Abstract
Repeated-epilation (Er) mutation in the mouse is inherited as an autosomal and semidominant mutation. Major defects in heterozygous adults and homozygous fetuses were associated with skin and were caused by abnormal ectodermal differentiation. Heterozygous mice are characterized by repeated hair loss and regrowth, and homozygous fetuses die at birth with severe abnormality in skin, limb, tail, and face. To identify the gene causing Er mutation, we have performed gene-expression profiles of skins and mouse embryonic fibroblasts from WT and mutant Er mice by using Affymetrix (Santa Clara, CA) chip analysis. By analyzing the candidate genes generated from gene-expression profiling, we identified a Sfn mutation in Er mice. A single nucleotide insertion in the Sfn (Stratifin, also called 14-3-3σ) coding region results in a truncated protein lacking 40 amino acid residues at the C terminus. The mutation is linked with phenotypes of Er-heterozygous and -homozygous mice. Ectopic overexpression of WT 14-3-3σ in Er/Er keratinocytes rescues defects in keratinocyte differentiation. Our study demonstrates that 14-3-3σ is a crucial regulator for skin proliferation and differentiation.
Keywords: epidermal development, genetics, IKK1, stratifin, Er
The skin is composed of an external epidermis of stratified squamous keratinizing epithelium and the underlying dermis of supporting connective tissue. The epidermis consists of the keratinocytes at various stages of differentiation, based on which, they form at least four different cell layers. These are stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. The cells in the basal layer of epidermis proliferate by a mechanism of self-renewal, and, once activated, they withdraw from the cell cycle and start to differentiate. After detaching from the basement membrane, the cells migrate toward the outer epidermal layers, a process during which they progressively differentiate into terminally cornified cells. Epidermal cell proliferation and differentiation is controlled by a regulatory coordination of both the dermal–epidermal interaction and epidermal interaction. Many genes have recently been identified to participate in this regulation (1, 2). Genetic alteration of genes in mice by transgenic and knockout technologies has provided a powerful approach for studying the genes involved in skin homeostasis. However, natural or induced mutations in the mouse also offer useful tools for studying phenotype-driven genetics. One such mouse model for skin development and differentiation is the repeated-epilation (Er) mutation mouse. Er mutation in the mouse was induced by γ irradiation of spermatogonia and mapped to chromosome 4 (3) and is inherited as an autosomal and semidominant mutation (3, 4). Er/+ heterozygous mice are viable and fertile, with repeated loss of hair. Mice homozygous for the Er gene die at birth because of respiratory stress, develop skin characterized by hyperproliferation and failure to undergo terminal differentiation, and have a fused oral cavity and stumpy legs and tail. The molecular mechanism that contributes to these mutant phenotypes is unknown (3–7) Similar phenotypes were also observed in two other mutant mice, pupoid fetus (pf) and IKK1 (8–10). The pf mutation was mapped to chromosome 4, but it was not the same allele as the Er mutation (9). IKK1 is a Ser-Thr kinase mapped to chromosome 19 in the mouse. Thus, it does not appear that IKK1 is the candidate gene for the Er mutation. To identify gene(s) responsible for the Er mutation, we performed RNA profiling studies in Er/Er skin and fibroblast samples. In comparison with the control mouse (+/+), we identified transcriptional changes in a number of genes on chromosome 4. One of the genes identified was Sfn/14-3-3σ. We further identified a single nucleotide insertion in the Sfn gene resulting in a truncated mutant 14-3-3σ protein. We report here that loss of functional 14-3-3σ protein associates with the Er phenotypes.
Materials and Methods
Animals. Mating pairs of Er/+ mutant mice were ordered from The Jackson Laboratory. Er/+ heterozygous mice were generated and cross-mated to generate Er/Er homozygous fetuses. The mice were given food and water ad libitum. All procedures met National Institutes of Health guidelines, with the approval of The Salk Institute Animal Care and Use Committee.
Histology. All histological procedures were described in ref. 10. Whole embryos were embedded in optimal cutting temperature medium. Frozen sections were cut and fixed with 4% paraformaldehyde and stained with hematoxylin and eosin. The antibodies against keratin-5 (K5), K6, K10, K14, filaggrin, and loricrin were purchased from Covance Research Products (Denver, PA), and an antibody against the C terminus of 14-3-3σ (C-18) was from Santa Cruz Biotechnology.
