Abstract
Bromelain, a mixture of cysteine proteases from pineapple stems, blocks signaling by the mitogen-activated protein (MAP) kinases extracellular regulated kinase 1 (ERK-1) and ERK-2, inhibits inflammation, and protects against enterotoxigenic Escherichia coli infection. In this study, we examined the effect of bromelain on Salmonella enterica serovar Typhimurium infection, since an important feature of its pathogenesis is its ability to induce activation of ERK-1 and ERK-2, which leads to internalization of bacteria and induction of inflammatory responses. Our results show that bromelain dose dependently blocks serovar Typhimurium-induced ERK-1, ERK-2, and c-Jun NH2-terminal kinase (JNK) activation in Caco-2 cells. Bromelain also blocked signaling induced by carbachol and anisomycin, pharmacological MAP kinase agonists. Despite bromelain inhibition of serovar Typhimurium-induced MAP kinase signaling, it did not prevent subsequent invasion of the Caco-2 cells by serovar Typhimurium or alter serovar Typhimurium -induced decreases in resistance across Caco-2 monolayers. Surprisingly, bromelain also did not block serovar Typhimurium-induced interleukin-8 (IL-8) secretion but synergized with serovar Typhimurium to enhance IL-8 production. We also found that serovar Typhimurium does not induce ERK phosphorylation in Caco-2 cells in the absence of serum but that serovar Typhimurium-induced invasion and decreases in monolayer resistance are unaffected. Collectively, these data indicate that serovar Typhimurium-induced invasion of Caco-2 cells, changes in the resistance of epithelial cell monolayers, and IL-8 production can occur independently of the ERK and JNK signaling pathways. Data also confirm that bromelain is a novel inhibitor of MAP kinase signaling pathways and suggest a novel role for proteases as inhibitors of signal transduction pathways in intestinal epithelial cells.
Bromelain is a mixture of cysteine proteases obtained from pineapple stems that protects animals from enterotoxigenic Escherichia coli (ETEC)-induced diarrhea (3, 27). Bromelain exerts a dual antidiarrhea action. First, it temporarily inactivates enterocyte ETEC receptors such that bacteria cannot colonize the small intestine (3, 30). Second, bromelain inhibits intestinal secretion induced by ETEC heat-labile and heat-stable toxins by blocking host cell signal transduction downstream from the generation of cyclic nucleotides (28).
In further studies investigating the action of bromelain on intracellular signal transduction pathways, we showed that bromelain blocked activation of extracellular signal-regulated protein kinase 1 (ERK-1) and ERK-2 (29). In ThO cells, bromelain blocked anti-CD3ɛ monoclonal antibody (MAb; T-cell receptor dependent)- and combined phorbol 12-myristate 13-acetate (PMA) and calcium ionophore (receptor independent)-mediated activation of ERK-2. The inhibitory activity of bromelain was dependent on its proteolytic activity, as ERK-2 inhibition was abrogated by E-64 [ℓ-trans-epoxysuccinyl-leucylamido(4-guanidino)butane], a selective cysteine protease inhibitor. However, inhibitory effects were not caused by nonspecific proteolysis, as the protease trypsin had no effect on ERK. Bromelain does not act on ERK-2 directly, as it also inhibits activation of p21Ras, an effector molecule upstream from ERK-2 in the Raf-1/MEK/ERK-2 kinase signaling cascade.
Since bromelain prevents ERK-2 signaling in T cells, the current study was undertaken to investigate whether bromelain can, in addition, block ERK-2 signaling in other cell types, namely, intestinal cells. Also, since bromelain blocks pathogenesis due to ETEC, we investigated whether bromelain would affect pathogenesis due to a different pathogen in which ERK-2 signaling is thought to play a crucial role. Hence, we investigated the effect of bromelain on Salmonella enterica serovar Typhimurium-induced ERK-2 signaling and invasion of Caco-2 cells and its effect on serovar Typhimurium-induced IL-8 production. Salmonella is an important intracellular pathogen that causes a variety of illnesses, ranging from localized gastroenteritis to severe, life-threatening typhoid fever. To initiate disease, salmonellae first adhere to and then invade intestinal epithelial cells by activating host cell signal transduction pathways (9). Salmonella-induced epithelial cell signaling leads to reorganization of the host cytoskeleton, membrane ruffles at the point of bacterium-cell contact leading to internalization of the bacteria (reviewed in reference 41), and production of several proinflammatory mediators, including the chemokine IL-8 (14). The clinical manifestations of salmonellosis may be attributed to the host inflammatory response to this pathogen, in which IL-8 production leads to the recruitment and transepithelial migration of polymorphonuclear leukocytes (12, 17, 24).
A key element in the signaling pathways involved in transducing serovar Typhimurium-initiated signals to cellular responses is the family of mitogen-activated protein (MAP) kinases (14). Serovar Typhimurium infection of cultured intestinal epithelial cells leads to activation of MAP kinases ERK-1 and ERK-2, c-Jun NH2-terminal kinases (JNK), and p38 (4, 14, 32, 36). ERK activation is dependent on the GTP-binding protein p21Ras, with subsequent activation of the Raf-1/MEK1/ERK kinase cascade (15). JNK and p38 activation requires signals generated by members of the Rho family of GTP-binding proteins such as CDC42 and Rac-1, which play an essential role in mediating bacterium-induced actin cytoskeleton rearrangements (4). Activated ERK, JNK, and p38 mediate induction of the transcription factors AP-1 and NF-κB, resulting in IL-8 synthesis (14). Serovar Typhimurium activation of JNK is mediated by an effector molecule called SopE, a substrate of the serovar Typhimurium type III protein secretion system (13). The Salmonella effector(s) of ERK and p38 activation is not known.
Our results show that serovar Typhimurium induces serum-dependent ERK-1, ERK-2, and JNK activation in Caco-2 cells in a time- and dose-dependent manner. Increased intestinal epithelial cell (IEC) signaling also coincided with a concomitant increase in serovar Typhimurium invasion and a decrease in IEC monolayer resistance. In serum-deprived Caco-2 cells, serovar Typhimurium did not induce ERK or JNK signaling but bacterial internalization and changes in IEC monolayer resistance were not affected. These data suggest that serovar Typhimurium internalization can occur independently of ERK and JNK signaling. Bromelain inhibited serovar Typhimurium-induced ERK and JNK activation and also inhibited MAP kinase activation induced by the pharmacological agents carbachol and anisomycin. However, bromelain pretreatment of IEC monolayers did not prevent subsequent invasion of the epithelial cells by serovar Typhimurium nor did this pretreatment alter serovar Typhimurium-induced resistance across the monolayers. Collectively, these data indicate that both serovar Typhimurium-induced invasion of IEC and changes in the resistance of IEC monolayers can occur independently of the ERK and JNK signaling pathways. Surprisingly, bromelain did not inhibit IL-8 production but actually synergized with serovar Typhimurium to enhance IL-8 production in IEC. These data suggest that serovar Typhimurium stimulation of IL-8 secretion in Caco-2 cells may also occur independently of ERK and JNK activation. These results also show that bromelain is a novel inhibitor of several MAP kinase signal transduction pathways that may have a role in preventing pathophysiology in intestinal cells, where MAP kinases have a critical role.
MATERIALS AND METHODS
Antibodies.
