Abstract
We have previously identified the STK receptor tyrosine kinase as a key regulator of macrophage activation and cell-mediated immune responses. Here we demonstrate that, although MSP activation of STK inhibits NO production by macrophages in response to heat-killed Listeria monocytogenes, STK-deficient mice exhibit increased susceptibility to infection with Listeria.
The STK receptor tyrosine kinase (15, 23), a member of the MET family of RTKs, is expressed on a variety of epithelial cell populations, hematopoietic progenitor cells, and tissue-resident macrophages (15, 16, 22). STK is the murine homologue of the human RON receptor and the ligand for STK, as well as RON, is macrophage-stimulating protein (MSP) (31). MSP is expressed primarily in the liver (6), where it is synthesized and secreted as an inactive, single-chain precursor. This pro-MSP can be activated by proteolytic conversion to a heterodimer by enzymes of the coagulation cascade (32). Stimulation of peritoneal macrophages by MSP results in shape change, chemotaxis, and phagocytosis (27, 28). Moreover, MSP has been shown to suppress nitric oxide (NO) production by activated peritoneal macrophages in vitro (30). The ability of MSP to inhibit NO production by macrophages in response to cytokines and lipopolysaccharide (LPS) has been shown to require phosphatidylinositol 3-kinase (1) and is associated with decreased transcriptional activity of IRF-1, resulting in decreased expression of inducible nitric oxide synthase (iNOS) (17).
Knockout studies have suggested a role for STK in placental and ovarian development (19, 29). Furthermore, mice with a targeted deletion in exon 1 of the gene encoding STK are resistant to Friend virus-induced erythroleukemia, confirming the identity of STK as the Friend virus susceptibility gene, Fv2 (20). Previously, we and others have shown that activated macrophages from STK-deficient mice (3, 19, 29) produce elevated levels of NO, both in vitro and in vivo, rendering them more susceptible to endotoxic shock. Based on these observations, we proposed a model in which MSP becomes activated after LPS administration, thereby activating STK signaling in macrophages and limiting the tissue damage associated with septic shock by attenuating NO synthesis.
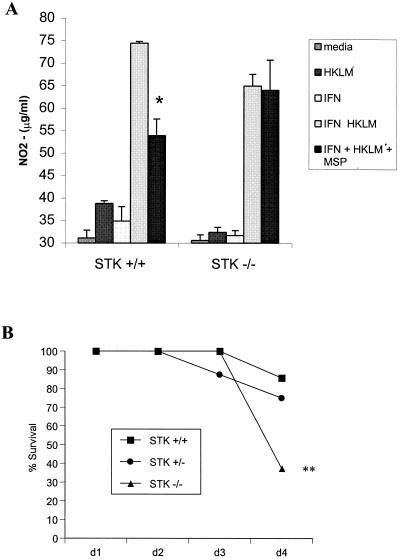
While MSP has been shown to inhibit NO production by peritoneal macrophages in response to stimulation with gamma interferon (IFN-γ) and LPS, the ability of MSP to regulate NO production in response to gram-positive bacteria has not been previously demonstrated. In order to determine whether MSP inhibits NO production in response to Listeria spp., we harvested primary peritoneal macrophages from wild-type and STK-deficient mice and stimulated them with 100 U of IFN-γ/ml plus heat-killed Listeria monocytogenes (HKLM) (50 bacteria/macrophage), in the presence or absence of 100 ng of MSP/ml. After 36 h, the levels of NO in the supernatant were assayed by reacting 100 μl of supernatant with an equal volume of Greiss reagent as described previously (3). MSP alone has no effect on the production of NO (data not shown). However, the data from these studies demonstrate that, in addition to its role in regulating NO production in response to LPS, MSP inhibits NO production in response to IFN-γ plus HKLM (Fig. 1A) and that this regulation requires the STK receptor.
FIG. 1.
