Abstract
Mycobacterium bovis BCG, the only presently available vaccine against tuberculosis, was obtained from virulent M. bovis after serial passages in vitro. The vaccine strain retained at least some of its original virulence, as it persists in immune-competent hosts and occasionally may cause fatal disease in immune-deficient hosts. Mycobacterial persistence in vivo is thought to depend on anaerobic metabolism, an apparent paradox since all mycobacteria are obligate aerobes. Here we report that M. bovis BCG lacking anaerobic nitrate reductase (NarGHJI), an enzyme essential for nitrate respiration, failed to persist in the lungs, liver, and kidneys of immune-competent (BALB/c) mice. In immune-deficient (SCID) mice, however, bacilli caused chronic infection despite disruption of narG, even if growth of the mutant was severely impaired in lungs, liver, and kidneys. Persistence and growth of BCG in the spleens of either mouse strain appeared largely unaffected by lack of anaerobic nitrate reductase, indicating that the role of the enzyme in pathogenesis is tissue specific. These data suggest first that anaerobic nitrate reduction is essential for metabolism of M. bovis BCG in immune-competent but not immune-deficient mice and second that its role in mycobacterial disease is tissue specific, both of which are observations with important implications for pathogenesis of mycobacteria and vaccine development.
Mycobacterium tuberculosis claims more human lives each year than any other bacterial pathogen. Mycobacterium bovis BCG, the only presently available vaccine against tuberculosis, belongs phylogenetically to the M. tuberculosis complex. In humans, M. bovis BCG, like M. tuberculosis, forms granulomas and abscesses in various tissues. Following vaccination in immune-competent individuals, M. bovis BCG may persist for extended periods (34). In immune-compromised individuals the vaccine strain may even lead to fatal disease (2, 3, 10, 11, 14, 21, 27, 30, 36, 39).
Mycobacteria become firmly established within host tissues, adapting their metabolism to the available source of carbohydrates, nitrogen, and energy (4). Although the acquisition of essential nutrients by mycobacteria is an area of considerable interest, our knowledge of bacterial metabolism throughout the course of infection remains rudimentary. A recent study revealed that metabolism of fatty acids serves as a source of carbohydrates and is required for persistence of M. tuberculosis in mice and activated macrophages (25). Nitrate, through nitrate respiration, could provide energy for bacterial metabolism in an anaerobic environment, because anaerobic nitrate reductase (NarGHJI) couples the reduction of nitrate (NO3) to the generation of ATP by replacing oxygen as a terminal electron acceptor (29). Anaerobic nitrate reductase coding sequences (narGHJI) have been identified in both obligate aerobes such as Bacillus and Pseudomonas and facultative anaerobes such as Escherichia coli (5, 17, 28). However, a role of this enzyme in virulence was not established. In mycobacteria, a gene cluster homologous to narGHJI of Bacillus subtilis was first identified in the course of the M. tuberculosis genome project (12).
Previous studies of mycobacterial nitrate reduction have been limited to its role in classification and identification of the genus Mycobacterium, after an extensive study 40 years ago showed that M. tuberculosis reduces nitrate to nitrite, whereas M. bovis and M. bovis BCG have no discernible nitrate reductase activity (7, 35). Only recent reports revived interest in a possible physiological role by showing upregulation of enzymes involved in nitrate metabolism of M. tuberculosis and M. bovis BCG under oxygen restriction in vitro (19, 20, 37). We recently showed that both M. tuberculosis and M. bovis BCG express an anaerobic nitrate reductase (NarGHJI) activity and that a ΔnarG M. bovis BCG mutant lacked the ability to reduce nitrate under anaerobic conditions (38). The purpose of this study was to define the role of anaerobic nitrate reductase in the pathogenesis of M. bovis BCG.
MATERIALS AND METHODS
Bacteria.