Mouse anti-MAP kinase R2 (ERK-2) MAb was from UBI (Lake Placid, N.Y.). Goat anti-mouse and goat anti-rabbit immunoglobulin G (IgG) antibodies conjugated to horseradish peroxidase were from Bio-Rad (Hemel Hemstead, United Kingdom). Rabbit polyclonal phosphospecific MAP kinase IgG that detects ERK-1 and ERK-2 only when catalytically activated by phosphorylation at Thr202 and Tyr204 was from New England BioLabs (Hitchin, United Kingdom). A rabbit polyclonal phosphospecific JNK IgG that detects JNK-1 and JNK-2 only when activated by phosphorylation at Thr183/Tyr185 was also from New England BioLabs.
Reagents.
Bromelain (EC 3.4.22.32; activity; 1,541 nmol/min/mg) was purchased from Hong Mao Biochemicals (Rayong, Thailand). Phorbol 12-myristate 13-acetate (PMA), calcium ionophore A23187, carbachol, and anisomycin were from Sigma (Poole, United Kingdom). Other reagents were also from Sigma. Specific protease activity of bromelain was determined by monitoring the release of p-nitroaniline from the peptide–p-nitroanilide substrate Z-Arg-Arg-p-nitroanilide (31).
Bacteria.
Serovar Typhimurium wild-type strain SL1344 and isogenic mutant strain SL1344 invA, which is defective in the expression of Salmonella pathogenicity island 1, were provided by D. Pickard (Imperial College, London, United Kingdom). Serovar Typhimurium was grown overnight without shaking to late log phase (optical density of 0.90 at 650 nm; 5 × 108 bacteria per ml) in Luria-Bertani (LB) broth at 37°C. Cultures were centrifuged at 14,000 × g for 15 min and washed twice in sterile saline. Bacterial pellets were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) and adjusted to yield the desired concentration of bacteria to maintain a multiplicity of infection (MOI) of 10, 50, or 100 to 1 (bacterium-to-enterocyte ratio). Bacterial concentrations were confirmed by viable-cell counting on duplicate LB agar plates after serial dilution in phosphate-buffered saline and overnight culture at 37°C.
Cultured enterocytes.
Human Caco-2 cells (EAECC 2772) between passages 30 and 45 were cultured in 25 mM glucose-DMEM (Gibco, Paisley, United Kingdom) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, penicillin at 100 IU/ml, streptomycin at 100 μg/ml, 1% nonessential amino acids, 25 mM HEPES, and 4 mM ℓ-glutamine. For Salmonella invasion and experiments determining IL-8 secretion, Caco-2 cells were seeded at 4 × 104/cm2 onto Transwell-Clear polyester membrane filters (Corning Costar, High Wycombe, United Kingdom). Cells seeded onto filters were cultured for 14 days before use. For carbachol and anisomycin signaling experiments, cells were cultured in 75-cm2 flasks for 14 days postconfluence. All cells were maintained and experiments were conducted in a humidified atmosphere at 37°C and 5% CO2.
Invasion assays.
At 24 h before invasion assays, the Caco-2 cell culture medium was replaced with antibiotic-free medium or medium that was both antibiotic and serum free. Immediately before experimentation, the monolayers were washed twice with DMEM and the medium was replaced with medium that was both antibiotic and serum free. Bromelain has been shown to be inactivated by fetal calf serum (unpublished observations). Bromelain (0 to 50 μg/ml) diluted in DMEM was added to both the apical and basolateral surfaces of monolayers for 30 min at 37°C. Mock-treated monolayers were treated with an equal volume of DMEM. Monolayers were then washed three times with DMEM and resuspended in fresh antibiotic-free medium that either contained serum or was serum free, as appropriate. Monolayers were allowed to stabilize for 30 min at 37°C before invasion. Salmonellae suspended in DMEM were then added to the apical surface of Caco-2 monolayers for the times indicated later in the text and in the figure legends. At the desired time, bacteria were aspirated, the monolayers were washed twice with fresh DMEM, and the medium was replaced with serum-free culture medium containing gentamicin at 100 μg/ml to kill residual extracellular bacteria. After 1 h, the gentamicin was removed and the monolayers were washed twice with fresh DMEM. The monolayer support containing the epithelial cells was excised and immersed in ice-cold lysis buffer (25 mM Tris [pH 7.4], 75 mM NaCl, 0.4 mM EDTA, 0.5% Triton X-100, 0.4 mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM sodium pyrophosphate, leupeptin at 10 μg/ml, 740 μM phenylmethylsulfonyl fluoride, aprotinin at 10 μg/ml) for 30 min with continual rotation at 4°C. An aliquot of the lysate was used to quantify viable intracellular bacteria following serial dilution in phosphate-buffered saline and incubation overnight on LB agar at 37°C. The remaining lysate was clarified (14,000 × g for 10 min), and an equal volume of 2× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (50 mM Tris [pH 7], 700 mM 2-mercaptoethanol, 50% (vol/vol) glycerol, 2% [wt/vol] SDS, 0.01% [wt/vol] bromophenol blue) was added to postnuclear supernatants. Proteins were solubilized at 100°C for 5 min, and samples containing 106 cell equivalents were resolved by SDS-PAGE.
Stimulation of Caco-2 cells with PMA combined with ionophore, carbachol, or anisomycin.
Caco-2 cells (107) suspended in DMEM were treated with bromelain (0 to 50 μg/ml) for 30 min at 37°C. Mock-treated cells were treated with an equal volume of DMEM. At a high concentrations of bromelain (50 μg/ml), cell aggregation occurred, as noted previously (29). Following treatment, cell aggregates were gently dispersed, washed three times, and then resuspended in fresh DMEM. Cells were stimulated via the cell surface with carbachol (200 μM) or directly (receptor independent) with anisomycin (10 μg/ml) or PMA (80 to 320 nM) combined with ionophore (1 μM) for the times shown in the figure legends and the text. Stimulation was terminated by the addition of ice-cold lysis buffer as described above. Lysates were clarified, suspended in SDS-PAGE sample buffer, solubilized as described above, and used to investigate agonist-induced ERK and JNK activation.
Monolayer resistance measurements.
Transepithelial monolayer resistance was determined with an epithelial voltohmmeter (EVOM; World Precision Instruments, Stevenage, United Kingdom). Measurements were made at the times indicated later in the text and in the figure legends. Data are expressed as the percent decrease from the baseline in each individual monolayer.
Immunoblotting.
Separated proteins were transferred to nitrocellulose membranes (Bio-Rad), which were then blocked and immunoblotted with the appropriate antibodies, as shown in the figure legends. Immunoreactivity was determined with the ECL chemiluminescence detection system (Amersham Corp., Arlington Heights, Ill.). The relative amounts of substrate phosphorylation were quantitated by densitometry (Molecular Dynamics, Buckinghamshire, United Kingdom) with Phoretix 1-D software (Phoretix International, Newcastle, United Kingdom).
Measurement of IL-8 secretion.
At 24 h before assays were conducted, the Caco-2 cell culture medium was replaced with antibiotic-free culture medium that also contained serum. Monolayers were washed and treated with bromelain (25 μg/ml) or mock treated as described above. Salmonellae suspended in DMEM were added to the apical surface of monolayers for 1 h at 37°C. Bacteria were then aspirated, the monolayers were washed as described above, and the medium was replaced with culture medium containing serum and gentamicin (100 μg/ml). Monolayers were then incubated for a further 5 h at 37°C in 5% CO2 and a humid environment. The basolateral culture medium was then harvested, aliquoted, and stored at −20°C until required. IL-8 secretion was determined by ELISA (R&D Systems, Abingdon, Oxon, United Kingdom) as recommended by the manufacturer.
