age were unclear, Mathis et al. (11) postulated that a developmentally programmed wave of apoptosis in β cells between neonatal days 17 and 20 (which occurs in all rodents studied) is responsible for the abrupt onset of insulitis in BDC2.5 TCR tg mice at ≈3 weeks of age. This wave of apoptosis might also be related to the 3-week cutoff for the neonatal effects of TNF-α and anti-TNF-α.
The rationale for this study is also based on the following observations. First, Hawiger et al. (12) showed that presentation of antigenic proteins to T cells by immature resting dendritic cells (DCs) results in the induction of a form of tolerance, due either to an increase in regulatory T cells or to the induction of anergy. Second, Wu et al. (13) have shown that neonatal TNF-α treatment decreases the number of CD4+ CD25+ regulatory T cells, whereas neonatal anti-TNF-α treatment causes a 2- to 3-fold increase in the numbers of these cells.
In the present study, we hypothesized that the effects of TNF-α and anti-TNF-α in the neonatal period are mediated through the effects of TNF-α in activating, and anti-TNF-α in preventing, the maturation of DCs in the islets and pancreatic lymph nodes (PLNs). Here, we have explored possible mechanisms underlying the increase and decrease of disease activity by neonatal TNF-α and anti-TNF-α treatment, respectively, in wild-type NOD mice. We monitored DC maturation in PLNs and other lymph nodes (LNs) in NOD mice treated with TNF-α and anti-TNF-α and examined the impact on the activation of a pathogenic T cell response in an adoptive transfer model using BDC2.5 TCR tg T cells. Finally, we tested whether neonatal treatment with anti-TNF-α renders animals partially or almost completely immunologically tolerant. Our data indicate that, by keeping DCs immature, anti-TNF-α induces immunologic tolerance to islet cell proteins whereas TNF-α induces DC maturation, resulting in an increased immune response. We conclude that TNF-α plays a crucial role in the initiation of T1DM by modulating DC–T cell interactions via modulation of DC development.
Materials and Methods
Mice. NOD.Lt (NOD) mice were obtained from The Jackson Laboratory and bred in the Stanford University Animal Facility under barrier isolation conditions. Diabetes incidence in the colony is currently 80% in females at 22 weeks. All animals used were female newborns, unless specifically noted. Mice were injected i.p. neonatally with TNF-α or anti-TNF-α as described (9). There was no significant difference in weight between control and treated animals during or after the treatment period. BDC2.5/NOD TCR tg mice were the kind gift of Diane Mathis (Joslin Diabetes Center, Harvard University). The BDC2.5 tg mice express a Vα1Vβ4 TCR that recognizes an unknown β cell antigen presented by I-Ag7 (10). All animals were housed under barrier conditions in Stanford University animal facilities. All animal studies have been approved by Stanford University's Administrative Panel of Laboratory Animal Care.
Assessment of Diabetes. Mice were monitored three times per week for glycosuria. Mice were considered diabetic upon two consecutive positive readings. The onset of diabetes was dated from the first of the sequential measurements.
Antigens and Reagents. Insulin B:9-23 chain (SHLVEALYLVCGERG) was synthesized and purified by reverse-phase highperformance liquid chromatography and identified by mass spectroscopy (Genemed Synthesis, South San Francisco, CA). GAD65 in a baculovirus vector was used to generate recombinant baculovirus by standard method (Invitrogen). Recombinant protein was purified as described previously (14). Recombinant murine TNF-α was a generous gift from Centocor (Malvern, PA). Anti-TNF-α (clone TN3.19.12, a hamster IgG monoclonal antibody) was a generous gift from Robert Schreiber [Washington University, St. Louis (15)]. Hamster IgG (Pierce) is an Ig control for the anti-TNF-α.