Isolation and Culture of the Primary Mouse Embryonic Fibroblasts (MEFs) and Keratinocytes. MEFs were isolated from embryonic day (E)14.5 embryos. Briefly, the head and internal organs were removed and the main body tissues were minced to small pieces and digested with 0.25% trypsin at 37°C for 12 min. After inactivation of trypsin by adding DMEM containing 10% serum, a single-cell suspension was prepared by strong pipetting. The cells were cultured and further expanded in DMEM containing 10% serum.
For preparation of keratinocytes, the full-thickness skins taken from E18.5 embryos were digested with 0.05% trypsin overnight. The WT epidermal layer was peeled from the dermis. The keratinocytes were isolated from the inner surface of the WT epidermis by scraping cells from the epidermal sheet. The Er/Er keratinocytes were scraped from the outside surface of the skin. Keratinocytes were cultured in Defined Keratinocyte-SFM medium (Invitrogen).
RNA and cRNA Preparation for Affymetrix Analysis. Total RNA was extracted from MEF and skins of the E18.5 WT (+/+ or Er/+) and Er/Er embryos by using TRIzol reagent (Invitrogen). The A260/A280 ratio of all RNA samples was >2.0, and the RNA quality was checked on 1% agarose gel. Double-stranded cDNA was reverse-transcribed with a modified oligo(dT) primer with a 5′ T7 RNA polymerase promoter sequence and the SuperScript Choice system for cDNA synthesis (Invitrogen). The cRNA probes were transcriptionally biotin labeled from 10 μg of double-stranded cDNA with the ENZO kit (Affymetrix) and purified on an affinity column (RNEasy, Qiagen, Valencia, CA). Hybridization on mouse-genome array MG U74Av2 (Affymetrix) was performed by the Microarray Core Facility at The Salk Institute. The data were analyzed with the microarray suite 5.0 gene-expression-analysis program (Affymetrix).
Northern Blot Analysis. Ten micrograms of total RNA was fractionated on formaldehyde–agarose gels, blotted onto Gene-Screen Plus membrane (NEN Life Science Products), and hybridized with a 32P-labeled probe from the coding region of 14-3-3σ cDNA.
PCR, Sequencing, and Cloning. Genomic DNAs were extracted from +/+, Er/+, or Er/Er mice. We amplified genomic DNA by PCR and purified the PCR products using QIAquick purification columns (Qiagen). PCR primers used for amplifying the 14-3-3σ genomic fragment were 5′-cgcggatcccatatggagagagccagtctgatc-3′ (forward, 5′ end containing the BamHI site) and 5′-ccgctcgagtcagctctggggctcctccgg-3′ (reverse, 5′ end containing the XhoI site). PCR products purified with QIAquick purification columns were sequenced by using the same primers. For cloning, PCR products were digested with BamHI and XhoI and ligated into the BamHI and XhoI sites of the lentiviral vectors (11).
Lentiviral Vector Production. The lentiviral expression vectors for production of either WT or mutant 14-3-3 isoforms were constructed by inserting the PCR-generated genomic DNA into a lentivector. Lentiviral vectors were produced by a four-plasmid transfection system as described in refs. 12 and 13. Briefly, 293T cells were transfected with lentiviral expression vector and packaging plasmids, and the supernatants, containing recombinant pseudolentiviral particles, were collected from culture dishes at the second and third days after transfection. The lentiviral-vector titers were estimated by measuring the amount of the HIV p24 gag antigen with an ELISA kit (PerkinElmer). The high-titer preparation was used for transducing the primary keratinocytes. The expression of 14-3-3σ protein was confirmed by Western blotting.
Western Analysis. Tissues from the liver, intestine, kidney, heart, lung, and skin of the E18.5 embryos were homogenized and lysed in cold RIPA buffer (20 mM Tris·HCl, pH 7.4/100 mM NaCl/0.2% each of deoxycholate, Triton X-100, and Nonidet P-40) and a protease-inhibitor-mixture tablet (Roche Diagnostics). Equal amounts of whole-cell lysates were separated on 10% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and blotted with anti-14-3-3σ (N-14) (Santa Cruz Biotechnology), anti-filaggrin (Covance Research Products), and anti-actin (Sigma). The secondary antibody was conjugated with horse-radish peroxidase (Amersham Pharmacia). The blots were visualized with an enhanced chemiluminescence (ECL) system (Amersham Pharmacia).