RESULTS
Serovar Typhimurium induces JNK and ERK activation in Caco-2 cells.
Several reports have described serovar Typhimurium-induced activation of ERK and JNK in the cultured epithelial cell lines Henle-407, HeLa, and A431 (14, 36, 39). However, to date there are no reports on serovar Typhimurium-induced ERK and JNK activation in Caco-2 cells. This is despite the facts that Caco-2 cells have been described as an ideal in vitro model for the study of Salmonella pathogenesis and that these cells more closely resemble normal small-intestinal enterocytes—the site of serovar Typhimurium invasion—than do the other cell lines studied (8, 11). Therefore, initial studies were first carried out to investigate the capacity of serovar Typhimurium to induce ERK and JNK signaling pathways in Caco-2 cells and we correlated these signaling events with invasion of enterocytes by the bacteria and changes in monolayer resistance.
Caco-2 cells cultured as monolayers on semipermeable supports were infected apically with serovar Typhimurium strain SL1344 or isogenic serovar Typhimurium SL1344 invA at an MOI of 100 bacteria per host cell. At increasing times after infection, changes in IEC monolayer resistance were recorded. At 1, 2, 3, and 5 h after infection, the monolayer support containing epithelial cells was excised and immersed in a lysis buffer. On the exact same cell lysate, we investigated Salmonella-induced ERK and JNK phosphorylation and quantified the number of internalized Salmonella bacteria.
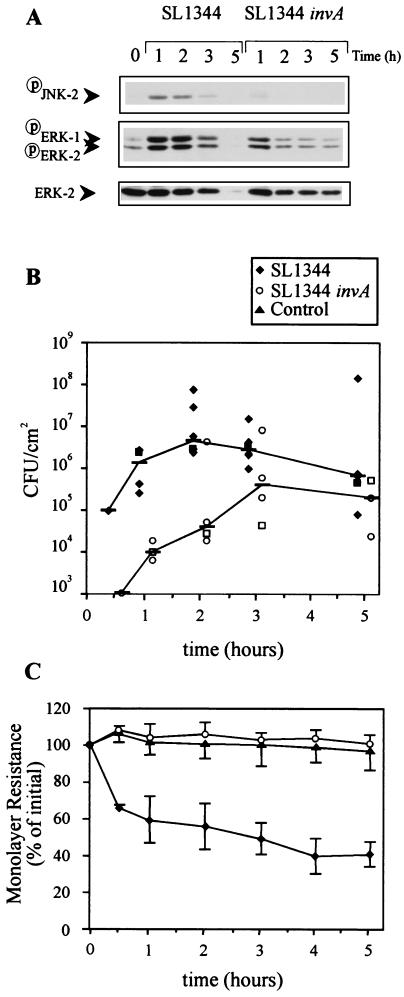
As shown in Fig. 1A, wild-type SL1344 induced phosphorylation of ERK-1, ERK-2, and JNK-2 1 h postinfection. As expected, phosphorylation of host proteins also coincided with an increase in SL1344 invasion and a decrease in IEC monolayer resistance (Fig. 1B and C), consistent with earlier reports (8). At 5 h postinfection, ERK and JNK phosphorylation and IEC monolayer resistance were completely abolished. There also appeared to be a reduction in the number of bacteria that invaded. This apparent decrease in cell phosphorylation and IEC monolayer resistance was most likely a result of SL1344-induced destruction of Caco-2 cells and their detachment from the monolayer support. This notion is supported by the observation that the level of ERK protein in SL1344-infected monolayers at 5 h postinfection is negligible (Fig. 1A). Detachment of IEC might also explain why there were fewer bacteria in monolayers at 5 h postinvasion. The reduction in bacterial numbers might also have been caused by disruption of the barrier function of the plasma membranes such that gentamicin penetrated the Caco-2 cells and killed internalized bacteria.
FIG. 1.
Effect of serovar Typhimurium SL1344 and SL1344 invA on MAP kinase activation, invasion, and monolayer resistance of Caco-2 cells. Confluent Caco-2 cells cultured in the presence of serum on semipermeable supports were infected apically with serovar Typhimurium strain SL1344 or isogenic serovar Typhimurium SL1344 invA at an MOI of 100. (A) At 1, 2, 3, and 5 h postinfection, extracellular bacteria were killed by gentamicin, monolayers were washed, and the monolayer support containing epithelial cells was excised and immersed in cell lysis buffer. Postnuclear supernatants of cell lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific MAP kinase. The blots were then stripped and reimmunoblotted with phosphospecific JNK antibody and anti-ERK-2 MAb. The results shown are from one experiment that is representative of the five conducted. (B) The numbers of internalized Salmonella bacteria in the cell lysates shown in panel A were quantified by serial dilution on LB agar. Each datum point represents the mean of duplicate agar plates, and lines link the median of the five experiments performed. The square symbol at each time point represents the result of the experiment shown in panel A. (C) Changes in monolayer resistance were recorded at the postinfection times indicated. Each datum point represents the mean percent change in initial monolayer resistance plus the standard deviation of the five experiments performed.
Strain SL1344 invA also induced ERK activation 1 h postinfection, but activation was substantially less than that by parent strain SL1344. JNK was only marginally activated at 1 h postinfection. SL1344 invA did not affect IEC monolayer resistance and invaded IEC 10- to 100-fold less than did the wild-type strain. These data show that Caco-2 cells cultured as monolayers on semipermeable supports are a suitable model for investigation of serovar Typhimurium-induced activation of JNK and ERK. They also show that induction of MAP kinase phosphorylation in Caco-2 cells, particularly JNK, is mediated in part by the Salmonella pathogenicity island 1 invasion locus.
Serovar Typhimurium-induced JNK and ERK activation in Caco-2 cells is dependent on the presence of serum.
To investigate whether lower rates of invasion are also capable of activating ERK and JNK in Caco-2 cells, we conducted invasion experiments with salmonellae at an MOI of 10. In addition, we also conducted studies with cells that had been serum starved for 24 h before infection. In several studies investigating the effects of specific agonists on mammalian cell signaling, cells were cultured in the absence of serum before signaling studies were conducted (37). Withdrawal of serum serves two purposes, (i) to synchronize cells into the G0 phase and (ii) to distinguish between specific agonist-induced signaling and that mediated by lysophosphatidic acid (LPA). LPA is a potent mitogenic serum phospholipid that binds albumin and activates several signaling pathways to mediate cell proliferation via Ras and ERK and cytoskeletal rearrangement via Rac and Rho signaling (26). The effects of purified LPA on ERK activity are similar to the effects of 10% fetal calf serum (6).
Since the LPA present in serum activates ERK and other signaling pathways, we investigated the effect of SL1344 on ERK and JNK signaling in cells that had been deprived of serum for 24 h before invasion experiments were conducted. We also investigated whether removal of serum affects serovar Typhimurium-induced changes in IEC monolayer resistance or internalization of bacteria. SL1344 (MOI, 10) was applied to the apical surface of Caco-2 monolayers, and experiments measuring JNK and ERK phosphorylation, monolayer resistance, and internalized bacteria were done as described above.