Flow Cytometric Analysis of DCs. LNs were harvested, teased, and digested in Hanks' balanced salt solution/2–5% FCS buffer containing 1.3 units/ml collagenase P (Roche) at 37°C for 20 min (16). Digested material was pipetted several times and filtered through nylon membrane in cold wash buffer containing 5–10 mM EDTA. After blocking with purified α-FcγR (2.4G2), single cell suspensions were stained for multicolor flow cytometry. Monoclonal antibodies specific for MHC class II (10-3.6), CD40 (3/23), CD54 (3E2), CD86 (GL1), CD11c (HL3), and CD119 (IFN-γ receptor, α-chain) were purchased from BD Biosciences, and antibodies specific for B220 (CD45R), CD11b (M1/70), and CD8 (CT-CD8) were purchased from Caltag (Burlingame, CA). Directly conjugated isotype controls included mouse IgG2a, rat IgG2a, and Armenian hamster IgG (BD Biosciences). For dead cell exclusion, samples were incubated with Hoescht 33342 (HO, Calbiochem) for 5 min on ice before analysis. Cell suspensions were analyzed immediately after staining to avoid maturation and/or fixation artifacts. Dead cells (HOhi) and B220hi cells were excluded during analysis. For DC analysis, samples were analyzed with MoFlo (DakoCytomation, Fort Collins, Colorado) and analyzed with flowjo software (FlowJo, Ashland, Oregon). The DC subsets were divided into four subsets based on the expression of CD11c and CD11b. Four different subsets were further analyzed for the expression profiles of DC maturation markers.
Adoptive Transfer of BDC2.5 T Cells into Treated NOD Mice. Lymphocytes from peripheral LNs and spleens of 3- to 4-week-old BDC2.5 NOD mice were labeled with 4 μM CFSE (2 × 107 cells per ml) at 37°C for 15 min (17). The cells were centrifuged through FCS, washed, and resuspended, and 2 × 107 cells in 200 μl were transferred i.v. into NOD mice that had been treated with TNF-α, PBS, or anti-TNF-α. LNs were harvested ≈66 h after transfer, and single cell suspensions were prepared by homogenizing with glass slides. The extent of T cell proliferation was determined by FACS analysis of CFSE dilution.
LN cells were stained with antibodies against Vβ4, CD4, and CD44 (IM7), CD69 (H1.2F3), and CD62L (MEL-14) for flow cytometric measurement of T cell activation. Data were acquired by using Coulter instrumentation (BD Biosciences) and analyzed with cellquest software (Becton Dickinson).
Immunization and T Cell Proliferation Assay. NOD mice were treated with anti-TNF-α or PBS in the first 3 weeks of life and then immunized at 8–9 weeks of age. Mice received footpad or base-of-tail injections of 100 μl containing a mixture of 100 μg of insulin B:9-23 peptide or GAD65 protein dissolved in 50 μl of saline and emulsified in an equal volume of incomplete Freund's adjuvant (Difco). Seven to 10 days after immunization, inguinal LNs (ILNs) and popliteal LNs were removed, suspended, and placed in 96-well plates (106 cells per well) with 2 μg of insulin B:9-23 or 4 μg of GAD65 in 200 μl of medium. [3H]Thymidine was added 72 h later at 0.5 μCi (1 Ci = 37 GBq) per well, and cells were further incubated for 12–16 h before scintillation counting. Stimulation index was defined by cpm in the presence of antigen divided by cpm in the absence of antigen.
Statistics. Significant differences between groups were assessed by Student's t test. A criterion of P < 0.05 was accepted as significant.
Results
We previously reported that systemic administration of nontoxic doses of TNF-α i.p. from birth to day 21 resulted in an increased incidence of diabetes, whereas anti-TNF-α treatment in the same time period prevents diabetes in NOD mice (9). We first tested whether the effects of TNF-α and anti-TNF-α in the neonatal period are mediated through their effects on the maturation of DCs in the islets and PLNs. First, we analyzed DC maturation markers in different subsets of DCs. NOD mice were treated with TNF-α, PBS, or anti-TNF-α from birth to day 21 and killed at day 24. We compared the composition of DC subsets from different treatment groups using six-color cytofluorimetry. Single cell suspensions from PLNs and ILNs were analyzed without preenrichment of DCs to prevent any loss of potentially important cells as reported by Turley et al. (18). Instead, we stained cells with appropriate reagents to exclude dead cells and lymphocytes. We examined the surface expression of DC maturation markers (CD86, CD40, CD54, MHC class II, and CD119) in four subsets of DCs found in PLNs and ILNs subdivided based on CD11b and CD11c expression levels (Fig. 1A) as follows: subset 1, CD11b++CD11c+++; subset 2, CD11b++CD11c-; subset 3, CD11b++CD11c++; subset 4, CD11b-CD11c++. In the CD11b++CD11c+++ subset (gate 1 in Fig. 1 A), the expression of MHC class II, CD86, CD54, and CD40 was up-regulated in the TNF-α-treated mice and down-regulated in the anti-TNF-α-treated mice (Fig. 1B). CD119, the IFN-γ receptor, known to be down-regulated upon DC maturation, showed a significant decrease in expression in TNF-α-treated mice compared with anti-TNF-α-treated mice (Fig. 1B). The expression profiles of these DC markers on cell subsets 2–4 (Fig. 1 A) were not significantly different between TNF-α-treated and anti-TNF-α-treated mice (Table 1). Similar changes in DC maturation were also seen in ILNs (data not shown), suggesting that i.p. injections of TNF-α and anti-TNF-α have a systemic effect on DC maturation.