M. bovis bacillus Calmette-Guérin (BCG) Pasteur (Pasteur vaccine strain; Statens Serum Institute, Copenhagen, Denmark) was used in this study. 7H9 broth or 7H10 plates (Difco Laboratories, Inc., Detroit, Mich.) supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% ADS (0.5% bovine albumin fraction V, 0.2% glucose, 140 mM NaCl) were used for culturing of mycobacteria unless indicated otherwise. Deletion of a 700-bp fragment of narG through allelic exchange has been described elsewhere (38).
In vitro tests.
Strains were grown in 7H9 broth supplemented as indicated above for 5 to 7 days to an optical density at 600 nm of 0.7 and were washed twice with MB medium supplemented as indicated below, but without nitrate. For persistence in vitro under anaerobic conditions, strains were kept in an anaerobic jar (anaerobic conditions were achieved with the AnaeroGen anaerobic system from Oxoid Ltd., Basingstoke, Hampshire, England) in MB medium with 0.5 mM MgCl2, 0.5 mM CaCl2, 0.2% glycerol, 0.05% Tween 80, 10% ADS, and 10 mM NO3 (1 liter of MB medium contained 1 g of KH2PO4, 2.5 g of Na2HPO4, 2.0 g of K2SO4, and 2 ml of trace elements; 1 liter of trace elements contained 40 mg of ZnCl2, 200 mg of FeCl3 · 6H2O, 10 mg of CuCl2 · 4H2O, 10 mg of MnCl2 · 4H2O, 10 mg of Na2B4O7 · 10H2O, and 10 mg of (NH4)6Mo7O24 · 4H2O). As recommended by the manufacturer, an indicator strip was used to confirm anaerobic conditions. Bacteria were cultivated in medium without nitrate, with 10 mM nitrate, or with 10 mM nitrate plus 10 mM ammonia. At various time points, an aliquot was plated for counts of CFU.
Infection of BALB/c and SCID mice.
BALB/c and SCID mice (6 to 10 weeks old) were obtained from the Tierlaboratorium of the Medical School of Hannover (Hannover, Germany). Prior to infection of mice M. bovis BCG and ΔnarG M. bovis BCG were grown in 7H9 broth supplemented with Tween and ADS as described above. The infecting dose of 106 bacilli per mouse was established by plating aliquots for colony counts and confirmed by plating again immediately after injection of mice. Intravenous infection of mice was performed through needle puncture of the tail vein. Mice were anesthetized with ether and sacrificed by cervical dislocation. Organs were harvested at the indicated time and were homogenized in phosphate-buffered saline with 0.05% Tween 80 using a homogenizer (Ultra-Turrax T8; IKA-Werke, Staufen, Germany), diluted, and plated on 7H10 supplemented with ADS as indicated above. Three mice were sacrificed for each time point in experiments with BALB/c and SCID mice. For the last time point in experiments with SCID mice organs from three mice were harvested after mice succumbed to the infection. Animal work in this article has been approved by the ethics committee of the county government of Hannover, Germany, on 18 March 1999 (reference number 509c42502-99/163).
Histology.
Small pieces of lungs and liver were fixed for 24 h in 10% buffered (pH 7.0) formalin and embedded in paraffin. Sections of 3 to 4 μ m (thickness) were cut with steel knives using an ultramicrotome. The sections were stained for acid-fast bacteria with Ziehl-Neelsen stain and counterstained with hematoxylin.
RESULTS
Persistence of wild-type and ΔnarG strains of M. bovis BCG under anaerobic conditions in vitro.
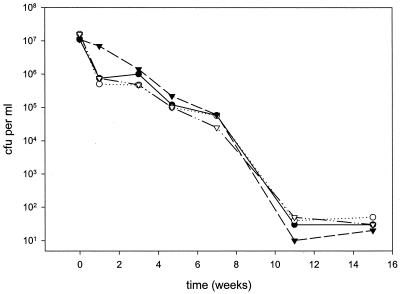
In one study, no difference in survival under hypoxic conditions was found between M. tuberculosis cells cultured in medium with nitrate and those in medium without nitrate (37). Therefore, we cultured wild-type and ΔnarG strains of M. bovis BCG in medium with nitrate and without nitrate under anaerobic conditions (Fig. 1). The mutant and the wild type were phenotypically indistinguishable, and bacterial viability gradually declined. After 15 weeks, cultures were almost sterile. We added ammonia as a second source of nitrogen to cultures supplemented with nitrate and repeated the experiments. Again, mutant and wild-type cells remained phenotypically indistinguishable (data not shown).