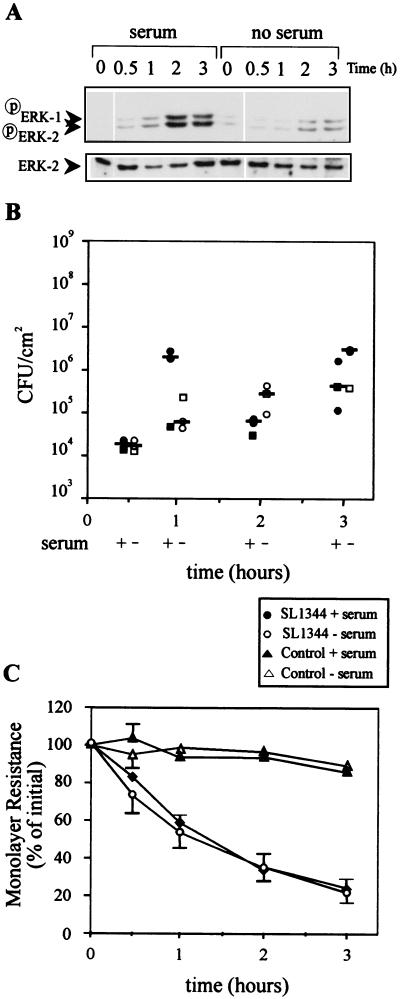
In Caco-2 cells cultured in the presence of serum and infected at an MOI of 10, serovar Typhimurium induced ERK, but not JNK (data not shown), phosphorylation in a time-dependent manner (Fig. 2A). The level of ERK phosphorylation reached the level observed when SL1344 was applied at an MOI of 100, but the ERK response was delayed (compare ERK activation at 1 h at an MOI of 100 [Fig. 1A] to that at 2 h at an MOI of 10 [Fig. 2A]). In Caco-2 cells from which serum was removed 24 h before the experiment (serum deprived), the ability of serovar Typhimurium to induce ERK phosphorylation was substantially reduced. The reduction in ERK phosphorylation was not caused by the ability of serum-deprived Caco-2 cells to respond to stimulation, since ERK-2 was equally phosphorylated when cells cultured in the presence and absence of serum were stimulated with carbachol (200 μM) (data not shown).
FIG. 2.
Serovar Typhimurium induction of ERK-1 and ERK-2 phosphorylation is dependent on the presence of serum. Confluent Caco-2 cells were cultured in the presence of serum or serum starved 24 h before apical infection with serovar Typhimurium SL1344 at an MOI of 10. (A) At 30 min and 1, 2, and 3 h postinfection, extracellular bacteria were killed by gentamicin and monolayers were washed and lysed. Cell lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific MAP kinase. Immunoblots were then stripped and reimmunoblotted with anti-ERK-2 MAb. The results shown are from one experiment that is representative of the three conducted. (B) The numbers of internalized Salmonella bacteria in the cell lysates shown in panel A were quantified by serial dilution on LB agar. Each datum point represents the mean of duplicate agar plates, and the bars show the median of the three experiments performed. Closed symbols represent Caco-2 cells cultured in the presence of serum; open symbols represent Caco-2 cells from which serum was removed 24 h before infection. Each square symbol represents an individual experiment shown in panel A. (C) Changes in monolayer resistance were recorded at the postinfection times indicated. Each datum point represent the mean percent change in initial monolayer resistance plus the standard deviation of the three experiments performed.
Despite the reduction in ERK activation observed in serum-deprived Caco-2 cells, the absence of serum did not affect the ability of SL1344 to invade IEC monolayers or affect their ability to reduce IEC monolayer resistance (Fig. 2B and C). We also observed that serovar Typhimurium at an MOI of 100 only marginally activated ERK signaling in serum-deprived Caco-2 cells but still equally invaded IEC in the presence and absence of serum (data not shown). The ability of serovar Typhimurium at an MOI 100 to invade equally cells cultured in the presence or absence of serum is consistent with an earlier report (36). The rate of invasion at an MOI of 10 was, however, 10- to 100-fold lower than that observed in Fig. 1B for SL1344 infection at an MOI of 100. This is despite the observation that at an MOI of 10, ERK was phosphorylated at a similar level, albeit after a delay.
These data indicate that since serovar Typhimurium is capable of invading Caco-2 cells in the absence of ERK activation, ERK activation may not be required for invasion. These data also suggest that factors present in serum, possibly LPA, might synergize with or induce serovar Typhimurium to induce ERK and JNK activation. It is interesting that serovar Typhimurium also does not induce IL-8 in Caco-2 cells cultured in the absence of serum (10), indicating that serum factors may also be important for serovar Typhimurium-induced IL-8 production.
Bromelain inhibits serovar Typhimurium-induced JNK and ERK activation but does not affect Salmonella invasion or Salmonella-induced changes in monolayer resistance.
We have previously shown that bromelain can block ERK signaling in T cells (29). Therefore, we next investigated whether bromelain can, in addition, block ERK signaling in intestinal cells. We studied bromelain’s effect on serovar Typhimurium-induced JNK and ERK signaling in Caco-2 cells and its effect on Salmonella-induced changes in IEC monolayer resistance and bacterial invasion.
Earlier, we showed that bromelain (50 μg/ml) pretreatment of T cells in suspension is effective at inhibiting ERK activation (29). However, when this dose level was used to pretreat Caco-2 cells cultured on permeable supports, there was an unacceptable decrease in IEC monolayer resistance of 25% in some monolayers incubated for periods of greater than 30 min (data not shown). Therefore, smaller doses of bromelain were used to study SL1344-induced JNK and ERK activation at incubation times of greater than 30 min.
Caco-2 cells cultured as monolayers in the presence of serum were washed and then treated on the apical and basolateral surfaces with bromelain (0, 10, 25, or 50 μg/ml) in medium without serum for 30 min. The monolayers were then rinsed with fresh DMEM and stabilized in fresh medium that contained serum before the addition of SL1344 (MOI, 100) to the apical surface. At either 30 or 60 min postinfection, the bacterium-containing medium was aspirated and replaced with fresh medium containing gentamicin, which was then incubated for 30 min. The monolayer support containing epithelial cells was then excised and immersed in a lysis buffer, whereupon ERK and JNK phosphorylation and SL1344 invasion were determined.