Fig. 1.
DC maturation in the LNs from TNF-α-treated (Left), anti-TNF-α-treated (Center), or PBS-treated (Right) NOD female mice. Mice were killed at 24 days of age. The fluorescence combination was as follows: maturation marker-FITC, CD11c-phycoerythrin, B220-phycoerythrin-Cy5.5, CD11b-phycoerythrin-Cy7, CD8-allophycocyanin, and Hoescht. (A) CD11c and CD11b expression by DCs gated on B220-HO- from TNF-α-, PBS-, or anti-TNF-α-treated mice. Dot plots are representative results from >16 mice. (B) Surface expression of indicated molecules by subset 1 cells (CD11b++CD11c+++) from PLNs of 24-day-old NOD mice. These results represent one of four independent experiments. **, P < 0.01; *, P < 0.05.
Table 1. Expression of MHC class II, CD40, and intercellular adhesion molecule 1 in subsets 2–4.
| MHC class II
|
CD40
|
Intercellular adhesion molecule 1
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subset | TNF | Anti-TNF | Control | TNF | Anti-TNF | Control | TNF | Anti-TNF | Control |
| 2 | 313 ± 45 | 289 ± 67 | 356 ± 89 | 613 ± 133 | 599 ± 67 | 732 ± 102 | 559 ± 131 | 353 ± 170 | 301 ± 49 |
| 3 | 922 ± 164 | 889 ± 89 | 950 ± 143 | 854 ± 78 | 794 ± 99 | 865 ± 90 | 1,200 ± 105 | 1,165 ± 99 | 1,237 ± 151 |
| 4 | 275 ± 48 | 375 ± 98 | 355 ± 110 | 455 ± 85 | 503 ± 92 | 486 ± 113 | 386 ± 74 | 466 ± 61 | 425 ± 88 |
Because Belz et al. (19) showed that CD8α+ DCs play an important role in crosspriming and cross-tolerance, we wanted to know how CD8α+ DCs behaved in the treated mice. We stained suspensions with antibodies specific for CD8α in combination with DC markers CD11c and CD11b. Within the B220-CD11chi population, we examined the expression of CD8α and CD11b. We did not observe significant changes in the total number of B220-CD11chi PLN-derived DCs in differently treated mice (data not shown). Surprisingly, the percentage of CD8α+CD11b-CD11chi DCs was significantly decreased in TNF-α-treated mice compared with PBS control mice (16.8% versus 36.9%) whereas anti-TNF-α-treated mice showed a significant increase in the CD8α+CD11b-CD11chi subset compared with PBS control (51.2% versus 36.9%) (P < 0.01) (Fig. 2A). This finding was observed in all mice in three separate repeat experiments. The mean percentage of CD8α+CD11b-CD11chi cells in PLNs is summarized in Fig. 2B. A corresponding decrease in CD54 expression was also observed on the CD8α+CD11b-CD11chi subset in the TNF-α-treated mice (Fig. 2C). These results indicate that neonatal treatment with TNF-α resulted in a decrease in the CD8α+CD11c+ DCs lacking CD11b.
Fig. 2.