FIG. 1.
Wild-type (closed symbols) and ΔnarG mutant (open symbols) bacteria were enumerated by plating for counts of CFU. Bacteria were cultured in medium without nitrate (circles) and with nitrate (triangles) under anaerobic conditions.
Histopathology of the lung of SCID and BALB/c mice infected with a ΔnarG mutant of M. bovis BCG.
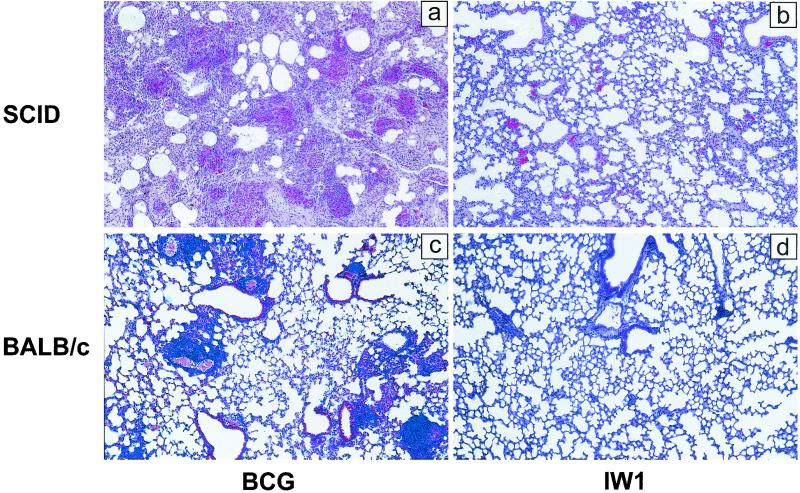
To define the role of anaerobic nitrate reductase in metabolism of M. bovis BCG in vivo, immune-deficient SCID mice and immune-competent BALB/c mice were infected with wild-type and ΔnarG mutant strains of M. bovis BCG. After 14 weeks SCID mice infected with the M. bovis BCG wild type succumbed to the infection. Histology revealed large coalescing lesions teeming with acid-fast bacilli replacing the entire lung and it seemed likely that mice had suffered fatal pulmonary failure (Fig. 2a). Mice infected with the ΔnarG mutant succumbed to the infection after 37 weeks (P < 0.05 for difference of survival by log rank test). Histology of lungs showed multiple small lesions filled with acid-fast bacilli scattered within healthy tissue (Fig. 2b). The results obtained from SCID mice suggested that despite pulmonary inflammation, the mutant was attenuated, as tissue destruction in the lung was substantially reduced and mice lived significantly longer. However, the mutant was obviously not avirulent. It remained unclear why mice infected with the mutant strain eventually developed a fatal disease. In immune-competent mice, the ability of the mutant to cause tissue distruction was profoundly different; in fact, the mutant was avirulent. Histology showed substantial tissue destruction in lungs of mice infected with the BCG wild type (Fig. 2c), but there were no signs of tissue damage in mice infected with the mutant (Fig. 2d) at 31 weeks postinfection. Thus, although M. bovis BCG lacking anaerobic nitrate reductase was attenuated in both immune-deficient and immune-competent mice, it was avirulent only in mice capable of generating a specific immune response, suggesting that the role of anaerobic nitrate metabolism in the pathogenesis of M. bovis BCG was linked to the immune response of the host.
FIG. 2.
