Abstract
Infection with an obligate intracellular bacterium, the Chlamydia trachomatis lymphogranuloma venereum (LGV/L2) strain or the guinea pig inclusion conjunctivitis serovar of Chlamydia psittaci, leads to apoptosis of host cells. The apoptosis is not affected by a broad-spectrum caspase inhibitor, and caspase-3 is not activated in infected cells, suggesting that apoptosis mediated by these two strains of Chlamydia is independent of known caspases. Overexpression of the proapoptotic Bcl-2 family member, Bax, was previously shown to induce caspase-independent apoptosis, and we find that Bax is activated and translocates from the cytosol to the mitochondria in C. psittaci-infected cells. C. psittaci-induced apoptosis is inhibited in host cells overexpressing Bax inhibitor-1 and is inhibited through overexpression of Bcl-2, which blocks both caspase-dependent and -independent apoptosis. As Bax and mitochondria are ideally located to sense stress-related metabolic changes emanating from the interior of an infected cell, it is likely that Bax-dependent apoptosis may also be observed in cells infected with other intracellular pathogens.
Cell death occurs through different mechanisms that can be distinguished morphologically and biochemically. The most common forms of cell death are necrosis and apoptosis (24, 57, 58). The hallmarks of apoptosis are condensation of the nuclear chromatin and cytoplasm, activation of cysteine proteases (caspases) and endonucleases, loss of plasma membrane phosphatidylserine asymmetry, cleavage of the DNA into oligonucleosomal fragments, and segmentation of the dying cell into membrane-bound apoptotic bodies. During apoptosis in vivo, the apoptotic bodies are phagocytosed by neighboring cells in such a way that the contents are degraded intracellularly, usually without provoking inflammation. A number of viral, bacterial, and parasitic infections affect viability of the host cell, either inhibiting or promoting host cell apoptosis (4, 47, 54). Some microbes may protect infected cells against apoptosis induced by effector cells and soluble mediators of the immune system, while other microbes may trigger apoptosis of immune effector cells (21, 54).
In the nematode Caenorhabditis elegans, apoptosis is regulated by genes named for their cell death-defective (CED) quality (16). Two of the genes, ced-3 and ced-4, are necessary for execution of apoptosis. The mammalian homolog of CED-3 is a family of caspases, which contains at least 14 members (35, 48). In mammalian cells, caspases are present as inactive procaspases and are proteolytically processed into active caspases. Specific caspase inhibitors, consisting of smalltri- or tetrapeptides, have been developed that inhibit most types of apoptosis studied to date (22).
Caspases can be activated through engagement of cell surface receptors such as the tumor necrosis factor alpha receptor and Fas (39) or release of cytochrome c from mitochondria (31). Dysfunction of mitochondria can lead to redistribution of cytochrome c to the cytosol, which can activate caspase-9 in the presence of dATP and the CED-4 homolog, the procaspase-processing factor Apaf-1 (33). Caspase-9 in turn activates the effector caspase-3 that is responsible for many of the morphological and biochemical features of apoptosis (35, 48).
Mitochondria can be induced to release cytochrome c through a myriad of weak stress signals emanating from the interior of the cell or following activation of caspases stimulated by ligation of surface receptors (26). The integrity of the mitochondrial outer membrane and cytochrome release are regulated by the Bcl-2 family of proteins, which consist of antiapoptotic factors such as Bcl-2 and Bcl-xL, and proapoptotic proteins such as Bax and Bak (1). These proteins can heterodimerize with each other and interact with mitochondria, where they play a key role in deciding whether a cell will live or die (26, 27). Thus, Bcl-2 proteins prevent apoptosis by preventing the release of mitochondrial interspace proteins, including cytochrome c and the apoptosis inducing factor, while Bax stimulates release of cytochrome c from mitochondria, resulting in apoptosis (46, 50).
Recent studies demonstrate that some types of cell death can take place in the absence of caspase activation. In a growing number of cell death models, specific caspase inhibitors were unable to inhibit apoptosis induced by proapoptotic stimuli (14, 36, 60) and caspase activation was not sufficient for initiating apoptosis (8). In addition, overexpression of Bax or Bak induces cell death without the involvement of caspases (44, 60), suggesting that factors other than caspases can also mediate apoptosis. Some of these factors may reside normally in mitochondria. Apoptosis inducing factor, for example, promotes mitochondrial swelling, chromatin condensation, and cytochrome c release in the absence of caspase activation (52).
Most apoptotic pathways characterized to date have been triggered via surface receptors or induced with unphysiological ligands such as staurosporine, and most have relied on caspase activation for cell death to occur. Pathways initiated within the cell under physiological conditions have attracted less attention, and there are few models for caspase-independent pathways.
We have recently reported that infection in vitro by an obligate intracellular bacterium, the guinea pig inclusion conjunctivitis (GPIC) strain of Chlamydia psittaci or the mouse pneumonitis (MoPn) strain of Chlamydia trachomatis, results in morphological and biochemical changes in infected epithelial cells and macrophages that are characteristic of apoptosis, such as DNA fragmentation, condensation of nuclei, and positive staining with the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling technique, and that infection by the MoPn strain of C. trachomatis leads to shedding of apoptotic cells into the genital tract of infected mice in vivo (42, 45). In addition, apoptosis has been observed to occur in the genital tract of guinea pigs following infection with C. psittaci (12).