Results
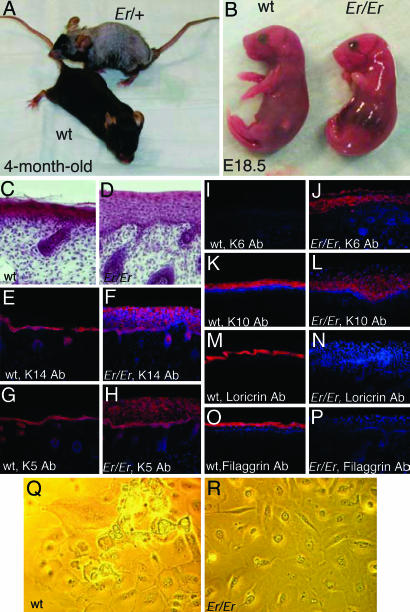
Er Mutation Blocks Skin Differentiation. The Er mutation in the mouse is genetically autosomal and semidominant (1, 2). Er/+ heterozygous mice are viable and fertile, with repeated hair loss in adults (Fig. 1A). Er/Er homozygous mice died at birth because of respiratory stress and skin defects, with characteristics of hyperplastic epidermis, failure of keratinocyte terminal differentiation, and abnormal craniofacial development, featuring fusion of the epithelium of the oral cavity and stumpy legs and tail (Fig. 1B). The thick epidermis lacks stratum corneum and stratum granulosum (Fig. 1 C and D). In an effort to better characterize the abnormal development of the mutant epidermis, we investigated a few differential markers by immunohistochemstry. The expressions of keratins K14 and K5 were limited in the basal cells and hair buds of WT epidermis but expanded to the suprabasal layer in Er/Er epidermis (Fig. 1 E–H). More significantly, the hyperplasic marker K6 was strongly stained in the suprabasal epidermis of the Er/Er skin, in contrast to the negative staining in normal skin (Fig. 1 I and J). The early differentiation-specific keratin K10 was stained in the WT suprabasal cells, and its expression was expanded in Er/Er epidermis because of its suprabasal expansion (Fig. 1 K and L). However, the Er/Er epidermis cells did not differentiate into mature granular and cornified cells that normally express loricrin and filaggrin (Fig. 1 M–P). The normal primary keratinocytes can differentiate in culture with association of specific morphological changes featuring cell rounding-up and detaching and floating from plates (Fig. 1Q). In contrast, the keratinocytes from Er/Er epidermis failed to undergo such differentiation-specific morphological changes (Fig. 1R).
Fig. 1.
Phenotypes of Er mutant mice. (A)An Er/+ heterozygous mouse (top) shows repeated hair loss and regrowth. (B) An Er/Er fetus at E18.5 (right) shows abnormal morphology, including shining skin with short limbs and tail. (C and D) Hematoxylin and eosin-stained sagittal sections of the dorsal skin from E18.5 embryos. Immunostaining of the sagittal sections of the dorsal skins from WT (E, G, I, K, M, and O) and Er/Er (F, H, J, L, N, and P) E18.5 embryos using anti-K14 (E and F), anti-K5 (G and H), anti-K6 (I and J), anti-K10 (K and L), antiloricrin (M and N), and anti-filaggrin (O and P) antibodies. Positive cells are stained red. Nuclei stained with DAPI are blue. (Q and R) Primary keratinocyte culture from WT (Q) and Er/Er (R) E18.5 embryos.
Transcriptional Level of 14-3-3σ on Chromosome 4 Was Altered in Er/Er Skin. To identify the gene(s) differentially expressed in the Er/Er mutation, we undertook systematic analyses of gene-expression profiles of the skin tissues isolated from two pairs of WT (+/+ or Er/+) and Er/Er E18.5 embryos. The labeled cRNA was synthesized in vitro from double-strand cDNA generated from reverse transcription of the total RNA isolated from those tissues and hybridized to Affymetrix mouse gene chips MG-U74Av2 containing 12,385 mouse genes. Expression comparison of mutant vs. WT was analyzed with Affymetrix microarray suite 5.0 software. There were potentially 513 transcripts identified as differentially expressed genes with fold change ratios >1.4 in two comparisons of Er/Er vs. WT. We assumed that, if a transcriptional change resulted from a genomic mutation, then the change would be persistent in all tissues where it is expressed. To identify the genomic mutation(s) responsible for Er/Er phenotypes, we compared these 513 differentially expressed candidates with those obtained by gene-expression comparison of the MEF cells isolated from the E14.5 WT and Er/Er embryos. We limited our candidate genes to those that simultaneously either up- or down-regulated in both skin and MEFs and also to those that were present only in skin but not in MEFs, to avoid exclusion of the candidates that were not expressed by MEFs. In a further effort to target the genes causing Er/Er mutation, we focused on the genes located on chromosome 4, on which the Er/Er allele has been mapped (3). By these efforts, eight genes were chosen as candidates, among which five were down-regulated and three were up-regulated (Table 1).