Increased susceptibility of STK−/− mice to infection with L. monocytogenes. (A) Resident peritoneal macrophages from STK+/+ and STK−/− mice were treated with HKLM in the presence or absence of 100 U of IFN-γ and 100 ng of MSP/ml. After 36 h of stimulation, the levels of NO in the supernatant were assayed by using Greiss reagent. (B) STK+/+ (n = 7), STK+/− (n = 8), and STK−/− (n = 8) mice were injected intravenously with an infectious dose of 2 × 105 to 4 × 105 L. monocytogenes, and the survival of these animals was monitored for 4 days. ✽, P < 0.01; ✽✽, P < 0.02.
To determine whether the ability of STK to suppress NO production in response to HKLM would result in increased resistance to infection in mice with a targeted mutation in STK, we challenged the STK−/− mice and their control littermates with L. monocytogenes. One day prior to inoculation, serial 1:10 dilutions of L. monocytogenes EDG were grown in Trypticase soy broth at 37°C in shaking cultures (125 rpm) for 15 h. On the day of infection, the optical densities of the Listeria dilutions were determined, and an infectious dose of 4.82 × 105 in 0.2 ml of phosphate-buffered saline (PBS) was administered by tail vein injection. STK−/− mice exhibited increased susceptibility to infection with 2 × 105 to 4 × 105 Listeria organisms compared to their littermate controls (Fig. 1B). The mortality in these animals occurred primarily between days 3 and 4 after the initial infection, corresponding to the time that T-cell-mediated immunity is triggered.
To determine the bacterial load, the livers and spleens were homogenized on day 3 or 4 after infection, in 8 and 9 ml of PBS, respectively, for 30 s at speed 5. Serial dilutions of the homogenate were plated on Trypticase soy agar, and the number of bacteria/organ was determined by colony counts. As shown in Table 1, the increased susceptibility of the STK-deficient mice to 2 × 105 to 4 × 105 L. monocytogenes was accompanied by an increase in the bacterial load in livers, but not the spleens, of these animals on day 3. However, the bacterial burden in the livers of surviving STK-deficient animals on day 4 was indistinguishable from that of wild-type controls. These data suggest that the difference in susceptibility may lie in the innate ability of macrophages to maintain the bacterial load at a level which is not lethal until the onset of T-cell-mediated events. At a lower Listeria dose (1.3 × 104), we failed to observe a statistically significant difference in the bacterial burden in either the livers or the spleens of STK-deficient mice.
TABLE 1.
Increased bacterial burden in the livers of STK−/− micea
| Dose | Day | Organ | Mean bacterial burden ± SD (n) in:
|
||
|---|---|---|---|---|---|
| STK+/+ mice | STK+/− mice | STK−/− mice | |||
| 2 × 105–4 × 105 | 3 | Liver | 6.8 ± 1.4 (10) | 7.2 ± 1.0 (11) | 8.4 ± 1.1* (10) |
| Spleen | 7.1 ± 0.7 (10) | 7.3 ± 0.6 (11) | 7.5 ± 0.5 (10) | ||
| 4 | Liver | 5.8 ± 1.3 (8) | 6.1 ± 1.7 (10) | 6.2 ± 1.0 (6) | |
| Spleen | 6.5 ± 0.9 (8) | 6.6 ± 1.1 (10) | 7.1 ± 0.7 (6) | ||
| 1.3 × 104 | 3 | Liver | 4.71 ± 1.91 (8) | 5.04 ± 1.90 (8) | 5.22 ± 1.60 (7) |
| Spleen | 6.04 ± 1.15 (8) | 5.59 ± 1.25 (8) | 5.82 ± 1.31 (7) | ||
STK+/+, STK+/−, and STK−/− mice were injected intravenously with an infectious dose of L. monocytogenes. The bacterial burden (log10/organ) in the liver and spleen was determined on days 3 and 4 after infection. *, P < 0.01.
Targeted mutations in a number of genes involved in the regulation of NO production also affect susceptibility to infection with Listeria. Mutations in genes that encode members of the IFN-γ (14, 18) and tumor necrosis factor alpha (TNF-α) pathways (21, 24, 25) render mice more susceptible to Listeria. Both of these signaling pathways appear to be essential for optimal activation of iNOS expression and subsequent NO production by macrophages. Furthermore, disruption of the iNOS gene itself renders mice more susceptible to infection with Listeria (33), highlighting the pivotal role for NO in the immune response to Listeria. The observation that the STK-deficient mice are more susceptible to infection with Listeria, despite the role of STK in limiting NO production, suggests that the enhanced sensitivity of these mice to Listeria could be secondary to increased inflammatory damage. Alternatively, the STK-deficient mice may harbor a defect in other pathways essential for efficient bacterial clearance.