Chlamydia species are causative agents of a large spectrum of diseases in humans and animals (7, 9, 49). There are four known species of Chlamydia: C. trachomatis, C. psittaci, C. pneumoniae, and C. pecorum. The C. trachomatis serovars are responsible for infections of ocular tissue and sexually transmitted diseases in humans. C. psittaci elicits different symptoms, depending on its host. It causes intestinal infections in birds and abortion in pregnant ewes, but in humans it manifests itself as pneumonia. C pneumoniae is responsible for about a tenth of all pneumonias in humans and has recently been linked to atherosclerosis. C. pecorum is associated with infectious pneumonitis in domestic animals. However, in spite of the clinical and economic importance of Chlamydia infections, the biology of the infections is not well characterized (6, 51). The bacteria exist in two developmental forms: the infectious extracellular elementary bodies (EB) attach to the host cell and are internalized into a vacuole that avoids fusion with host cell lysosomes. Within 8 to 10 h, the EB differentiate into multiplicative intracellular reticulate bodies, which proliferate within the same membrane-bound vacuole. After several divisions, the reticulate bodies differentiate back into EB, and after 2 to 3 days, depending on the Chlamydia strain, the EB are released from the infected cell through unknown mechanisms and begin a new cycle of infection (38). The apoptosis observed at the end of the infection cycle could thus be involved in release of EB from the host cell and could partially contribute to the inflammatory response of the host, since macrophages undergoing apoptosis secrete inflammatory cytokines (28).
As C. psittaci-induced apoptosis was not sensitive to a specific inhibitor of caspase-3, we had proposed that the apoptosis may be independent of caspases (42). We now confirm the caspase independence by demonstrating that a broad-spectrum caspase inhibitor has no effect on the apoptosis during infection by C. psittaci or the C. trachomatis lymphogranuloma venereum (LGV/L2) strain and that caspase-3 is not activated during the infection. In order to characterize the molecular basis for the apoptosis, we studied the activation and distribution of Bax during Chlamydia infection and the influence of Bax inhibitor-1 (BI-1) and Bcl-2 on Chlamydia-induced apoptosis.
MATERIALS AND METHODS
Materials, cells, and bacteria.
Cytochalasin D, leupeptin, pepstatin A, TPEN [N,N,N′,N′-tetrakis (2-pyridylmethyl)ethylenediamine], and staurosporine were from Sigma (St. Louis, Mo.). The caspase inhibitor z-VAD-fmk [z-Val-Ala-Asp-(OMe)-fmk] was from Enzyme Systems Products (Livermore, Calif.). The human cervical adenocarcinoma cell line, HeLa 229; the human monocytic cell line, U937; and the C. trachomatis strain of the lymphogranuloma venereum (LGV/L2) strain were from the American Type Culture Collection (Manassas, Va.). The GPIC serovar of C. psittaci was from Roger Rank (University of Arkansas, Little Rock). Bacteria were prepared and cells were infected as previously described (42).
Transfections and clones.
The pcDNA3-BI-1-HA (61) and the pcDNA3-sbcl-2 plasmid (2), the stable U937 cell lines U937 overexpressing Bcl-2 (U937 CMV-s bcl-2), the control cell line U937pcDNA3 (2), and the stable HeLa cell lines overexpressing Bcl-2 (17) were previously described. DNA transfection of HeLa cell lines was performed using the FuGENE 6 Transfection Reagent kit (Boehringer Mannheim GmbH, Mannheim, Germany). Briefly, 2 × 105 HeLa cells/well were transfected in six-well plates (Costar) with 2 μg of plasmid DNA and 100 μl of FuGENE 6 Reagent diluted with serum-free Dulbecco modified Eagle medium. After incubation at room temperature for 15 min, the mixture was distributed in 35-mm-diameter culture dishes. The cells were infected 17 h after transfection. The expression level of pcDNA3-BI-1-HA and pcDNA3-Bcl-2 in HeLa cells and of Bcl-2 in the stable U937 transfectants was assessed by immunofluorescence microscopy and cytofluorimetry with rabbit polyclonal antibody (ΔC21) against human Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, Calif.) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (heavy and light chain) [IgG (H + L)] (Jackson Immuno Research Laboratories; West Grove, Pa.) or with antihemagglutinin (anti-HA) antibody (12CA5; kindly provided by Alain Israel, Institut Pasteur) and Fluorolink Cy3-labeled goat anti-mouse IgG (H + L) (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
Analysis of apoptosis.
Quantitative measurement of apoptosis was performed by cytofluorimetry of detergent-permeabilized propidium iodide-stained cells as described (40, 42). Both adherent cells and cells in suspension were collected for analysis. For cells transfected with the BI-1 or Bcl-2 constructs, apoptosis was quantified by immunofluorescence microscopy. Condensed nuclei were counted in Hoechst-stained cells that overexpressed Bcl-2, as observed with the anti-Bcl-2 antibody, or BI-1-HA, as revealed with the anti-HA antibody.