Table 1. Genes on chromosome 4 are transcriptionally changed in Er/Er skin (similarly changed or not expressed in Er/Er MEF).
| Gene | Gene ID | Fold changes |
|---|---|---|
| Orm | 435786 | –22.6, –24.3 |
| Wnt4 | 22417 | 1.7, 2.1 |
| Gjb3 | 14620 | –2.8, –3.2 |
| Akp2 | 11647 | –2.3, –2.6 |
| Elovl1 | 54325 | –2.1, –2.3 |
| Sfn | 55948 | 1.87, 2.46 |
| Map17 | 67182 | –3.7, –5.3 |
| Tie1 | 21846 | 1.4, 3.0 |
Fold change from two pairs of comparisons. – means the expression is down-regulated in Er/Er skin.
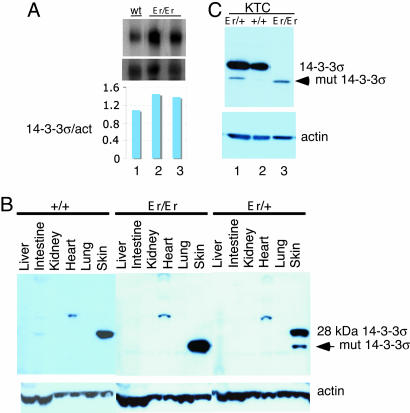
Based on the previous studies, one candidate 14-3-3σ (Stratifin/Sfn) was further pursued. It was first identified as an abundant protein in the stratifying keratinocytes (14), whose expression was induced during the exit of keratinocytes from the stem-cell compartment (15, 16). Functional inactivation of 14-3-3σ by antisense expression resulted in continued proliferation and immortalization of keratinocytes, presumably by preventing their exit from the stem-cell state and blocking keratinocyte differentiation (15). We initially tried to identify gene(s) by the loss-of-function mutation leading to the Er/Er phenotype in skin. To our surprise, the gene transcript of 14-3-3σ was up-regulated in Er/Er mutants, based on Affymetrix chip analysis (1.9- to 2.5-fold) and a further confirmation by Northern blot hybridization analysis (average 1.4-fold) (Fig. 2A).
Fig. 2.
Northern and Western analyses of 14-3-3σ expression in Er/Er skin and keratinocytes. (A) Northern analysis of RNA isolated from E18.5 embryonic skins with genotypes of normal littermates +/+ (lane 1) and Er/Er (lanes 2 and 3) skins showed increased 14-3-3σ transcripts in the Er/Er mutant mice. (Bottom) The quantified results of Northern analysis normalized by β-actin. (B) Western blotting of the tissue extracts shows that the full length of 28-kDa 14-3-3σ is expressed in +/+ skin (Left) but absent in Er/Er skin (Center). Instead, there is a 24-kDa protein recognized by 14-3-3σ antibody present in Er/Er skin (Center). The skins of Er/+ embryos from Er/+ intercrosses expressed both 28-kDa and 24-kDa isoforms (Right). (C) Western analysis shows that Er/Er keratinocytes express only a short 14-3-3σ isoform instead of the 28-kDa isoform, in comparison with the WT (+/+) control.
Loss of WT 14-3-3σ Protein Expression in Er/Er Skin and Keratinocytes. We examined by Western blotting the protein level in the tissues taken from E18.5 embryos by using an antibody raised against the N terminus of 14-3-3σ (N-14). The full-length 14-3-3σ protein with molecular mass of 28 kDa was detected in only the WT skin but not in the other WT tissues we examined (Fig. 2B Left). When we used the same antibody to examine different tissue samples from E18.5 Er/Er embryos, strikingly, we failed to detect the major 28-kDa band from all tissues we studied, but, instead, we detected a prominent smaller band with molecular mass of 24 kDa, present only in the skin sample (Fig. 2B Center). Both 28-kDa and 24-kDa isoforms were detected in the skin samples of some fetuses with normal-appearing morphology derived from Er/+ intercrossing, suggesting that those fetuses were Er/+ heterozygous (Fig. 2B Right).