Histology of the lungs of SCID mice (a and b) and BALB/c mice (c and d). Mice were infected with 106 CFU of wild-type (a and c) or ΔnarG (IW1) (b and d) bacteria. SCID mice succumbed to the infection after 14 weeks (a) and 37 weeks (b), whereas BALB/c mice were sacrificed 31 weeks after infection (c and d) and sections of the lung were stained with Ziehl-Neelsen and counterstained with hematoxylin.
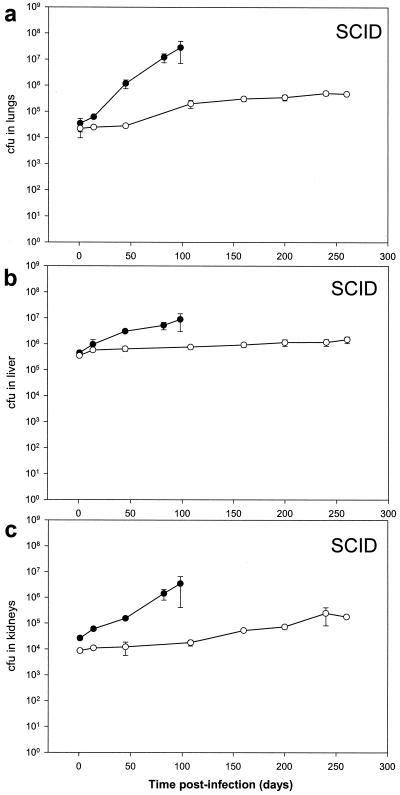
Bacillary load of ΔnarG strains of M. bovis BCG in lungs, liver, and kidneys of mice.
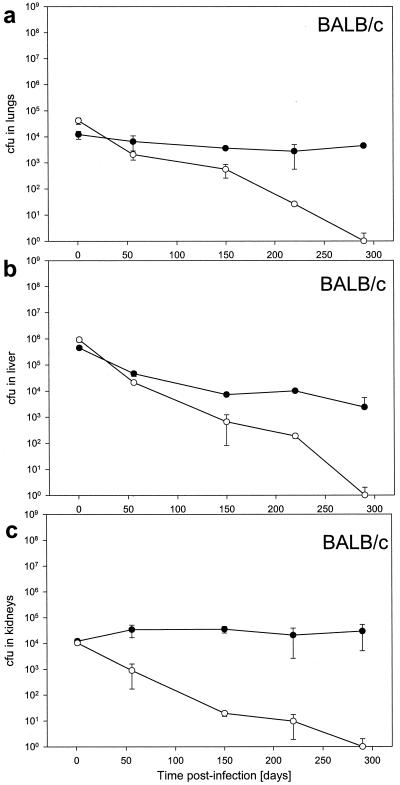
We further examined the phenotypic difference of wild-type and ΔnarG strains of M. bovis BCG in immune-deficient and immune-competent mice by comparing the bacillary load in various organs. Immune-deficient SCID mice were infected by intravenous infection with 106 bacteria. The M. bovis BCG wild-type strain grew progressively in the lungs, liver, and kidneys. In comparison, mice infected with the mutant showed little growth of bacilli, yet we observed no reduction of bacillary count at any time during the experiment (Fig. 3). On the one hand, this could represent a dynamic steady state in which the rate of bacillary killing just balances multiplication; on the other hand, it could represent a static phase in which bacilli divide seldom or never. Whatever the mechanism is, anaerobic nitrate reductase, although it facilitates progressive growth of bacilli, is not essential for metabolism of M. bovis BCG in the immune-deficient host. Immune-competent BALB/c mice were also infected intravenously with 106 bacteria. At all times during the experiment, mice showed no signs of clinical disease. The M. bovis BCG wild-type strain persisted in the lungs, liver, and kidneys. In comparison, the ΔnarG mutant was gradually cleared from these organs (Fig. 4). These results suggested that anaerobic nitrate reduction is essential for metabolism of M. bovis BCG in immune-competent, but not immune-deficient, mice.
FIG. 3.