Caspase-3 activity assay.
Whole-cell extracts were obtained by dissolving HeLa cells in lysis buffer containing 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-NaOH (pH 7.5), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 20 μM cytochalasin D, 1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), leupeptin (1 μg/ml), pepstatin A (1 μg/ml), and 1 mM phenylmethylsulfonyl fluoride (PMSF). Samples were vortexed and sonicated in a cold-water sonicator. After centrifugation, the pellet was discarded and supernatants were stored at −20°C until ready for use. Measurement of protein concentration was performed with the Bio-Rad Protein Assay (Bio-Rad Laboratories GmbH, Munich, Germany).
Lysates (25 μg of cytosolic protein) were diluted in 50 μl of buffer A (25 mM HEPES [pH 7.5], 5 mM MgCl2, 5 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DTT, pepstatin A [10 μg/ml], and leupeptin [10 μg/ml]) to which was added 225 μl of buffer B (25 mM HEPES [pH 7.5], 0.1% CHAPS, 10 mM DTT, 1 mM PMSF) containing 100 μM DEVD-AFC (Alexis, San Diego, Calif.). Samples were incubated for 4 h at 37°C. Cleavage of the substrate resulted in enhanced fluorescence, which was quantified in a Perkin-Elmer LS50 luminescence spectrometer (excitation wavelength, 360 nm; emission wavelength, 475 nm).
Immunofluorescence microscopy.
For immunocytochemistry analysis, cells were seeded onto glass coverslips and then infected or kept as uninfected controls. The cells were then washed in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 15 min. After washing, the cells were incubated for 1 h with the N-20 anti-Bax polyclonal antibody (Santa Cruz Biotechnology) at a 1:100 dilution in permeabilization buffer (bovine serum albumin [1 mg/ml] and 0.05% saponin in PBS), washed twice in PBS-bovine serum albumin buffer, and revealed with a FITC-conjugated goat anti-rabbit IgG (H+L) (Jackson Immuno Research Laboratories). A monoclonal antibody against mitochondrial hsp70 (Affinity Bioregents, Golden, Colo.) was used at a dilution of 1:100, followed by Alexa Fluor 546 goat anti-mouse second antibody (Molecular Probes, Eugene, Oreg.) at a 1:100 dilution. Hoechst dye (5 μg/ml) was added to the cells in order to detect apoptotic nuclei. Fluorescently labeled samples were examined under a fluorescent microscope (Axiophot; Zeiss, Jena, Germany) attached to a cooled charge-coupled device camera (Photometrics, Tucson, Ariz.), using X63 Neofluar lenses, or by confocal microscopy, as described (41).
RESULTS
Caspase independence of Chlamydia-induced cell death.
We have previously reported that C. psittaci infection leads to apoptosis in epithelial cells and in monocytes in vitro (42) and that the MoPn strain of C. trachomatis causes apoptosis both in vitro and in vivo in infected mice (45). Based on the ability of a caspase-3 inhibitor to block Fas-induced apoptosis but not Chlamydia-induced apoptosis, we had proposed that the apoptosis observed during infection may be independent of caspase-3 (42).
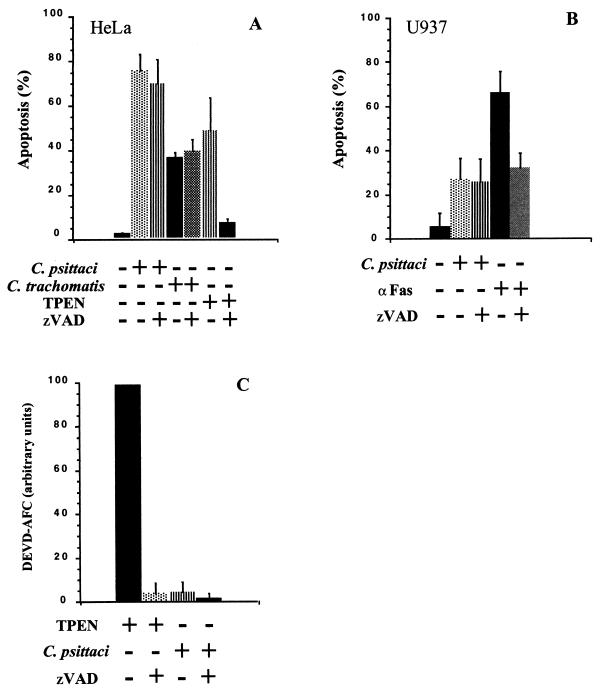
To determine whether other known caspases may be involved in apoptosis during infection, we studied the effects of the broad-spectrum caspase inhibitor, z-VAD-fmk, in two different human cell lines, HeLa epithelial cells and U937 monocytic cells. We also evaluated, as positive controls, the effect of this inhibitor on apoptosis due to incubation with the zinc chelator TPEN (30) or staurosporine (62) in HeLa cells, or with anti-Fas antibodies in U937 cells. After a 1-h incubation with 100 μM z-VAD-fmk, the cells were either infected for 48 h with a multiplicity of infection (MOI) of 0.7 (HeLa) and 1.0 (U937), treated for 17 h with 15 μM TPEN or for 24 h with 1 μM staurosporine (HeLa), or incubated for 16 h with anti-Fas antibody (0.5 μg/ml) in the presence of cycloheximide (U937) (2 μg/ml). As nuclei of apoptotic cells undergoing DNA fragmentation contain subdiploid amounts of DNA, apoptosis was quantified by cell cycle analysis with propidium iodide-stained cells (40). The inhibitor protected cells from apoptosis due to incubation of HeLa cells with TPEN (Fig. 1A) or staurosporine (not shown) or incubation of U937 cells with anti-Fas antibodies (Fig. 1B), suggesting that the caspase inhibitor was used at a sufficiently high concentration to decrease caspase-dependent apoptosis in HeLa or U937 cells. In contrast, z-VAD-fmk had no effect during infection with either C. psittaci or the LGV/L2 strain of C. trachomatis (Fig. 1A and B).