We next investigated the expression profiles of 14-3-3σ isoforms in the primary keratinocytes isolated from the mice with genotypes of Er/+, +/+, and Er/Er. Consistent with the data obtained from the whole-skin tissue lysates, the major 28-kDa WT band was detected only in the +/+ and Er/+ keratinocytes but not in the Er/Er cells (Fig. 2C). Instead, the short 24-kDa bands appeared in both Er/Er and Er/+ keratinocytes. This smaller 24-kDa band in Er/Er or Er/+ skin samples was, conceivably, caused by either protein degradation or translational truncation.
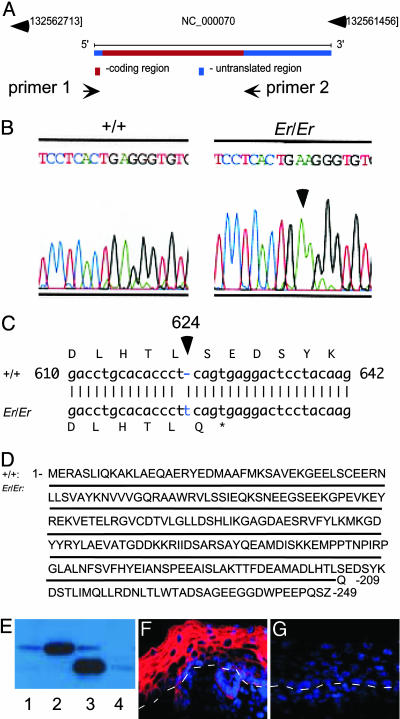
Er Mutation Was Caused by a Single Nucleotide Insertion in the Sfn Gene. To examine the possibility of truncation caused by a mutation resulting in a premature termination of the protein translation, we amplified by PCR and sequenced the genomic fragment spanning the 747-bp coding region of the single-exon Sfn gene (Fig. 3A). Remarkably, we identified a one-nucleotide insertion at nucleotide position 624 in the ORF (Fig. 3 B and C). This mutation causes a frameshift that can lead to the synthesis of a truncated 14-3-3σ isoform, lacking 40 amino acid residues at the C terminus (Fig. 3 C and D).
Fig. 3.
A-T pair insertion at nucleotide 624 of the14-3-3σ ORF resulted in a truncated form of protein lacking the C-terminal 40 amino acids. (A) Schematic view of the Sfn gene locus. The PCR primers 1 and 2 were designed to amplify the coding region of 14-3-3σ from genomic DNA. (B and C) Sequence comparison of PCR-amplified fragments from Er/Er and WT (+/+) control shows an A-T pair insertion at nucleotide 624 of the 14-3-3σ ORF. (D) WT 14-3-3σ sequence is shown on top. The mutation produced a truncated isoform of the 14-3-3σ lacking the C-terminal 40 amino acids. The solid line represents the same amino acid sequence in the mutant 14-3-3σ, with the last amino acid residue, Gln, in the place of Ser-209 before the end. (E) Expression of the WT 14-3-3σ ORF in the WT keratinocytes produced a full-length 14-3-3σ of 28 kDa (lane 2, compare with endogenous WT protein in lane 1). In contrast, expression of a mutant form of the 14-3-3σ isolated from Er/Er genomic DNA in the WT keratinocytes generated a truncated isoform of the 14-3-3σ protein with molecular mass of 24 kDa (lane 3, compare with Er/Er mutant endogenous 14-3-3σ in lane 4). (F and G) Immunohistochemical staining of the sagittal sections of the dorsal skins from the WT (F) and Er/Er (G) E18.5 embryos with an antibody specifically recognizing the C terminus of the 14-3-3σ. Positive cells are stained red. Nuclei stained with DAPI are purple. The white dashed lines represent the dermal–epidermal border.