Bacteria in the lungs (a), livers (b), and kidneys (c) of intravenously infected SCID mice were enumerated by plating for counts of CFU. Bacterial loads in mice infected with 106 CFU of ΔnarG (open circles) or wild-type (closed circles) bacteria are shown.
FIG. 4.
Bacteria in the lungs (a), livers (b), and kidneys (c) of intravenously infected BALB/c mice were enumerated by plating for counts of CFU. Bacterial loads in mice infected with 106 CFU of ΔnarG (open circles) or wild-type (closed circles) bacteria are shown.
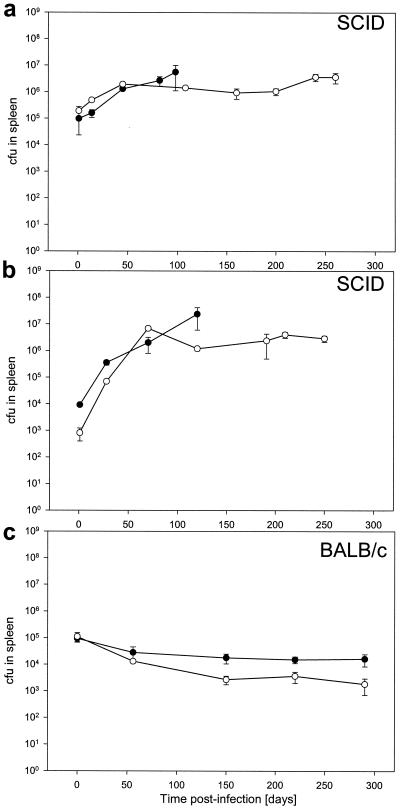
Growth and persistence of wild-type and ΔnarG strains of M. bovis BCG in the spleens of mice.
Interestingly, the mutant was not attenuated in the spleens of infected mice. In immune-deficient SCID mice the mutant and the wild type grew at the same rate, eventually reaching the same plateau (Fig 5a). Equal rates of multiplication were also seen when mice were infected with 104 bacteria (Fig. 5b). In immune-competent BALB/c mice wild-type and ΔnarG strains of M. bovis BCG persisted throughout the experiment (Fig. 5c). The spleen was enlarged both in mice infected with the wild type and in those infected with the ΔnarG mutant (data not shown). Thus, in the spleen of both immune-competent and immune-deficient mice M. bovis BCG lacking anaerobic nitrate reductase activity was phenotypically indistinguishable from the M. bovis BCG wild type.
FIG. 5.
Bacteria in the spleens of intravenously infected SCID (a and b) and BALB/c (c) mice were enumerated by plating for counts of CFU. Bacterial loads in mice infected with ΔnarG (open circles) or wild-type (closed circles) bacteria are shown. Mice were infected with 106 CFU (a and c) or 104 CFU (b) of bacteria.
DISCUSSION
For understanding mycobacterial pathogenesis, the presence of essential nutrients in the host and their metabolism by the pathogen remains an area of considerable interest. This study suggests that M. bovis BCG uses nitrate as a key nutrient for pathogenesis, supporting bacterial metabolism in the lungs, liver, and kidneys through reduction of nitrate to nitrite. Although we observed little expansion of the ΔnarG M. bovis BCG mutant in the lungs, liver, and kidneys of immune-deficient mice over time, the infection was not cleared from these organs, leading to a chronic infection. In immune-competent mice, by comparison, the mutant was unable to sustain an infection of these organs. Surprisingly, in the spleen anaerobic nitrate reduction appeared to be dispensable for mycobacterial metabolism. These results suggest first that anaerobic nitrate reduction is essential for metabolism of M. bovis BCG in immune-competent but not immune-deficient mice and second that its role in pathogenesis is tissue specific.