FIG. 1.
Caspase-independent cell death during infection of epithelial cells or monocytes with the GPIC strain of C. psittaci or the LGV/L2 strain of C. trachomatis. HeLa (A) and U937 (B) were infected with C. psittaci, at an MOI of 0.7 and 1.0, respectively, or with C. trachomatis at an MOI of 0.3, for 48 h in the presence or absence of 100 μM z-VAD-fmk. As positive controls for caspase-dependent cell death, HeLa cells were incubated with 15 μM TPEN for 17 h, and U937 cells were incubated with anti-Fas antibody (0.5 μg/ml) for 16 h. Apoptosis was measured by cytofluorimetry as described in Materials and Methods. The values represent the means and standard deviations (error bars) of three separate experiments. (C) Caspase-3 activity was measured in lysates from HeLa cells infected with C. psittaci for 48 h or treated with TPEN for 17 h, using the fluorescent substrate for caspase-3, DEVD-AFC, as described in Materials and Methods. The same level of apoptosis was measured in TPEN-treated or Chlamydia-infected cells under these conditions. The extent of DEVD-AFC cleavage in TPEN-treated cells was defined as 100, and the other values were normalized with respect to this positive control. The values represent the means and standard deviations (error bars) of three samples treated with TPEN or infected with Chlamydia on separate days.
Given the apparent caspase independence of apoptosis during Chlamydia infection, we examined the activation of caspase-3. A common step during late stages of many apoptotic processes is activation of caspase-3 (56), which we assayed by measuring the ability of lysates from infected or TPEN-treated HeLa cells to cleave the fluorogenic substrate of caspase-3, DEVD-AFC. In HeLa cells that had been treated with TPEN, there was a high level of caspase-3 activity, which was completely inhibited by z-VAD-fmk (Fig. 1C). However, under conditions in which the same percentage of cells is apoptotic, there was little if any caspase-3 activation after 48 h of infection with C. psittaci (Fig. 1C). These results are consistent with the observation that z-VAD-fmk does not have a significant effect on apoptosis during infection (Fig. 1A and B) and reinforce the interpretation that Chlamydia-induced apoptosis is independent of known caspases.
Bax activation during Chlamydia infection.
The proapoptotic protein Bax, a member of the Bcl-2 family, is activated in numerous cell lines in response to a variety of apoptotic stimuli (60) and translocates from the cytosol to mitochondria upon induction of apoptosis (55). In addition, overexpression of Bax induces apoptosis through mechanisms that are independent of caspases (60).
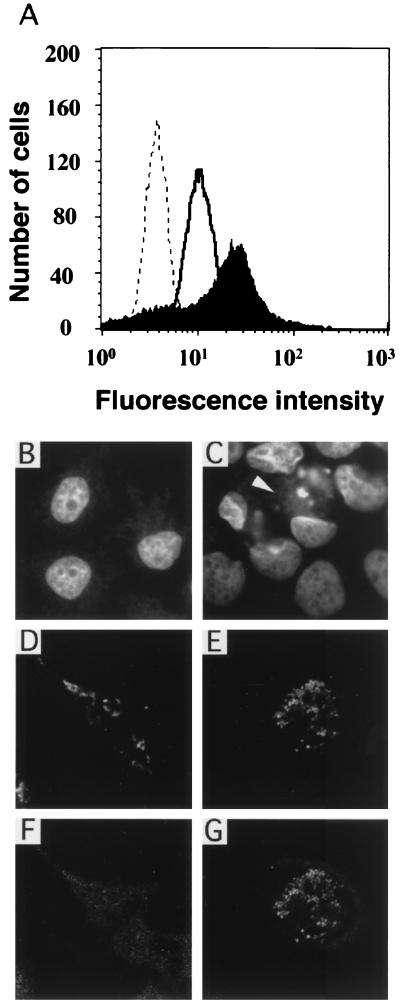
To determine whether Bax may play a role in C. psittaci-induced apoptosis, Bax activation was measured by cytofluorimetry. The anti-Bax antibody used, N-20, is specific for the Bax N terminus, which becomes exposed during Bax activation (15, 25, 29). Staining with this antibody increases after a 48-h infection with C. psittaci, compared to uninfected cells (Fig. 2A). To determine whether enhanced antibody binding to infected cells may be due to new synthesis of Bax, the cells were infected in the presence of a protein synthesis inhibitor. Cycloheximide did not decrease the level of Bax expression shown in Fig. 2A (data not shown), suggesting that preexisting Bax is activated during infection.