Composition of PLN CD8α DCs from TNF-α-, PBS-, or anti-TNF-α-treated NOD mice. (A) CD11b and CD8α expression by DC subsets in PLNs of 24-day-old NOD mice from different treatment groups. Dot plots (gated on B220-HO-CD11chi) are representative results from more than five experiments. (B) Percentage of CD8α+CD11chi cells in different treatment groups. The graph is a summary of several independent experiments. **, P < 0.01 compared with control group. (C) A decrease in CD54 expression in the CD8α+CD11b-CD11chi subset in the TNF-α-treated mice compared with the PBS group as indicated. Histograms represent results of four experiments.
We then examined whether the effects of TNF-α and anti-TNF-α treatment on DC maturation could influence the activation of autoreactive T cells in the pancreas. Splenocytes from 3- to 4-week-old BDC2.5/NOD mice, which lack any signs of T cell activation in the PLNs, were transferred into 24-day-old NOD mice that were treated with PBS, TNF-α, or anti-TNF-α from birth to day 21. In the transfer system, initial antigen encounter was measured by CFSE dilution due to cell proliferation (17). We found that TNF-α and anti-TNF-α treatment differentially modified the amplitude of this proliferation response (Fig. 3). In the PLNs, antigen-driven cell division was detected at 3 days after transfer in the control group as described previously (ref. 17 and data not shown). Adoptively transferred BDC2.5 T cells underwent multiple cell divisions in the PLNs of the PBS-treated mice (Fig. 3). NOD mice treated with TNF-α had a significant increase in the proliferation of BDC2.5 T cells whereas mice treated with anti-TNF-α showed a decrease in proliferation compared with control mice (Fig. 3). Proliferation was antigen-specific because it was absent in other LNs (mesenteric LNs, axillary LNs, and ILNs) (Fig. 3).
Fig. 3.
Proliferation of CFSE-labeled BDC2.5 spleen and LN cells after adoptive transfer into TNF-α-treated and anti-TNF-α-treated NOD mice. Flow cytometry was performed 3 days after transfer into NOD recipients treated with TNF-α, PBS, or anti-TNF-α in PLNs, mesenteric LNs, and axillary LNs plus ILNs. Proliferation percentage was determined 66 h after transfer by CFSE dilution in Vβ4+ CD4+ T cells.
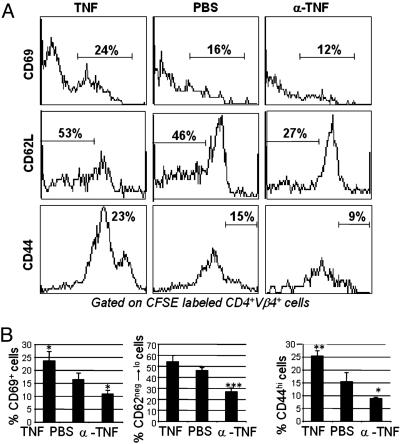
We examined the early events of BDC2.5 T cell activation by analyzing the expression of activation markers in PLNs 3 days after transfer. In TNF-α-treated recipients, increased numbers of donor T cells had an activated phenotype (CD69+, CD62Llo, CD44+), and in anti-TNF-α-treated recipients donor T cells had a decreased number of T cells with an activated phenotype when compared with control recipients (Fig. 4). In ILNs and mesenteric LNs, expression of activation markers did not differ significantly between groups (data not shown), consistent with the lack of antigen-specific proliferation in these LNs (Fig. 3). Collectively, our data suggest that neonatal treatment with TNF-α and anti-TNF-α is capable of altering the activation of autoreactive T cells in the PLNs.
Fig. 4.