Dissemination and subsequent persistence of bacteria are hallmarks of an infection with M. tuberculosis, accounting for reactivation of a latent focus and symptomatic disease later in life. Similarly M. bovis BCG may lead to dissemination and persistence after vaccination of infants, even of those with an intact immune system. In fact, dissemination of M. bovis BCG is a normal sequel as shown by autopsies on M. bovis BCG-vaccinated infants and children who died of unrelated causes; histological examination of tissues demonstrated granulomas in many distant organs, including lungs, liver, kidneys, and spleen. Organisms were found up to 3 years after vaccination without evidence of clinical disease (16, 34). Moreover, reactivation of M. bovis BCG in a patient with AIDS 30 years after vaccination has been reported (33). In the mouse model, “persistence” has been defined as the time when the number of bacteria has reached a plateau, despite numerous culturable bacilli (24). McKinney and his colleagues (25) demonstrated that isocitrate lyase, an essential enzyme for catabolism of fatty acids, is required for persistence of M. tuberculosis during the chronic phase of infection and that this requirement was dependent on an intact immune response of the host. An intact immune response leads to the formation of granulomas, which creates a hostile environment in which restricted access to nutrients and reduced oxygen tension may force mycobacteria to adapt their metabolic activity (4). Our results show that anaerobic nitrate reduction is required for persistence of M. bovis BCG in the lungs, liver, and kidneys and that this requirement is dependent on an intact immune response of the host, suggesting that nitrate might play an important role in mycobacterial persistence. Polar effects on the expression of genes other than the one targeted by specific deletion have not been documented for mycobacteria. Nonetheless, in future experiments we will address the interesting issue of whether altered expression of yet unknown genes contributes to the phenotype of the narG mutant by complementation studies using, for example, a single-copy integrating vector for rescue of the mutant phenotype in vivo.
Apparently nitrate is sufficiently provided in the lungs, liver, and kidneys of infected animals. The reported estimates of net nitrate synthesis by mammalian tissue vary greatly and range from 0.15 to 1 mM day−1 (22). Within tissue, nitrate is mainly a product of spontaneous degradation of nitric oxide. Nitric oxide, in contrast, is produced enzymatically by three different nitric oxide synthetases (32). An inducible nitric oxide synthetase is expressed in response to inflammatory and proinflammatory mediators (6). A variety of cells, including hepatocytes, can be induced to synthesize nitric oxide (9). Significant amounts of nitrate are detected in the urine of mice infected with bacteria, suggesting that nitrate is available in the kidney, especially in animals undergoing an inflammatory process (8, 15). It is intriguing to speculate that the inflammatory process due to mycobacterial infection in the lungs, liver, and kidneys might increase the amount of nitrate within granulomas and thereby provide an additional supply of this nutrient for anaerobic metabolism of the pathogen. Quite unexpected was the observation that anaerobic nitrate reductase activity was dispensable for metabolism of M. bovis BCG in the spleens of infected mice, at least for the first 250 days of infection. We do not believe that this discrepancy occurred simply due to a better supply of the spleen with oxygen, since compared to the lung, oxygen tension of the spleen is presumably lower. In the spleen, for some reason, production of nitrate might be reduced or absent, or the organ simply provides a different set of nutrients, including a component that could replace nitrate as a substrate for mycobacterial metabolism in vivo. Or there could be a second, possibly weak nitrate reductase activity of the mutant which might be sufficient for anaerobic metabolism in the spleen and which might also account for persistence of the narG mutant in SCID mice.
To our knowledge, gene disruption resulting in a selectively attenuated mutant has not been described for mycobacteria. In a practical sense, dissociation of mycobacterial nitrate metabolism between the spleen and other organs might prove beneficial to the development of M. bovis BCG as a safe live vaccine. Survival and growth of M. bovis BCG is necessary for eliciting protective immune responses, as early treatment of infected mice with isoniazid to inhibit bacillary growth prevents the development of effective acquired resistance. Similarly, vaccination with nonliving or nonreplicating bacilli is less effective than vaccination with bacilli capable of in vivo growth (see reference 26 and references therein). Moreover, M. bovis BCG has been proposed as a multivalent vaccine vehicle, delivering viral, bacterial, or protozoan antigens (1, 13, 18, 23, 31). A ΔnarG M. bovis BCG or a recombinant derivative expressing additional antigens, by selectively targeting the spleen and thereby inducing a protective immune response, might reduce the risk for developing disseminated disease with the vaccine strain, especially in immune-compromised individuals.