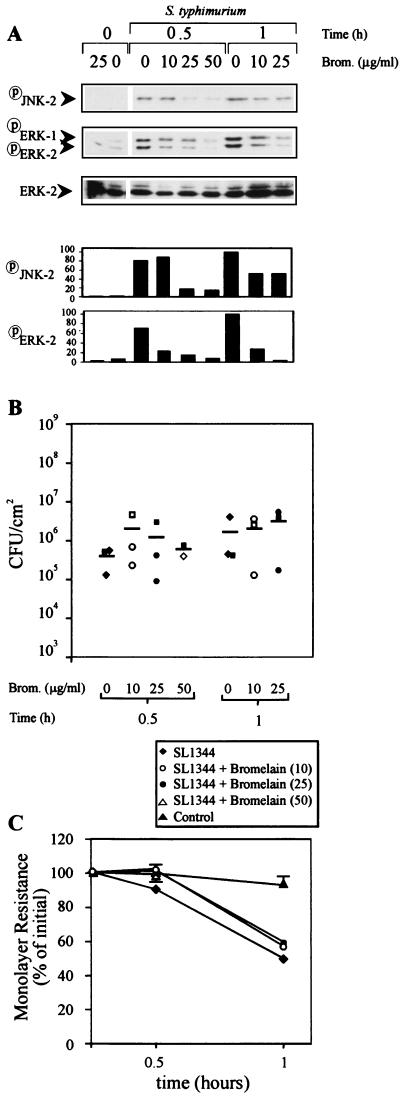
Immunoblots of Caco-2 cell lysates with specific anti-phosphospecific MAP kinase and anti-phospho-JNK antibodies revealed that bromelain dose dependently inhibited SL1344-induced ERK and JNK activation at 30 min postinfection. At the highest dose of bromelain (50 μg/ml), SL1344-induced ERK-2 and JNK-2 activation was almost completely blocked (Fig. 3A). At 1 h postinfection, bromelain (25 μg/ml) blocked SL1344-induced ERK-2 activation by 95% and JNK-2 activation by 62%. Similar results were obtained when SL1344 bacteria were inoculated at an MOI of 10 (data not shown). Despite bromelain inhibition of ERK and JNK activation, this treatment did not prevent invasion of IEC by SL1344 or SL1344-induced changes in IEC monolayer resistance at either infection rate (Fig. 3B and C). Since serovar Typhimurium can still equally invade cells with reduced ERK and JNK activation, collectively, these data further indicate that SL1344 invasion of Caco-2 monolayers and resistance changes induced by SL1344 can occur independently of the ERK and JNK signaling pathways.
FIG. 3.
Effect of bromelain on serovar Typhimurium SL1344-induced MAP kinase activation, invasion, and monolayer resistance of Caco-2 cells. Confluent Caco-2 cells cultured in the presence of serum were treated with bromelain (0, 10, 25, or 50 μg/ml) for 30 min on the apical and basolateral surfaces, washed, and then infected apically with serovar Typhimurium SL1344 at an MOI of 100. (A) At 0 and 30 min and 1 h postinfection, extracellular bacteria were killed by gentamicin, monolayers were washed, and Caco-2 cell lysates were subjected to SDS-PAGE and immunoblotting with phosphospecific MAP kinase or phosphospecific JNK antibody. Columns show the intensities of ERK-2 and JNK-2 signals quantitated by densitometry. (B) The numbers of internalized Salmonella bacteria in the cell lysates shown in panel A were quantified by serial dilution on LB agar. Each datum point represents the mean of duplicate agar plates, and the bars show the mean of the three experiments performed. Each square symbol represents an individual experiment shown in panel A. (C) Changes in monolayer resistance were recorded at the postinfection times indicated. Each datum point represents the mean percent change in initial monolayer resistance plus the standard deviation of the five experiments performed.
Bromelain reduces carbachol- and anisomycin-induced ERK-2 activation in Caco-2 cells.
Since bromelain blocks Salmonella-induced ERK and JNK activation in Caco-2 cells, we next investigated whether bromelain can block signaling induced by other MAP kinase agonists. Caco-2 cells suspended in DMEM were stimulated with a combination of PMA plus ionophore, carbachol, or anisomycin. PMA activates ERK-2 by a direct agonist action on protein kinase C and p21Ras, while ionophore A23187 induces increased intracellular release of Ca2+ and therefore mimics the action of inositol 1,4,5-trisphosphate. Carbachol is a different calcium agonist that activates ERK via Gq-protein-coupled M2 and M3 muscarinic receptors (21). Anisomycin is a protein synthesis inhibitor that acts intracellularly to activate the JNK/SAPK MAP kinase pathway (2).
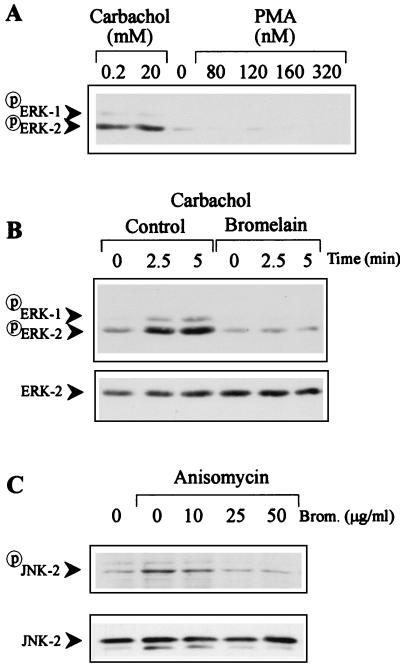
Immunoblots of Caco-2 cell lysates with specific anti-phosphospecific MAP kinase antibodies revealed that carbachol, but not combined PMA and ionophore, induced phosphorylation of ERK-2 (Fig. 4A and B). Even PMA at a concentration of 320 nM, a dose 3.2 times that previously used to induce ERK-2 activation in T84 cells (20), a different human intestinal epithelial cell line, failed to induce ERK-2 phosphorylation in Caco-2 cells (Fig. 4A). Stimulation for longer times with PMA also failed to induce ERK-2 activation (data not shown). These data show that despite the fact that PMA induces ERK-2 activation in several intestinal cell lines, including T84 (20), Intestine 407 (40), and IEC6 (18) cells, PMA does not activate ERK-2 in Caco-2 cells.
FIG. 4.
Bromelain inhibits ERK-1, ERK-2, and JNK phosphorylation in carbachol- or anisomycin-stimulated Caco-2 cells. (A) Caco-2 cells cultured in the presence of serum were stimulated with either carbachol (0 to 20 mM) or PMA (0 to 320 nM) plus ionophore (1 μM). (B and C) Caco-2 cells cultured in the presence of serum were suspended in fresh DMEM, treated with bromelain (50 μg/ml) for 30 min, and then washed. Cells were then stimulated with carbachol (200 μM; B) or anisomycin (10 μg/ml; C) for the times indicated. Cells were lysed, and postnuclear supernatants were subjected to SDS-PAGE and immunoblotted with phosphospecific MAP kinase, phosphospecific JNK antibody, or anti-ERK-2 MAb. The results shown are from one experiment that is representative of the three or four conducted.
Bromelain (50 μg/ml) pretreatment of Caco-2 cells for 30 min inhibited carbachol-induced activation of ERK-2 (Fig. 4B). Bromelain also dose dependently inhibited anisomycin-induced activation of JNK-2 (Fig. 4C). These data confirm that the inhibitory effects of bromelain on ERK-2 signaling are not restricted to T cells. These data also confirm that bromelain, in addition to inhibiting ERK activation, can also block activation of another MAP kinase pathway, the JNK/SAPK pathway.
Bromelain synergizes with serovar Typhimurium to increase IL-8 secretion in Caco-2 cells.
The MAP kinase pathways are thought to have a critical role in pathogen-induced IL-8 secretion, since selective inhibitors of the ERK-2 and p38 MAP kinases inhibit IL-8 secretion (14, 23, 38, 42). Since bromelain blocks the activation of several MAP kinase pathways, we investigated whether bromelain can, in addition, block IL-8 secretion.