Effect of TNF-α treatment on T cell activation. (A) CD69, CD62L, and CD44 expression on CD4+ Vβ4+ CFSE+ BDC2.5 T cells from PLNs of NOD mice described in Fig. 3 treated with TNF-α, PBS, or anti-TNF-α.(B) T cell activation markers in PLNs that represent the average of three experiments (mean ± standard deviation of 12 mice). ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Because anti-TNF-α treatment appears to prevent the maturation of DCs, it is reasonable to speculate that the prevention of T1D by anti-TNF-α administration is due to the induction of immunological tolerance. To investigate this possibility, mice were treated with anti-TNF-α in the first 3 weeks of life and then immunized in the hind footpads at 8–9 weeks of age with insulin B:9-23 or GAD65. Seven to 10 days after immunization, in vitro T cell proliferation assays were performed by using cells from ILNs and popliteal LNs. NOD mice treated with anti-TNF-α showed a 4- to 5-fold decrease in T cell proliferation after restimulation in vitro with 20 μg/ml GAD65 or 10 μg/ml insulin B:9-23 (Fig. 5 B and C). The total T cell number from anti-TNF-α- or PBS-treated mice immunized with GAD65 or insulin B:9-23 were comparable, indicating that the differential T cell responses in anti-TNF-α and PBS-treated mice were not due to cell death occurring in the anti-TNF-α group. The proliferative responses with anti-CD3 treatment were not significantly different between anti-TNF-α- and PBS-treated mice (Fig. 5A). These results suggest that anti-TNF-α treatment in the first 3 weeks of life can induce immune tolerance to islet cell proteins.
Fig. 5.
Effect of anti-TNF-α treatment on T cell proliferation. NOD newborn females were treated with anti-TNF-α or PBS for 3 weeks and then immunized at 8–9 weeks of age with GAD65 and insulin B:9-23. LN cells were harvested 10 days after immunization for in vitro proliferation assay. (A) As a positive control, LN cells were stimulated with anti-CD3 (1 μg/ml). (B and C) The proliferative responses of popliteal LN and ILN T cells from PBS (black bar) and anti-TNF-α-treated mice (gray bar), which were then immunized with GAD65 (B) or insulin B:9-23 (C) after restimulation with the individual peptides as indicated. The actual counts for medium only were 250–350 cpm, and the stimulated counts in the PBS group were 1,200–2,400 cpm. Results from representative triplicate experiments are shown as the mean stimulation index ± SD. **, P < 0.01.
Discussion
Our results indicate that neonatal treatment with TNF-α induced activation of DCs, which resulted in an increased activation of islet-specific T cells. In contrast, neonatal treatment with anti-TNF-α, by preventing maturation of DCs, resulted in a reduction in the T cell response to islet cell antigens and in partial immune tolerance (9). These studies have particular significance in the NOD mouse because of the well characterized 3-week checkpoint, originally described by Katz et al. (10) in the BDC2.5/NOD TCR tg mouse, and a similar 3-week checkpoint for the effect of TNF-α and anti-TNF-α. The underlying mechanism is thought to be the wave of programmed cell death at 3 weeks of age. This wave of apoptosis results in release of β cell proteins, which are then picked up by macrophages and DCs and transported to the draining PLNs.
We found that, in the CD11b++CD11c+++ subset (gate 1 in Fig. 1 A), the expression of DC maturation markers was up-regulated in the TNF-α-treated mice and down-regulated in the anti-TNF-α-treated mice (Fig. 1B). However, the expression profiles of these DC markers on cell subsets 2–4 (Fig. 1 A) were not significantly different between TNF-α- and anti-TNF-α-treated mice. This finding indicates that TNF-α matures DCs in the myeloid DC subset but not in the other subset (Table 1). We also observed that TNF-α treatment resulted in a skewed population of CD8α+CD11b- DCs. CD8α+ DCs are mostly LN resident cells; relatively few of these cells are derived from nonlymphoid tissue. Thus, the marked decrease in number of these cells in the TNF-α-treated group could be due to (i) accelerated death of CD8α+CD11c+ cells or (ii) a migration of CD8α- CD11c+ DCs into the LNs from pancreas, thus decreasing the relative number of CD8α+ DCs. The latter scenario could fit with the rest of our data showing an up-regulation of CD40 and CD54 on CD8α- DCs (Fig. 2). These “activated” CD8α- DCs may have been mobilized from the tissue by TNF-α treatment. Indeed, the absolute number of CD8α-CD11b+CD11c+ DCs is increased in the TNF-α-treated group when compared with PBS or anti-TNF-α, indicating that the CD11c+CD11b+ DC subset is expanding and maturing, thereby gaining immunostimulatory potential. Belz et al. (19) described that CD11c+CD8α+ DCs were responsible for inducing peripheral tolerance to tissue-associated antigens. It is thus of interest to investigate whether CD8α+ DCs, seen to be increased in anti-TNF-α-treated mice, have induced tolerance and whether tolerance induction by CD8α+ DCs is the default outcome, while additional stimuli are responsible for their activation and immunogenicity, as proposed by Belz et al. (19). To study this question, isolation of anti-TNF-α-treated PLN CD8α+ DCs is required. However, this experiment is extremely difficult because PLN CD8α+ DCs are not present in sufficient numbers for immune tolerance experiments, as shown previously (19).