Acknowledgments
We thank D. Bitter-Suermann for his continuing interest and support.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to F.-C.B.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351: 479–482. [DOI] [PubMed] [Google Scholar]
- 2.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280: 1432–1435. [DOI] [PubMed] [Google Scholar]
- 3.Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Doffinger, S. Lamhamedi, P. Drysdale, D. Scheel-Toellner, J. Girdlestone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barclay, R., and P. R. Wheeler. 1989. Metabolism of mycobacteria in tissues, p. 37–106. In C. Ratledge, J. Stanford, and J. M. Grange (ed.), Clinical aspects of mycobacterial disease. Academic Press, London, United Kingdom.
- 5.Blasco, F., C. Iobbi, G. Giordano, M. Chippaux, and V. Bonnefoy. 1989. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the alpha and beta subunits in iron binding and electron transfer. Mol. Gen. Genet. 218: 249–256. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12: 64–76. [DOI] [PubMed] [Google Scholar]
- 7.Bonicke, R., S. E. Juhasz, and U. Diemer. 1970. Studies on the nitrate reductase activity of mycobacteria in the presence of fatty acids and related compounds. Am. Rev. Respir. Dis. 102: 507–515. [DOI] [PubMed] [Google Scholar]
- 8.Boockvar, K. S., D. L. Granger, R. M. Poston, M. Maybodi, M. K. Washington, J. B. Hibbs, and R. L. Kurlander. 1994. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 62: 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, G. C. 1999. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta 1411: 351–369. [DOI] [PubMed] [Google Scholar]
- 10.Casanova, J. L., S. Blanche, J. F. Emile, E. Jouanguy, S. Lamhamedi, F. Altare, J. L. Stephan, F. Bernaudin, P. Bordigoni, D. Turck, A. Lachaux, M. Albertini, A. Bourrillon, J. P. Dommergues, M. A. Pocidalo, F. Le Deist, J. L. Gaillard, C. Griscelli, and A. Fischer. 1996. Idiopathic disseminated bacillus Calmette-Guerin infection: a French national retrospective study. Pediatrics 98: 774–778. [PubMed] [Google Scholar]
- 11.Casanova, J. L., E. Jouanguy, S. Lamhamedi, S. Blanche, and A. Fischer. 1995. Immunological conditions of children with BCG disseminated infection. Lancet 346: 581. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393: 537–544. [DOI] [PubMed] [Google Scholar]
- 13.Connell, N. D., E. Medina-Acosta, W. R. McMaster, B. R. Bloom, and D. G. Russell. 1993. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc. Natl. Acad. Sci. USA 90: 11473–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emile, J. F., N. Patey, F. Altare, S. Lamhamedi, E. Jouanguy, F. Boman, J. Quillard, M. Lecomte-Houcke, O. Verola, J. F. Mousnier, F. Dijoud, S. Blanche, A. Fischer, N. Brousse, and J. L. Casanova. 1997. Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J. Pathol. 181: 25–30. [DOI] [PubMed] [Google Scholar]
- 15.Flesch, I. E., and S. H. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59: 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gormsen, H. 1956. On the occurrence of epitheloid granulomas in the organs of BCG-vaccinated human beings. APMIS 29: 117–120. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, T., B. Troup, A. Szabo, C. Hungerer, and D. Jahn. 1995. The anaerobic life of Bacillus subtilis: cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol. Lett. 131: 219–225. [DOI] [PubMed] [Google Scholar]