FIG. 2.
Bax activation during infection with Chlamydia. (A) Bax activation was measured in HeLa cells by cytofluorimetry with the anti-Bax antibody (N-20). The dotted line represents the spectrum in the absence of N-20 antibody, the solid line corresponds to Bax staining in uninfected cells incubated with N-20 and FITC-conjugated second antibody, and filled spectrum shows Bax staining in cells infected for 48 h and incubated with N-20 and second antibody. Bax activation and intracellular distribution were also evaluated by confocal microscopy. Nuclei stained with Hoechst of uninfected HeLa cells (B) or cells infected with C. psittaci for 48 h (C), showing fragmenting nuclei in infected cells, are shown. The arrowhead indicates a Chlamydia inclusion. Subcellular localization of Bax (F) and mitochondria (D) in uninfected cells is shown. Subcellular localization of Bax (G) and mitochondria (E) in cells undergoing apoptosis in infected sample is also shown.
Finally, we investigated the subcellular distribution of Bax in HeLa cells infected with C. psittaci for 48 h. Normal nuclei were observed in uninfected cells (Fig. 2B), while segmenting nuclei were found in many cells containing Chlamydia inclusions (Fig. 2C). In uninfected cells from the same experiment, diffuse cytoplasmic staining was observed with the anti-Bax antibody (Fig. 2F), which had a distribution different from that of mitochondria (Fig. 2D). In cells infected with C. psittaci (Fig. 2G), the staining of Bax became punctate and more intense, especially in cells displaying condensed nuclei, and colocalized with mitochondria (Fig. 2E).
Effect of BI-1 or Bcl-2 overexpression on Chlamydia-induced apoptosis.
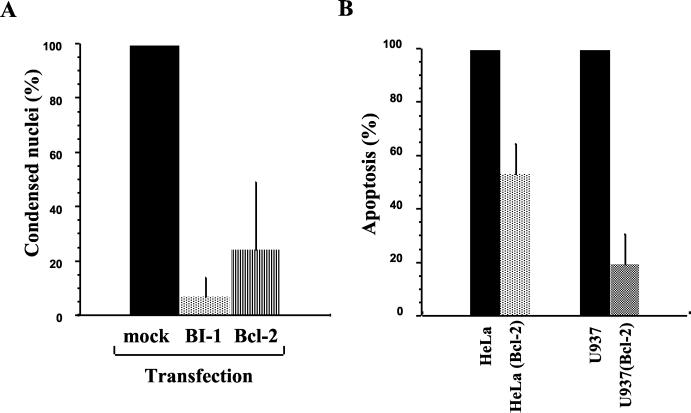
Since BI-1 interacts with Bcl-2 family members and inhibits apoptosis mediated by Bax but not by Fas (61), we infected HeLa cells that expressed transiently the BI-1 protein. As shown in Fig. 3A, BI-1 overexpression resulted in a 90% inhibition of apoptosis during infection, suggesting that Chlamydia-induced apoptosis may require the activity of Bax.
FIG. 3.
Inhibition of Chlamydia-induced apoptosis in cells overexpressing Bax inhibitor-1 (BI-1) or Bcl-2. (A) HeLa cells were transfected transiently with constructs encoding BI-1 or Bcl-2 for 1 day and then infected with C. psittaci for 48 h. Apoptosis was measured by immunofluorescence, as described in Materials and Methods, and the level of apoptosis was normalized to 100 for untransfected cells. (B) The stable U937 or HeLa clones overexpressing Bcl-2 and the corresponding clones transfected with control expression vector were infected with C. psittaci for 48 h. Apoptosis was measured by cytofluorimetry, and the level of apoptosis was normalized to 100 for untransfected cells. Each series of experiments was performed at least three times.
Bax is an antagonist of the antiapoptotic protein Bcl-2 (1), and overexpression of Bcl-2 can inhibit caspase-independent apoptosis (3, 43). We therefore investigated whether Bcl-2 overexpression has any effect on C. psittaci-induced apoptosis. Compared to untransfected cells, transient overexpression of Bcl-2 led to 80% specific inhibition of apoptosis due to infection with C. psittaci, and there was also inhibition in epithelial or monocytic clones overexpressing Bcl-2 permanently (Fig. 3B). Thus, Bcl-2 blocks the Bax-dependent, caspase-independent apoptosis due to Chlamydia infection.
DISCUSSION
We have previously observed apoptosis during infection of epithelial cells and macrophages with C. psittaci GPIC or C. trachomatis MoPn in vitro (42, 45) and in the genital tract of mice infected with C. trachomatis MoPn in vivo (45). To evaluate the relationship between Chlamydia infection and cell death, we investigate here the involvement of caspases and the role of the Bcl-2 family of proteins during Chlamydia infection.