We have shown that systemic TNF-α or anti-TNF-α plays an important role in DC development in the PLNs and ILNs (Fig. 1). BDC2.5 T cells proliferated only in the PLNs, presumably because endogenous islet antigens are found only in the PLNs (Fig. 3). There are several possible explanations for the observation of increased activation of transferred BDC2.5 T cells in the PLNs of TNF-α-treated mice compared with those in PBS- and anti-TNF-α-treated mice. First, endogenous antigens available from the islets may be increased in TNF-α-treated mice. Second, TNF-α can mature DCs in vivo and thus enhance their antigen presentation ability. Third, TNF-α can activate other antigen-presenting cells, such as B cells and macrophages, which can further activate T cells. We observed that TNF-α-treated young NOD mice showed increased proliferation and expression of CD69hi, CD62Llo, and CD44hi markers on BDC2.5 T cells found in the PLNs (Figs. 3 and 4). In contrast, anti-TNF-α-treated young NOD mice showed virtually no stimulation of cell division in the PLNs. This finding is even more striking in view of the fact that anti-TNF-α has been shown to cause an increase in T cell response in HNT-TCR tg T cells and in human T cells as well (20). These results with anti-TNF-α are compatible with our finding that neonatal treatment with anti-TNF-α increases CD8α+ DCs that are CD11b-. Neonatal treatment with anti-TNF-α may decrease apoptosis and prevent DC maturation, resulting in the induction of tolerance to β cell proteins. We observed that neonatal treatment with anti-TNF-α induced immunologic tolerance to islet cell proteins released by developmental apoptosis (Fig. 5).
We have shown that neonatal anti-TNF-α made the animals specifically susceptible to tolerance to islet proteins that were released by programmed cell death. This result is significant because our previous results (9) showed that tolerance to foreign proteins such as hen egg lysozyme (HEL) and ovalbumin (OVA) was not observed in the neonatal mice treated with anti-TNF-α. Instead, repeated treatment with anti-TNF-α resulted in a 2- to 3-fold increase in response to immunization with HEL and OVA. Although anti-TNF-α treatment suppressed T cell proliferation 4- to 5-fold in the immune tolerance experiment, it should be noted that the proliferative response in control mice to GAD65 or insulin B:9-23 was much less than in immunized cells restimulated with anti-CD3 in vitro. This observation may be due to the low frequency of antigen-specific T cells in NOD mice. Indeed, an earlier report by Tisch et al. (21) showed that splenic T cells from 4- to 6-week-old NOD mice showed only a modest proliferative response (stimulation index = 4- to 6-fold) to GAD65. Thus, neonatal anti-TNF-α-induced immune tolerance to both GAD65 and insulin B:9-23 is significant and specific.
Finally, we have shown that neonatal treatment with TNF-α altered DC phenotype and altered the ratio between DC subsets. Treatment with TNF-α also resulted in an increased ability of antigen-presenting cells to present pancreatic antigens, as demonstrated by the adoptive transfer of BDC2.5 TCR tg cells in the TNF-α-treated mice.
In summary, the studies presented here show that neonatal TNF-α-induced activation of DCs results in an increased activation of islet-specific T cells, whereas neonatal anti-TNF-α suppresses the T cell response to islet cell antigens, most likely by preventing maturation of DCs and thereby inducing partial immune tolerance to β islet cell proteins.
Acknowledgments
We thank Dr. Seon-Kyeong Kim for critical readings and discussions about this work. This work was supported by National Institutes of Health (NIH) Career Development Award K01DK064656 (to L.-F.L.), NIH Grants RO1 DK51705 and 1-2002-209 (to H.O.M.), and funds from the Juvenile Diabetes Research Foundation (to H.O.M.).