- 18.Honda, M., K. Matsuo, T. Nakasone, Y. Okamoto, H. Yoshizaki, K. Kitamura, W. Sugiura, K. Watanabe, Y. Fukushima, S. Haga, et al. 1995. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guerin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc. Natl. Acad. Sci. USA 92: 10693–10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutter, B., and T. Dick. 1999. Up-regulation of narX, encoding a putative “fused nitrate reductase” in anaerobic dormant Mycobacterium bovis BCG. FEMS Microbiol. Lett. 178: 63–69. [DOI] [PubMed] [Google Scholar]
- 20.Hutter, B., and T. Dick. 2000. Analysis of the dormancy-inducible narK2 promoter in Mycobacterium bovis BCG. FEMS Microbiol. Lett. 188: 141–146. [DOI] [PubMed] [Google Scholar]
- 21.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335: 1956–1961. [DOI] [PubMed] [Google Scholar]
- 22.Kelm, M. 1999. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta 1411: 273–289. [DOI] [PubMed] [Google Scholar]
- 23.Langermann, S., S. Palaszynski, A. Sadziene, C. K. Stover, and S. Koenig. 1994. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature 372: 552–555. [DOI] [PubMed] [Google Scholar]
- 24.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6: 1327–1329. [DOI] [PubMed] [Google Scholar]
- 25.McKinney, J. D., B. K. Honerzu, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406: 735–738. [DOI] [PubMed] [Google Scholar]
- 26.McKinney, J. D., W. R. Jacobs, and B. R. Bloom. 1998. Persisting problems in tuberculosis, p. 51–139. In R. M. Krause (ed.), Emerging infections. Academic Press, London, United Kingdom.
- 27.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335: 1941–1949. [DOI] [PubMed] [Google Scholar]
- 28.Philippot, L., A. Clays-Josserand, R. Lensi, I. Trinsoutreau, P. Normand, and P. Potier. 1997. Purification of the dissimilative nitrate reductase of Pseudomonas fluorescens and the cloning and sequencing of its corresponding genes. Biochim. Biophys. Acta 1350: 272–276. [DOI] [PubMed] [Google Scholar]
- 29.Philippot, L., and O. Hojberg. 1999. Dissimilatory nitrate reductases in bacteria. Biochim. Biophys. Acta 1446: 1–23. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeldt, V., A. Paerregaard, and N. H. Valerius. 1997. Disseminated infection with bacillus Calmette-Guerin in a child with advanced HIV disease. Scand. J. Infect. Dis. 29: 526–527. [DOI] [PubMed] [Google Scholar]
- 31.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351: 456–460. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr, D. J. 1999. Mammalian nitric oxide synthases. Biochim. Biophys. Acta 1411: 217–230. [DOI] [PubMed] [Google Scholar]
- 33.Talbot, E. A., M. D. Perkins, S. F. Silva, and R. Frothingham. 1997. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin. Infect. Dis. 24: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 34.Trevenen, C. L., and R. D. Pagtakhan. 1982. Disseminated tuberculoid lesions in infants following BCG vaccination. Can. Med. Assoc. J. 127: 502–504. [PMC free article] [PubMed] [Google Scholar]
- 35.Virtanen, S. 1960. A study of nitrate reduction by mycobacteria. Acta Tuberc. Scand. 47: 1–116. [PubMed] [Google Scholar]
- 36.von Reyn, C. F., C. J. Clements, and J. M. Mann. 1987. Human immunodeficiency virus infection and routine childhood immunisation. Lancet 2: 669–672. [DOI] [PubMed] [Google Scholar]
- 37.Wayne, L. G., and L. G. Hayes. 1998. Nitrate reduction as a marker for hypoxic shiftdown of Mycobacterium tuberculosis. Tuber. Lung Dis. 79: 127–132. [DOI] [PubMed] [Google Scholar]
- 38.Weber, I., C. Fritz, S. Ruttkowski, A. Kreft, and F. C. Bange. 2000. Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice. Mol. Microbiol. 35: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 39.Weltman, A. C., and D. N. Rose. 1993. The safety of Bacille Calmette-Guerin vaccination in HIV infection and AIDS. AIDS 7: 149–157. [DOI] [PubMed] [Google Scholar]