To test whether this point mutation indeed caused a premature termination of the translation that produced a truncated protein with molecular mass of 24 kDa, we reintroduced an Er/Er genomic DNA fragment covering the 14-3-3σ coding region into keratinocytes by using a lentiviral vector as a delivery vehicle. This genomic fragment produced a short 14-3-3σ isoform with the size same as the one we detected in the Er/Er keratinocytes (Fig. 3E, compare lanes 3 and 4). In contrast, when we infected the cell with a lentiviral vector containing a WT 14-3-3σ genomic DNA fragment, a protein with a normal size of 28 kDa was produced (Fig. 3E, compare lanes 2 and 1). Furthermore, an antibody specifically recognizing the C terminus of 14-3-3σ detected a high-level expression of 14-3-3σ in the epidermal layer of the skin from WT E18.5 embryos (Fig. 3F) but not in the Er/Er skin (Fig. 3G).
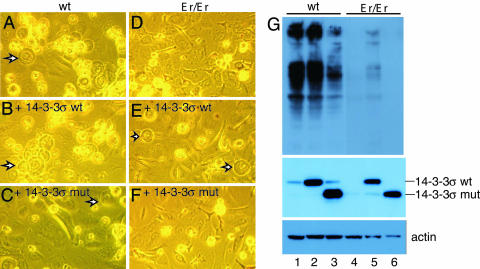
Overexpression of WT 14-3-3σ Rescued Er Phenotype in Keratinocytes. To further examine whether WT 14-3-3σ would rescue the normal differentiation of keratinocytes from Er/Er mice, we expressed a WT 14-3-3σ protein in cultured Er/Er keratinocytes. WT keratinocyte differentiation was readily detected after culturing in dishes for 4–5 days. Upon differentiation, the WT keratinocytes appeared rounded-up and detached from the plate, with bubble-like shapes, and shed into medium (Fig. 4A). The shed cells expressed filaggrin, an epidermal differentiation marker (Fig. 4G, lane 1). In contrast, Er/Er keratinocytes did not differentiate, based on morphological features (Fig. 4D), and shed cells did not express filaggrin (Fig. 4G, lane 4). Forced overexpression of the WT 14-3-3σ protein indeed rescued the differentiation phenotypes of the Er/Er keratinocytes, based on cell morphology (Fig. 4, compare E and D) and filaggrin expression (Fig. 4G, compare lanes 5 and 4). Previous studies have shown that 14-3-3 functions as a dimer, with the N terminus mainly responsible for dimer formation (17–19) and the C terminus providing the ligand-binding and phosphorylation sites for upstream kinases (20). Deletion of the C-terminal 38 residues in 14-3-3τ impaired macrophage stimulating protein-induced association of the receptor RON and β4-intergrin that was important for signaling keratinocyte migration (21). The truncated 14-3-3σ isoform likely has a dominant-negative effect on the function of endogenous WT protein. This appears to be the case, because overexpression of truncated 14-3-3σ in the cultured keratinocytes not only failed to rescue the undifferentiating phenotype in mutant keratinocytes (Fig. 4F) but also partially blocked the normal differentiation in the WT cells (Fig. 4C). Expression of the WT and mutant 14-3-3σ proteins was verified by anti-14-3-3σ antibody (Fig. 4G Middle). However, we cannot definitively conclude whether the dominant-negative effect of the mutant 14-3-3σ isoform or haploinsufficiency is the cause for the hair-loss phenotype in Er heterozygous mice.
Fig. 4.
The WT 14-3-3σ rescues the defective phenotype of the Er/Er keratinocytes. (A and D) Keratinocytes from E18.5 WT (A) and Er/Er (D) skins were cultured in SFM-keratinocyte medium for 8 days. (B and E) Keratinocytes from E18.5 WT (B) and Er/Er (E) skins at 5 days of transduction by a WT 14-3-3σ expression lentivector. (C and F) Keratinocytes from E18.5 WT (C) and Er/Er (F) skins at 5 days of transduction by a mutant 14-3-3σ expression lentivector. The arrows indicate differentiated keratinocytes in A–C and E.(G) Western analysis shows the expression of the keratinocyte differentiation marker filaggrin in the shed keratinocytes (Top), the expression of delivering WT and mutant 14-3-3σ in transduced keratinocytes (Middle), and actin expression (Bottom) as loading control. Samples 1–6 are protein extracts of keratinocytes shown in A–F.