Caco-2 cells cultured as monolayers on semipermeable supports were treated with bromelain (25 μg/ml) as described above. Monolayers were then washed, stabilized, and infected apically with serovar Typhimurium (MOI, 10, 50, or 100) for 1 h. Bacteria were then aspirated, residual extracellular bacteria on monolayers were killed with gentamicin (100 μg/ml), and monolayers were incubated for a further 5 h. Basolateral supernatants were assayed for IL-8 secretion by ELISA.
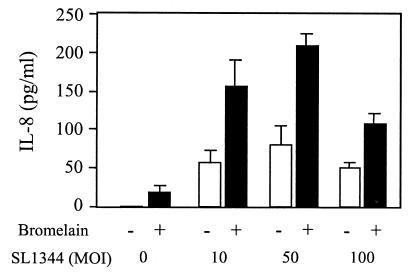
Data in Fig. 5 show that, as expected, serovar Typhimurium dose dependently increased IL-8 secretion by Caco-2 cells. The reduced amount of IL-8 secreted by cells infected with serovar Typhimurium at an MOI of 100 was presumably caused by bacterium-induced disruption of the monolayers, as described above. Treatment of monolayers with bromelain alone induced a negligible but detectable amount of IL-8. Surprisingly, bromelain significantly increased IL-8 secretion by Caco-2 cells that were infected with serovar Typhimurium (P < 0.01) at all of the infection levels tested. The amount of IL-8 produced was greater than the sum of the serovar Typhimurium-induced IL-8 and the IL-8 induced by bromelain alone. These results indicate that bromelain acts synergistically with serovar Typhimurium to increase IL-8 production by Caco-2 cells. Preliminary data also indicate that bromelain synergizes with carbachol to induce IL-8 secretion (data not shown). These data also indicate that serovar Typhimurium-induced IL-8 secretion may occur in the absence of ERK and JNK activation.
FIG. 5.
Effect of bromelain on serovar Typhimurium SL1344-induced IL-8 production. Confluent Caco-2 monolayers cultured in the presence of serum were treated with bromelain (0 or 25 μg/ml) for 30 min on the apical and basolateral surfaces, washed, and then infected apically with serovar Typhimurium SL1344 at an MOI of 10, 50, or 100. At 1 h postinfection, extracellular bacteria were killed with gentamicin (100 μg/ml) and the monolayers were incubated for a further 5 h. Basolateral supernatants were assayed for IL-8 secretion by ELISA. Columns represent the mean plus the standard deviation of three experiments performed on duplicate monolayers. The bromelain-induced increase in IL-8 is significant (P < 0.01 [Student’s paired t test]).
DISCUSSION
An important feature of Salmonella pathogenesis is its ability to activate host cell signal transduction pathways. Serovar Typhimurium induces activation of the ERK, JNK, and p38 MAP kinase pathways, which leads to rearrangement of the host cell cytoskeleton and internalization of the bacteria and IL-8 secretion (4, 14, 32, 36). In this study, we examined the effect of bromelain, a substance known to prevent ETEC pathogenesis (3, 28) and inhibit ERK signaling in T cells (29), on serovar Typhimurium-induced MAP kinase activation, internalization of bacteria, changes in monolayer resistance, and IL-8 secretion by cultured intestinal epithelial cells. We selected Caco-2 cells as our study model, since fully differentiated Caco-2 cells closely resemble the small-intestinal epithelium, the site of Salmonella invasion.
Our results show that serovar Typhimurium induces ERK-1, ERK-2, and JNK activation in Caco-2 cells in a time- and dose-dependent manner. Increased Caco-2 cell signaling also coincided with increased internalization of salmonellae and decreased monolayer resistance. An invasion-impaired serovar Typhimurium mutant (invA) also induced ERK and JNK activation and invaded Caco-2 cells, albeit at levels substantially lower than those of the wild-type strain. The invA mutant did not, however, affect Caco-2 monolayer resistance. The ability of Salmonella invA mutants to activate host cell signaling is consistent with earlier reports of studies conducted with Henle-407 cells (36) and BAC-1.2F5 macrophages (34) but contrasts with another study in which serovar Typhimurium invA mutants did not activate ERK-2 or JNK in HeLa or HenLe-407 cells (14).
Bromelain dose dependently reduced serovar Typhimurium-induced JNK and ERK activation but had no effect on Salmonella internalization into epithelial cells or IEC monolayer resistance. These data are consistent with earlier reports that show that ERK inhibitors do not inhibit serovar Typhimurium invasion (35, 36, 39). Rosenshine et al. (35) showed that the protein tyrosine kinase inhibitors genistein and tyrphostin-AG34 (which block ERK) prevent Yersinia enterocolitica and Y. pseudotuberculosis internalization into HeLa cells but not serovar Typhimurium internalization. They then confirmed these results in later studies with different cell lines (A431, Henle-407) (36). In separate studies, Tang et al. (39) compared the invasion of Listeria monocytogenes and serovar Typhimurium in the presence of PD98059, a highly selective inhibitor of MEK-1 and thus ERK. Again, data show that inhibition of ERK does not affect serovar Typhimurium invasion. PD98059 did, however, block L. monocytogenes invasion. These data suggest that ERK-2 signaling is not essential for serovar Typhimurium invasion of epithelial cells. However, it is clear that invasion by some pathogens is, indeed, dependent on ERK activation. Interestingly, studies investigating the effects of PD98059 on the internalization of serovar Typhimurium into macrophages show that this ERK inhibitor blocks Salmonella- and lipopolysaccharide-induced MEK and ERK phosphorylation but not phagocytosis (33). Those authors concluded that Salmonella phagocytosis and ERK activation are entirely independent phenomena. Since bromelain also blocks serovar Typhimurium-induced JNK activation, data also suggest that JNK is not essential for serovar Typhimurium invasion. Furthermore, since IEC monolayer resistance was not affected following bromelain treatment, data indicate that resistance changes induced by Salmonella may occur independently of JNK and ERK activation.
Further evidence that ERK is not essential for serovar Typhimurium invasion is the observation that Salmonella induction of host substrate phosphorylation is dependent on the presence of serum in the cell culture medium. The absence of serum, however, does not affect the ability of serovar Typhimurium to invade Caco-2 monolayers or affect its ability to reduce Caco-2 monolayer resistance. Many previous reports have shown that serovar Typhimurium induces ERK activation in epithelial cells when cells are cultured in the presence of serum for up to 2 h or immediately before experimentation (Fig. 2A) (25, 32, 35, 39). Our results obtained with Caco-2 cells cultured in the presence of serum are consistent with these data. In contrast, however, serovar Typhimurium fail to activate ERK in cells that had been deprived of serum 24 h before experimentation. These data suggest that ERK activation may not be required for internalization and changes in monolayer resistance. Data also suggest that factors present in serum, possibly LPA, a potent activator of ERK that mimics the effects of 10% serum (6), might induce serovar Typhimurium to activate ERK in Caco-2 cells.