We have previously shown that caspase-1 is activated during infection and participates in processing of interleukin-1β in monocytes, although caspase-1 is not involved in C. psittaci-induced apoptosis. An inhibitor of caspase-3 also had no effect on the level of apoptosis, suggesting that this caspase is not required for this pathway of apoptosis (42). In the present study, we show that the broad-spectrum caspase inhibitor, z-VAD-fmk, has no effect on apoptosis during C. psittaci GPIC or C. trachomatis LGV/L2 infection, even though it inhibits cell death pathways that rely on caspases. Furthermore, there was essentially no activation of caspase-3 during Chlamydia infection, compared to the levels observed in the same cells treated with the caspase-dependent apoptosis trigger, TPEN, whose apoptosis could be inhibited by z-VAD-fmk. These results suggest that caspases do not participate in the apoptosis observed during infection by C. psittaci GPIC or C. trachomatis LGV/L2 and that caspases are not activated during infection or, as previously proposed (18), are inhibited by the infection.
We next evaluated the role that two Bcl-2 family proteins (the proapoptotic protein, Bax, and the antiapoptotic protein, Bcl-2) may play during Chlamydia-induced apoptosis. We observed that the Bax protein redistributes to mitochondria in cells undergoing apoptosis and that the intensity of staining increases. Enhanced staining was confirmed by cytofluorimetry and was not affected by the protein synthesis inhibitor cycloheximide. These results are thus consistent with the observation that cycloheximide has no effect on apoptosis during C. psittaci infection (42) and are reminiscent of reports that Bax or Bak overexpression results in a cell death that does not depend on caspases (44, 60). Apoptosis during C. psittaci infection could be blocked by overexpression of the Bax inhibitor, BI-1, suggesting that Bax activation is also required for this cell death.
Many apoptotic stimuli cause relocalization of cytochrome c from the mitochondria to the cytosol, resulting in activation of the effector caspases, including caspase-3, in the presence of Apaf-1, pro-caspase-9 and ATP (34, 64). Bcl-2 normally interferes with this caspase activation (11), but it can also partially inhibit apoptosis at a postmitochondrial step, in cells that have been injected with cytochrome c (63). More importantly, Bcl-2 can suppress both caspase-dependent and caspase-independent pathways of apoptosis (11, 37, 43). We therefore studied the effect of Bcl-2 overexpression on apoptosis of monocytic or epithelial cells infected with C. psittaci. With either host cell type, apoptosis due to infection was inhibited significantly by Bcl-2.
Our results on the caspase independence of apoptosis during Chlamydia infection are consistent with a report that C. trachomatis infection inhibits release of cytochrome c from mitochondria and inhibits caspase activation when host cells are treated with staurosporine or anti-Fas antibodies (13, 18). It is thus likely that, at least for some strains, Chlamydia infection may both protect infected cells against apoptosis due to external ligands and induce apoptosis towards the end of the infection cycle (21). C. pneumoniae has also been reported to protect infected peripheral blood mononuclear cells against apoptosis, but through a different mechanism, involving secretion of interleukin-10 from infected cells (23). Apoptosis has now been described for infections with C. psittaci serovar GPIC, C. trachomatis MoPn strain, and C. trachomatis LGV/L2 strain (references 42 and 45 and this work), but an uncharacterized host cell death due to infection with low multiplicities of Chlamydia has also been previously reported for fibroblasts (L cells) and peritoneal macrophages infected with the meningopneumonitis strain, the 6BC strain, and the LW-613 strains of C. psittaci (10, 19, 53, 59), with a time course similar to what has been reported for apoptosis (onset of cell death beginning 20 to 36 h after the beginning of infection). In a plaque assay for infection with over 100 isolates of Chlamydia, all isolates of avian, mammalian, and human origin were cytotoxic for L cells, with the exception of slow-growing trachoma-inclusion-conjunctivitis agents (IC-Cal-3 and Apache 2) (5). While it is tempting to speculate that some of these strains cause host cell death through apoptosis, it is also possible that only some strains induce apoptosis. A careful study of host cell death and survival of cells infected with different strains should allow us to determine if the pro- and antiapoptotic activities are a general characteristic of Chlamydia infections.
Both inhibition and induction of apoptosis have been previously reported for other intracellular pathogens, including herpes simplex virus 1 (20). In the case of cells infected with a herpes simplex virus 1 mutant, apoptosis could take place in the presence of caspase inhibitors, similarly to apoptosis due to Chlamydia infection. Likewise, infection by the human adenovirus induces apoptosis through a mechanism that involves activation of caspases but is not inhibitable by the broad-spectrum caspase inhibitor z-VAD-fmk. However, adenovirus-mediated apoptosis is inhibited by overexpression of Bcl-2 or Bcl-xL (32). Inhibition of apoptosis due to external ligands may therefore protect infected cells against proapoptotic effectors of the immune system. Conversely, Bax activation during later stages of infection may be a general phenomenon occurring during other types of intracellular infections and could reflect a stress response in the infected cell (6).