Discussion
We have shown that Sfn mutation is the most likely cause of skin defects in Er mice. Sfn encodes stratifin/14-3-3σ, which belongs to a family of closely related adaptor proteins that act by interacting with various target proteins through its specific phospho-Ser/phospho-Thr-binding activity in a sequence-specific manner (22). The 14-3-3 binding alters the cellular localization, stability, phosphorylation activity, and interaction of the target proteins. Proteins that have been found to associate with 14-3-3 include transcription factors, enzymes, signal-transduction components, apoptosis regulators, and tumor suppressors. In mammals, there are seven isoforms (22). The 14-3-3σ is unique among the family, with its expression primarily in the epithelial cells and preferentially forming homodimers (23). The 14-3-3σ is involved in the differentiation of keratinocyte stem cells (16) and may also function as a tumor-suppressor gene, and its protein levels are significantly reduced or completely lost in a variety of primary tumors with epithelial origin, including many breast cancers (24) and hepatocellular carcinoma cells (25). The 14-3-3σ is strongly induced by ionizing radiation and DNA damage through a p53-responsive promoter element and has been shown to be involved in G2M checkpoint control after DNA damage (26, 27).
In line with these functions, we have shown here that Er/Er keratinocytes with the 14-3-3σ mutation are highly proliferative and fail to differentiate. Er/+ heterozygous mice have been demonstrated to exhibit an increased sensitivity to induction by 7,12,-dimethylbenz(a)anthracene and 12-O-tetradecanolphorbol-13-acetate to develop skin papillomas (28). Clearly, 14-3-3σ is a key mediator for keratinocyte growth, proliferation, and differentiation, and its functional disruption may contribute to skin cancer.
Recent studies have shown that secretion of 14-3-3σ from differentiated keratinocytes induces matrix metalloproteinase 1 expression in dermal fibroblasts (29, 30). This finding provides a potential mechanism for epidermal cells to influence the dermis. It is conceivable that the loss-of-function of 14-3-3σ in epithelium could result in not only epidermal defects but also mesodermal defects, because secreted 14-3-3σ from epidermis regulates dermal fibroblast function. Additional studies are needed to elucidate the mechanism of how 14-3-3σ regulates epidermal, skeletal, and craniofacial morphogenesis. Interestingly, the identical phenotypes are observed between Er/Er and IKB kinase 1 (IKK1)-deficient mice (10). Our study suggests that IKK1 and 14-3-3σ may be involved in the regulation of the same cellular function in keratinocytes.
Acknowledgments
Q. Li was supported, in part, by a grant from Wyeth. I.M.V. is an American Cancer Society Professor of Molecular Biology and is supported, in part, by grants from the National Institutes of Health, the Larry L. Hillblom Foundation, the Lebensfeld Foundation, and Merck Research Laboratories.
Author contributions: Q. Li and Q. Lu designed research; Q. Li, Q. Lu, and G.E. performed research; Q. Li, Q. Lu, and I.M.V. analyzed data; Q. Li, Q. Lu, and I.M.V. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: En, embryonic day n; MEF, mouse embryonic fibroblast.
References
- 1.Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199-209. [DOI] [PubMed] [Google Scholar]
- 2.Koster, M. I. & Roop, D. R. (2004) Eur. J. Cell Biol. 83, 625-629. [DOI] [PubMed] [Google Scholar]
- 3.Guenet, J. L., Salzgeber, B. & Tassin, M. T. (1979) J. Hered. 70, 90-94. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. S., Harne, L. C., Holbrook, K. A. & Dale, B. A. (1982) Prog. Clin. Biol. Res. 94, 251-264. [PubMed] [Google Scholar]