Data described in a recent report might appear to conflict with our data that show that serovar Typhimurium requires serum to activate ERK. In a study investigating the effects of serovar Typhimurium on ERK in Henle-407 and HeLa cells, serum was removed 24 h before conducting invasion experiments (14), as in our studies. Hobbie et al. (14) noted that serovar Typhimurium induces ERK-2 activation in serum-starved, 50% confluent cells at 45 min postinfection. We noted only negligible activation of ERK in serum-starved Caco-2 cells. One possible explanation for the ability of serovar Typhimurium to strongly activate ERK-2 in Henle-407 and HeLa cells in the absence of serum, but not in Caco-2 cells, may reflect differences in the cell lines used. Differences in ERK-2 activation may also reflect differences in the functional states of the cells used in the two studies. Hobbie et al. (14) conducted studies with cell lines that had achieved 50% confluence, whereas our Caco-2 cells were used at least 15 days postconfluence (100% confluent), when cells have fully differentiated to a small-bowel phenotype. The differences in the results may also be attributed to the activation state of ERK. Recent studies suggest that cell density regulates the activity of ERKs (1). Aliaga et al. (1) showed that serum stimulation of ERK-1 and ERK-2 in subconfluent IEC-6 jejunal cells is rapid and maximal within 5 min, whereas the level of ERK stimulation by serum in confluent cells is significantly lower. Further data obtained with Caco-2/15 cells show that constitutive ERK activity varies substantially between subconfluent (undifferentiated) and confluent (differentiated) cells, while the abundance of ERK protein remains unchanged (1). Constitutive levels of active ERK-1 and ERK-2 were shown to be very high in subconfluent Caco-2/15 cells, while ERK activity dramatically decreased as Caco-2/15 cells reached confluence and ERK became inactive by 15 days postconfluence. These data indicate that the levels of ERK activation and their responsiveness to stimuli diminish as cells reach confluence and resemble those of the small intestine. Taken together, these data suggest that ERKs in confluent cells are less responsive to serovar Typhimurium stimulation. Thus, the effects of serovar Typhimurium on signaling in intestinal cells may vary, depending on the cell type, differentiation status, and degree of basal phosphorylation in the monolayers studied. The precise relevance of the effects of serum or the level of confluence of cells on serovar Typhimurium-induced host cell signal transduction remains to be elucidated. Data suggest that future studies be designed to distinguish effects mediated by serum, LPA, and Salmonella.
Data presented in this study also show that bromelain inhibits JNK and ERK signaling induced by the pharmacological agents carbachol and anisomycin. Since anisomycin is a lipid-soluble antibiotic, these results support our earlier studies that show that the inhibitory action of bromelain on cell signaling is not simply caused by a degradative action on cell surface molecules that alters receptor-ligand interactions (28, 29). The precise mechanism of action of bromelain remains to be determined. Earlier studies with T cells showed that bromelain exerts its inhibitory effect on ERK signaling at least at the level of p21Ras (29). Since p21Ras is an effector of both the JNK and ERK MAP kinase pathways, it is possible that bromelain’s ability to block ERK and JNK in Caco-2 cells is also at the level of p21Ras.
A surprising finding in this study is that despite bromelain’s blocking of MAP kinase activation, it did not inhibit IL-8 production. Earlier studies using inhibitors of MAP kinases have shown that ERK-2 is required for IL-8 production (23, 38). In contrast, we noted that bromelain synergized with serovar Typhimurium-induced signals to significantly (P < 0.01) enhance IL-8 secretion. These data suggest the existence of serovar Typhimurium-activated, ERK-independent pathways involved in IL-8 production in Caco-2 cells. Bromelain’s ability to synergize with exogenous signals to enhance cytokine production has also been observed in other cell types. Recently, bromelain was shown to enhance IFN-γ-mediated nitric oxide and tumor necrosis factor alpha production by both RAW 264.7, a macrophage cell line, and primary murine macrophages (7). Bromelain’s effect was independent of endotoxin receptor activation and was not caused by direct modulation of IFN-γ receptors. Instead, it either enhanced or acted synergistically with IFN-γ receptor-mediated signals. Bromelain also increased IL-2-plus-IL-12-mediated IFN-γ production by mouse-derived natural killer cells. Others have also shown that bromelain can increase IFN-γ-dependent tumor necrosis factor alpha, IL-1β, and IL-6 production by human peripheral blood mononuclear cells (5).
Since the clinical manifestations of salmonellosis may be attributed to the host inflammatory response to the pathogen (12), bromelain might be expected to exacerbate serovar Typhimurium-induced disease by increasing inflammatory responses. However, this possibility is in direct contrast to reports that describe the use of bromelain as an anti-inflammatory agent. Bromelain has been used therapeutically for the treatment of inflammation, including ulcerative colitis (19) and urogenital inflammation (22). Bromelain has also been shown to inhibit intestinal responses to prostaglandin E2, a proinflammatory mediator (28). Since inhibitors of ERK-1 and ERK-2 have been shown to elicit effective anti-inflammatory responses in vivo (16), the ability of bromelain to block ERK-1 and ERK-2 is consistent with an anti-inflammatory role for bromelain. Therefore, clearly, the role of bromelain as a pro-inflammatory or anti-inflammatory agent needs to be resolved. Indeed, it is well known that bromelain is composed of several different proteases (31) and preliminary testing of these isolated proteases indicates that the specific ERK-inhibitory and proinflammatory effects of bromelain are attributable to distinct proteases (Mynott et al., unpublished data). Studies are planned to investigate the role of these proteases in serovar Typhimurium infection in vivo.
In summary, we have shown that bromelain, a mixture of cysteine proteases, blocks serovar Typhimurium-induced ERK-1, ERK-2, and JNK activation in Caco-2 cells. Bromelain also inhibits carbachol- and anisomycin-induced MAP kinase signaling. These data extend our previous findings and show that bromelain is a novel inhibitor of MAP kinase pathways in intestinal cells. Despite bromelain inhibition of serovar Typhimurium-induced MAP kinase signaling, this treatment did not prevent subsequent invasion of Caco-2 cells by Salmonella or prevent the serovar Typhimurium-induced reduction of IEC monolayer resistance or IL-8 production. In serum-deprived Caco-2 cells, serovar Typhimurium did not induce ERK or JNK signaling, while bacterial internalization and changes in IEC monolayer resistance were not affected. Other studies have reported that in serum-deprived Caco-2 cells, serovar Typhimurium also fails to induce IL-8 production (10). Collectively, these data indicate that invasion of IEC by serovar Typhimurium, resistance changes induced by Salmonella, and IL-8 secretion can occur independently of the ERK-1, ERK-2, and JNK signaling pathways. These results also show that bromelain and cysteine proteases are novel inhibitors of MAP kinase signaling pathways in intestinal cells and may have a role in preventing diseases in the pathophysiology of which MAP kinases have a critical role.
Acknowledgments
This work was supported by a sponsored research grant from Provalis plc, Newtech Square, Deeside, Clwyd, United Kingdom.