Acknowledgments
We thank Philippe Souque for excellent technical assistance, Anne Roumier for help with the confocal microscope, and Philippe Kourilsky for support and encouragement.
This work was supported by the Institut Pasteur, Université Paris 7, and INSERM.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 2.Aillet, F., H. Masutani, C. Elbim, H. Raoul, L. Chêne, M.-T. Nugeyre, C. Paya, F. Barré-Sinoussi, M.-A. Gougerot-Pocidalo, and N. Israël. 1998. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4+ T or monocytic cell lines. J. Virol. 72: 9698–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarante-Mendes, G. P., D. M. Finucane, S. J. Martin, T. G. Cotter, G. S. Salvesen, and D. R. Green. 1998. Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 5: 298–306. [DOI] [PubMed] [Google Scholar]
- 4.Ameisen, J. C., J. Estaquier, and T. Idziorek. 1994. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol. Rev. 142: 9–51. [DOI] [PubMed] [Google Scholar]
- 5.Banks, J., B. Eddie, J. Schachter, and K. F. Meyer. 1970. Plaque formation by Chlamydia in L cells. Infect. Immun. 1: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bavoil, P. M., R.-C. Hsia, and D. M. Ojcius. 2000. Closing in on Chlamydia and its intracellular bag of tricks. Microbiology 146: 2723–2731. [DOI] [PubMed] [Google Scholar]
- 7.Bavoil, P. M., R.-C. Hsia, and R. G. Rank. 1996. Prospects for a vaccine against Chlamydia genital disease. I. Microbiology and pathogenesis. Bull. Inst. Pasteur 94: 5–54. [Google Scholar]
- 8.Boise, L. H., and C. B. Thompson. 1997. Bcl-x(L) can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc. Natl. Acad. Sci. USA 94: 3759–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, L. A., C. C. Kuo, and J. T. Grayston. 1998. Chlamydia pneumoniae and cardiovascular disease. Emerg. Infect. Dis. 4: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, G. T., and J. W. Moulder. 1978. Loss of inorganic ions from host cells infected with Chlamydia psittaci. Infect. Immun. 19: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosulich, S. C., P. J. Savory, and P. R. Clarke. 1999. Bcl-2 regulates amplification of caspase activation by cytochrome c. Curr. Biol. 9: 147–150. [DOI] [PubMed] [Google Scholar]
- 12.Darville, T., C. W. Andrews, and R. G. Rank. 2000. Does inhibition of tumor necrosis factor-α affect chlamydial genital tract infection in mice and guinea pigs? Infect. Immun. 68: 5299–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69: 2442–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deas, O., C. Dumont, M. MacFarlane, M. Rouleau, C. Hebib, F. Harper, F. Hirsch, B. Charpentier, G. M. Cohen, and A. Senik. 1998. Caspase-independent cell death induced by anti-CD2 or staurosporine in activated human peripheral T lymphocytes. J. Immunol. 161: 3375–3383. [PubMed] [Google Scholar]
- 15.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J.-C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis, R. E., J. Y. Yuan, and H. R. Horvitz. 1991. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7: 663–698. [DOI] [PubMed] [Google Scholar]
- 17.Estoppey, S., I. Rodriguez, R. Sadoul, and J.-C. Martinou. 1997. Bcl-2 prevents activation of CPP32 cysteine protease and cleavage of poly (ADP-ribose) polymerase and U1-70 kD proteins in staurosporine-mediated apoptosis. Cell Death Differ. 4: 34–38. [DOI] [PubMed] [Google Scholar]
- 18.Fan, T., H. Lu, L. Shi, G. A. McCarthy, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friis, R. R. 1972. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J. Bacteriol. 180: 706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95: 3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, L.-Y., and Y. Abu Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8: 306–313. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Calvo, M., E. P. Peterson, B. Leiting, R. Ruel, D. W. Nicholson, and N. A. Thornberry. 1998. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273: 32608–32613. [DOI] [PubMed] [Google Scholar]
- 23.Geng, Y., R. B. Shane, K. Berencsi, E. Gonczol, M. H. Zaki, D. J. Margolis, G. Trinchieri, and A. H. Rook. 2000. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J. Immunol. 164: 5522–5529. [DOI] [PubMed] [Google Scholar]
- 24.Golstein, P., D. M. Ojcius, and J. D. Young. 1991. Cell death mechanisms and the immune system. Immunol. Rev. 121: 29–65. [DOI] [PubMed] [Google Scholar]
- 25.Goping, I. S., A. Gross, J. N. Lavoie, M. Nguyen, R. Jemmerson, K. Roth, S. J. Korsmeyer, and G. C. Shore. 1998. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 143: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 27.Gross, A., J. M. McDonnell, and S. Korsmeyer. 1999. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 28.Hogquist, K. A., M. A. Nett, E. R. Unanue, and D. D. Chaplin. 1991. Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. USA 88: 8485–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaled, A. R., K. Kim, R. Hofmeister, K. Muegge, and S. K. Durum. 1999. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc. Natl. Acad. Sci. USA 96: 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolenko, V., T. Bloom, P. Rayman, R. Budowski, E. Hsi, and J. Finke. 1999. Inhibition of NF-kB activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J. Immunol. 163: 590–598. [PubMed] [Google Scholar]