- 5.Holbrook, K. A., Dale, B. A. & Brown, K. S. (1982) J. Cell Biol. 92, 387-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tassin, M. T., Salzgeber, B. & Guenet, J. L. (1983) J. Craniofac. Genet. Dev. Biol. 3, 289-307. [PubMed] [Google Scholar]
- 7.Salzgeber, B. & Guenet, J. L. (1984) J. Craniofac. Genet. Dev. Biol. 4, 95-114. [PubMed] [Google Scholar]
- 8.Fisher, C., Jones, A. & Roop, D. R. (1987) J. Cell Biol. 105, 1807-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher, C. (2000) Trends Genet. 16, 482-484. [DOI] [PubMed] [Google Scholar]
- 10.Li, Q., Lu, Q., Hwang, J. Y., Buscher, D., Lee, K. F., Izpisua-Belmonte, J. C. & Verma, I. M. (1999) Genes Dev. 13, 1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marr, R. A., Rockenstein, E., Mukherjee, A., Kindy, M. S., Hersh, L. B., Gage, F. H., Verma, I. M. & Masliah, E. (2003) J. Neurosci. 23, 1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi, H., Blomer, U., Takahashi, M., Gage, F. H. & Verma, I. M. (1998) J. Virol. 72, 8150-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D. & Naldini, L. (1998) J. Virol. 72, 8463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leffers, H., Madsen, P., Rasmussen, H. H., Honore, B., Andersen, A. H., Walbum, E., Vandekerckhove, J. & Celis, J. E. (1993) J. Mol. Biol. 231, 982-998. [DOI] [PubMed] [Google Scholar]
- 15.Dellambra, E., Golisano, O., Bondanza, S., Siviero, E., Lacal, P., Molinari, M., D'Atri, S. & De Luca, M. (2000) J. Cell Biol. 149, 1117-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrini, G., Dellambra, E., Golisano, O., Martinelli, E., Fantozzi, I., Bondanza, S., Ponzin, D., McKeon, F. & De Luca, M. (2001) Proc. Natl. Acad. Sci. USA 98, 3156-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, D., Bienkowska, J., Petosa, C., Collier, R. J., Fu, H. & Liddington, R. (1995) Nature 376, 191-194. [DOI] [PubMed] [Google Scholar]
- 18.Xiao, B., Smerdon, S. J., Jones, D. H., Dodson, G. G., Soneji, Y., Aitken, A. & Gamblin, S. J. (1995) Nature 376, 188-191. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe, M. B., Rittinger, K., Volinia, S., Caron, P. R., Aitken, A., Leffers, H., Gamblin, S. J., Smerdon, S. J. & Cantley, L. C. (1997) Cell 91, 961-971. [DOI] [PubMed] [Google Scholar]
- 20.Dubois, T., Rommel, C., Howell, S., Steinhussen, U., Soneji, Y., Morrice, N., Moelling, K. & Aitken, A. (1997) J. Biol. Chem. 272, 28882-28888. [DOI] [PubMed] [Google Scholar]
- 21.Santoro, M. M., Gaudino, G. & Marchisio, P. C. (2003) Dev. Cell 5, 257-271. [DOI] [PubMed] [Google Scholar]
- 22.Fu, H., Subramanian, R. R. & Masters, S. C. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 617-647. [DOI] [PubMed] [Google Scholar]
- 23.Wilker, E. W., Grant, R. A., Artim, S. C. & Yaffe, M. B. (2005) J. Biol. Chem. 280, 18891-18898. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe, M. B. (2002) FEBS Lett. 513, 53-57. [DOI] [PubMed] [Google Scholar]
- 25.Iwata, N., Yamamoto, H., Sasaki, S., Itoh, F., Suzuki, H., Kikuchi, T., Kaneto, H., Iku, S., Ozeki, I., Karino, Y., et al. (2000) Oncogene 19, 5298-5302. [DOI] [PubMed] [Google Scholar]
- 26.Hermeking, H., Lengauer, C., Polyak, K., He, T. C., Zhang, L., Thiagalingam, S., Kinzler, K. W. & Vogelstein, B. (1997) Mol. Cell 1, 3-11. [DOI] [PubMed] [Google Scholar]
- 27.Chan, T. A., Hermeking, H., Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1999) Nature 401, 616-620. [DOI] [PubMed] [Google Scholar]
- 28.Lutzner, M. A., Guenet, J. L. & Breitburd, F. (1985) J. Natl. Cancer Inst. 75, 161-166. [PubMed] [Google Scholar]
- 29.Ghahary, A., Marcoux, Y., Karimi-Busheri, F., Li, Y., Tredget, E. E., Kilani, R. T., Lam, E. & Weinfeld, M. (2005) J. Invest. Dermatol. 124, 170-177. [DOI] [PubMed] [Google Scholar]
- 30.Ghahary, A., Karimi-Busheri, F., Marcoux, Y., Li, Y., Tredget, E. E., Taghi Kilani, R., Li, L., Zheng, J., Karami, A., Keller, B. O. & Weinfeld, M. (2004) J. Invest. Dermatol. 122, 1188-1197. [DOI] [PubMed] [Google Scholar]