Author contributions: L.-F.L. and H.O.M. designed research; L.-F.L., B.X., G.F.B., and T.W. performed research; L.-F.L., S.A.M., G.F.B., and S.T. analyzed data; L.-F.L. and H.O.M. wrote the paper; and B.X., S.A.M., T.W., and S.T. contributed new reagents/analytic tools.
Conflict of interest statement: No conflict declared.
Abbreviations: tg, transgenic; LN, lymph node; ILN, inguinal LN; PLN, pancreatic LN; NOD, nonobese diabetic; DC, dendritic cell; TCR, T cell receptor; T1D, type 1 diabetes.
References
- 1.Castano, L. & Eisenbarth, G. S. (1990) Annu. Rev. Immunol. 8, 647-679. [DOI] [PubMed] [Google Scholar]
- 2.Tisch, R. & McDevitt, H. (1996) Cell 85, 291-297. [DOI] [PubMed] [Google Scholar]
- 3.Todd, J. A., Bell, J. I. & McDevitt, H. O. (1987) Nature 329, 599-604. [DOI] [PubMed] [Google Scholar]
- 4.Vyse, T. J. & Todd, J. A. (1996) Cell 85, 311-318. [DOI] [PubMed] [Google Scholar]
- 5.Leiter, E. H. & Serreze, D. V. (1992) Reg. Immunol. 4, 263-273. [PubMed] [Google Scholar]
- 6.Makino, S., Kunimoto, K., Muraoka, Y., Mizushima, Y., Katagiri, K. & Tochino, Y. (1980) Exp. Anim. 29, 1-13. [DOI] [PubMed] [Google Scholar]
- 7.Stratmann, T., Apostolopoulos, V., Scott, C. A., Garcia, K. C., Kang, A. S., Wilson, I. A., Teyton, L. & Corper, A. L. (2000) Science 288, 505-511. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich, S., Weyers, M., Gong, J. H., Sprenger, H., Nain, M. & Gemsa, D. (1988) J. Immunol. 140, 1511-1518. [PubMed] [Google Scholar]
- 9.Yang, X. D., Tisch, R., Singer, S. M., Cao, Z. A., Liblau, R. S., Schreiber, R. D. & McDevitt, H. O. (1994) J. Exp. Med. 180, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. (1993) Cell 74, 1089-1100. [DOI] [PubMed] [Google Scholar]
- 11.Mathis, D., Vence, L. & Benoist, C. (2001) Nature 414, 792-798. [DOI] [PubMed] [Google Scholar]
- 12.Hawiger, D., Inaba, K., Dorsett, Y., Guo, M., Mahnke, K., Rivera, M., Ravetch, J. V., Steinman, R. M. & Nussenzweig, M. C. (2001) J. Exp. Med. 194, 769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu, A., Hua, H., Munson, S. H. & McDevitt, H. O. (2002) Proc. Natl. Acad. Sci. USA 99, 12287-12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao, C. C. & McDevitt, H. O. (1997) Immunogenetics 46, 29-34. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan, K. C., Pinckard, J. K., Arthur, C. D., Dehner, L. P., Goeddel, D. V. & Schreiber, R. D. (1995) J. Exp. Med. 181, 607-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vremec, D. & Shortman, K. (1997) J. Immunol. 159, 565-573. [PubMed] [Google Scholar]
- 17.Hoglund, P., Mintern, J., Waltzinger, C., Heath, W., Benoist, C. & Mathis, D. (1999) J. Exp. Med. 189, 331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turley, S., Poirot, L., Hattori, M., Benoist, C. & Mathis, D. (2003) J. Exp. Med. 198, 1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belz, G. T., Wilson, N. S., Villadangos, J. A., Shortman, K., Carbone, F. R. & Heath, W. R. (2003) J. Immunol. 170, 4437-4440. [DOI] [PubMed] [Google Scholar]
- 20.Cope, A. P., Liblau, R. S., Yang, X. D., Congia, M., Laudanna, C., Schreiber, R. D., Probert, L., Kollias, G. & McDevitt, H. O. (1997) J. Exp. Med. 185, 1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tisch, R., Yang, X. D., Singer, S. M., Liblau, R. S., Fugger, L. & McDevitt, H. O. (1993) Nature 366, 72-75. [DOI] [PubMed] [Google Scholar]