- 31.Kroemer, G., B. Dallaporta, and M. Resche-Rigon. 1998. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60: 619–642. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie, J. N., M. Nguyen, R. C. Marcellus, P. E. Branton, and G. C. Shore. 1998. E4orf4, a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J. Cell Biol. 140: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489. [DOI] [PubMed] [Google Scholar]
- 34.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157. [DOI] [PubMed] [Google Scholar]
- 35.Los, M., S. Wesselborg, and K. Schulze-Osthoff. 1999. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10: 629–639. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy, N. J., M. K. B. Whyte, C. S. Gilbert, and G. I. Evan. 1997. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J. Cell Biol. 136: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monney, L., I. Otter, R. Olivier, H. L. Ozer, A. L. Haas, S. Omura, and C. Borner. 1998. Defects in the ubiquitin pathway induce caspase-independent apoptosis blocked by Bcl-2. J. Biol. Chem. 273: 6121–6131. [DOI] [PubMed] [Google Scholar]
- 38.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55: 143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata, S. 1997. Apoptosis by death factor. Cell 88: 355–365. [DOI] [PubMed] [Google Scholar]
- 40.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139: 271–279. [DOI] [PubMed] [Google Scholar]
- 41.Ojcius, D. M., F. Niedergang, A. Subtil, R. Hellio, and A. Dautry-Varsat. 1996. Immunology and the confocal microscope. Res. Immunol. 147: 175–188. [DOI] [PubMed] [Google Scholar]
- 42.Ojcius, D. M., P. Souque, J.-L. Perfettini, and A. Dautry-Varsat. 1998. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 161: 4220–4226. [PubMed] [Google Scholar]
- 43.Okuno, S., S. Shimizu, T. Ito, M. Nomura, E. Hamada, Y. Tsujimoto, and H. Matsuda. 1998. Bcl-2 prevents caspase-independent cell death. J. Biol. Chem. 273: 34272–34277. [DOI] [PubMed] [Google Scholar]
- 44.Pastorino, J. G., S. T. Chen, M. Tafani, J. W. Snyder, and J. L. Farber. 1998. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 273: 7770–7775. [DOI] [PubMed] [Google Scholar]
- 45.Perfettini, J.-L., T. Darville, G. Gachelin, P. Souque, M. Huerre, A. Dautry-Varsat, and D. M. Ojcius. 2000. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect. Immun. 68: 2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossé, T., R. Olivier, L. Monney, M. Rager, S. Conus, I. Fellay, B. Jansen, and C. Borner. 1998. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391: 496–499. [DOI] [PubMed] [Google Scholar]
- 47.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53: 577–628. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen, G. S., and V. M. Dixit. 1997. Caspases: intracellular signaling by proteolysis. Cell 91: 443–446. [DOI] [PubMed] [Google Scholar]
- 49.Schachter, J., and H. D. Caldwell. 1980. Chlamydiae. Annu. Rev. Microbiol. 34: 285–310. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu, S., M. Narita, and Y. Tsujimoto. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487. [DOI] [PubMed] [Google Scholar]
- 51.Stephens, R. S. 1993. Challenge of Chlamydia research. Infect. Agents Dis. 1: 279–293. [PubMed] [Google Scholar]
- 52.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446. [DOI] [PubMed] [Google Scholar]
- 53.Todd, W. J., and J. Storz. 1975. Ultrastructural cytochemical evidence for the activation of lysosomes in the cytocidal effect of Chlamydia psittaci. Infect. Immun. 12: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53: 155–187. [DOI] [PubMed] [Google Scholar]
- 55.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo, M., R. Hakem, M. S. Soengas, G. S. Duncan, A. Shahinian, D. Kägi, A. Hakem, M. McCurrach, W. Khoo, S. A. Kaufman, G. Senaldi, T. Howard, S. W. Lowe, and T. W. Mak. 1998. Essential contribution of caspase-3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12: 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyllie, A. H. 1997. Apoptosis: an overview. Br. Med. Bull. 53: 451–465. [DOI] [PubMed] [Google Scholar]
- 58.Wyllie, A. H., J. F. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68: 251–305. [DOI] [PubMed] [Google Scholar]
- 59.Wyrick, P. B., E. A. Brownridge, and B. E. Ivins. 1978. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect. Immun. 19: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiang, J., D. T. Chao, and S. J. Korsmeyer. 1996. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93: 14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, Q., and J. C. Reed. 1998. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1: 337–346. [DOI] [PubMed] [Google Scholar]
- 62.Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. I. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 63.Zhivotovsky, B., S. Orrenius, O. T. Brustugun, and S. O. Døskeland. 1998. Injected cytochrome c induces apoptosis. Nature 391: 449–450. [DOI] [PubMed] [Google Scholar]
- 64.Zou, H., W. J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413. [DOI] [PubMed] [Google Scholar]