Editor: V. J. DiRita
REFERENCES
- 1.Aliaga, J. C., C. Deschenes, J. F. Beaulieu, E. L. Calvo, and N. Rivard. 1999. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am. J. Physiol. 277: G631–G641. [DOI] [PubMed] [Google Scholar]
- 2.Bogoyevitch, M. A., A. J. Ketterman, and P. H. Sugden. 1995. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J. Biol. Chem. 270: 29710–29717. [DOI] [PubMed] [Google Scholar]
- 3.Chandler, D. S., and T. L. Mynott. 1998. Bromelain protects piglets from diarrhoea caused by oral challenge with K88 positive enterotoxigenic Escherichia coli. Gut 43: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. M., S. Hobbie, and J. E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274: 2115–2118. [DOI] [PubMed] [Google Scholar]
- 5.Desser, L., A. Rehberger, E. Kokron, and W. Paukovits. 1993. Cytokine synthesis in human peripheral blood mononuclear cells after oral administration of polyenzyme preparations. Oncology 50: 403–407. [DOI] [PubMed] [Google Scholar]
- 6.Dixon, R. J., and N. J. Brunskill. 1999. Lysophosphatidic acid-induced proliferation in opossum kidney proximal tubular cells: role of PI 3-kinase and ERK. Kidney Int. 56: 2064–2075. [DOI] [PubMed] [Google Scholar]
- 7.Engwerda, C. R., D. Andrew, M. Murphy, and T. L. Mynott. 2001. Bromelain activates murine macrophages and natural killer cells in vitro. Cell. Immunol. 210: 5–10. [DOI] [PubMed] [Google Scholar]
- 8.Finlay, B. B., and S. Falkow. 1990. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 9.Galan, J. E., and J. B. Bliska. 1996. Cross-talk between bacterial pathogens and their host cells. Annu. Rev. Cell Dev. Biol. 12: 219–253. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz, A. T., B. McCormick, A. S. Neish, N. A. Petasis, K. Gronert, C. N. Serhan, and J. L. Madara. 1998. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J. Clin. Investig. 101: 1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannasca, K. T., P. J. Giannasca, and M. R. Neutra. 1996. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect. Immun. 64: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannella, R. A. 1979. Importance of the intestinal inflammatory reaction in Salmonella-mediated intestinal secretion. Infect. Immun. 23: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93: 815–826. [DOI] [PubMed] [Google Scholar]
- 14.Hobbie, S., L.-M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159: 5550–5559. [PubMed] [Google Scholar]
- 15.Izquierdo, M., S. J. Leevers, C. J. Marshall, and D. Cantrell. 1993. p21ras couples the T cell antigen receptor to extra signal regulated kinase in T cells. J. Exp. Med. 78: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffee, B. D., E. J. Manos, R. J. Collins, P. M. Czerniak, M. F. Favata, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 2000. Inhibition of MAP kinase kinase (MEK) results in an anti-inflammatory response in vivo. Biochem. Biophys. Res. Commun. 268: 647–651. [DOI] [PubMed] [Google Scholar]
- 17.Jung, H. C., L. Eckmann, S.-K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai, M., C. Mullen, and D. K. Podolsky. 1998. Intestinal trefoil factor induces inactivation of extracellular signal-regulated protein kinase in intestinal epithelial cells. Proc. Natl. Acad. Sci. USA 95: 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane, S., and M. J. Goldberg. 2000. Use of bromelain for mild ulcerative colitis. Ann. Intern. Med. 132: 680. [DOI] [PubMed] [Google Scholar]
- 20.Keely, S. J., J. M. Uribe, and K. E. Barrett. 1998. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J. Biol. Chem. 273: 27111–27117. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. Y., M. S. Yang, C. D. Oh, K. T. Kim, M. J. Ha, S. S. Kang, and J. S. Chun. 1999. Signaling pathway leading to an activation of mitogen-activated protein kinase by stimulating M3 muscarinic receptor. Biochem. J. 337: 275–280. [PMC free article] [PubMed] [Google Scholar]
- 22.Lotti, T., V. Mirone, C. Imbimbo, F. Corrado, G. Corrado, F. Garofalo, and I. Scaricabarozzi. 1993. Controlled clinical studies of nimesulide in the treatment of urogenital inflammation. Drugs 46(Suppl. 1): 144–146. [DOI] [PubMed] [Google Scholar]
- 23.Marie, C., S. Roman-Roman, and G. Rawadi. 1999. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 67: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signaling to subepithelial neutrophils. J. Cell Biol. 123: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills, S. D., and B. B. Finlay. 1994. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microb. Pathog. 17: 409–423. [DOI] [PubMed] [Google Scholar]
- 26.Moolenaar, W. H., O. Kranenburg, F. R. Postma, and G. C. Zondag. 1997. Lysophosphatidic acid: G-protein signaling and cellular responses. Curr. Opin. Cell Biol. 9: 168–173. [DOI] [PubMed] [Google Scholar]
- 27.Mynott, T. L., D. S. Chandler, and R. K. Luke. 1991. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect. Immun. 59: 3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mynott, T. L., S. Guandalini, F. Raimondi, and A. Fasano. 1997. Bromelain prevents secretion caused by Vibrio cholerae and Escherichia coli enterotoxins in rabbit ileum in vitro. Gastroenterology 113: 175–184. [DOI] [PubMed] [Google Scholar]
- 29.Mynott, T. L., A. Ladhams, P. Scarmato, and C. R. Engwerda. 1999. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J. Immunol. 163: 2568–2575. [PubMed] [Google Scholar]
- 30.Mynott, T. L., R. K. Luke, and D. S. Chandler. 1996. Oral administration of protease inhibits enterotoxigenic Escherichia coli receptor activity in piglet small intestine. Gut 38: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napper, A. D., S. P. Bennett, M. Borowski, M. B. Holdridge, M. J. Leonard, E. E. Rogers, Y. Duan, R. A. Laursen, B. Reinhold, and S. L. Shames. 1994. Purification and characterization of multiple forms of the pineapple-stem-derived cysteine proteinases ananain and comosain. Biochem. J. 301: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pace, J., M. J. Hayman, and J. E. Galan. 1993. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell 72: 505–514. [DOI] [PubMed] [Google Scholar]
- 33.Procyk, K. J., P. Kovarik, A. von Gabain, and M. Baccarini. 1999. Salmonella typhimurium and lipopolysaccharide stimulate extracellularly regulated kinase activation in macrophages by a mechanism involving phosphatidylinositol 3-kinase and phospholipase D as novel intermediates. Infect. Immun. 67: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Procyk, K. J., M. R. Rippo, R. Testi, F. Hoffmann, P. J. Parker, and M. Baccarini. 1999. Distinct mechanisms target stress and extracellular signal-activated kinase 1 and Jun N-terminal kinase during infection of macrophages with Salmonella. J. Immunol. 163: 4924–4930. [PubMed] [Google Scholar]
- 35.Rosenshine, I., V. Duronio, and B. B. Finlay. 1992. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect. Immun. 60: 2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenshine, I., S. Ruschkowski, V. Foubister, and B. B. Finlay. 1994. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect. Immun. 62: 4969–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosskopf, D., W. Daelman, S. Busch, M. Schurks, K. Hartung, A. Kribben, M. C. Michel, and W. Siffert. 1998. Growth factor-like action of lysophosphatidic acid on human B lymphoblasts. Am. J. Physiol. 274: C1573–C1582. [DOI] [PubMed] [Google Scholar]
- 38.Scherle, P. A., E. A. Jones, M. F. Favata, A. J. Daulerio, M. B. Covington, S. A. Nurnberg, R. L. Magolda, and J. M. Trzaskos. 1998. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 161: 5681–5686. [PubMed] [Google Scholar]
- 39.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Der Wijk, T., J. Dorrestijn, S. Narumiya, J. A. Maassen, H. R. Jonge, and B. C. Tilly. 1998. Osmotic swelling-induced activation of the extracellular-signal-regulated protein kinases Erk-1 and Erk-2 in Intestine 407 cells involves the Ras/Raf-signalling pathway. Biochem. J. 331: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36: 997–1005. [DOI] [PubMed] [Google Scholar]
- 42.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]