Interleukin-6-deficient mice are more susceptible to Listeria due to inefficient neutrophilia (5), and mice depleted for neutrophils exhibit exacerbated listeriosis in the liver but not in the spleen or peritoneal cavity (2). These data suggest a crucial role for neutrophils in the hepatic cell-mediated immune response to Listeria. Recent studies have also highlighted the role of reactive oxygen intermediates in the course of listeriosis (7, 10). In addition, studies utilizing the TNF receptor p55 knockout mice have found that, despite normal leukocyte recruitment, inflammatory cytokine production, nitric oxide synthesis, and oxidative burst formation, these mice are highly susceptible to Listeria infection, suggesting a role for NO- and reactive oxygen intermediate-independent mechanisms of killing (9). Furthermore, IFN-γ treatment of peritoneal macrophages has been shown to restrict listerial growth due to limited escape of the Listeria from the phagocytic vacuole by a reactive nitrogen-independent mechanism (4). Therefore, antibacterial effector mechanisms other than NO could be targets of the MSP/STK signaling pathway.
In addition to a potential role in the cytotoxic response of macrophages to infection, STK could also regulate susceptibility to Listeria by influencing the uptake of Listeria by host cells. MSP has been shown to promote phagocytosis of C3bi-coated sheep erythrocytes through the CR3 receptor (27). We have shown that the STK receptor associates with CR3 and that MSP-induced CR3-mediated phagocytosis requires the STK receptor (M. A. Lutz and P. H. Correll, unpublished data). Listeria that are phagocytosed by macrophages via the CR3 receptor are readily killed in the absence of inflammatory mediators (8). Furthermore, we have recently demonstrated upregulation of scavenger receptor A (SR-A) by primary peritoneal macrophages in response to MSP (A. C. Morrison and P. H. Correll, unpublished data). SR-A knockout mice are more susceptible to endotoxic shock (13) and infection with Listeria (8), a phenotype strikingly similar to the phenotype of the STK-deficient mice. In addition to traditional routes of entry, the MET receptor has recently been shown to mediate uptake of Listeria by hepatocytes and other epithelial cells by an InB-dependent mechanism (26). Listeria lacking InB fail to effectively colonize the liver, and STK has recently been shown to interact with the MET receptor (11, 12). Therefore, the possibility exists that STK regulates the uptake of Listeria by hepatocytes through interactions with MET. Future studies will be aimed at further delineating the mechanism by which STK regulates the host response to infection with Listeria.
Acknowledgments
This work was supported by Public Health Service grant AI43367 from the National Institute of Allergy and Infectious Disease (P.H.C.) and by a Terry Fox Program Project Grant of the National Cancer Institute of Canada (A.B.).
Editor: V. J. DiRita
REFERENCES
- 1.Chen, Y. Q., J. H. Fisher, and M. H. Wang. 1998. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phosphatidylinositol-3 kinase is required for RON-mediated inhibition of iNOS expression. J. Immunol. 161: 4950–4959. [PubMed] [Google Scholar]
- 2.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll, P. H., A. Iwama, S. Tondat, G. Mayrhofer, T. Suda, and A. Bernstein. 1997. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Function 1: 69–83. [DOI] [PubMed] [Google Scholar]
- 4.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158: 5297–5304. [PubMed] [Google Scholar]
- 5.Dalrymple, S. A., L. L. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63: 2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degen, S. J., L. A. Stuart, S. Han, and C. S. Jamison. 1991. Characterization of the mouse cDNA and gene coding for a hepatocyte growth factor-like protein: expression during development. Biochemistry 30: 9781–9791. [DOI] [PubMed] [Google Scholar]
- 7.Dinauer, M. C., M. B. Deck, and E. R. Unanue. 1997. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infeciton with Listeria monocytogenes. J. Immunol. 158: 5581–5583. [PubMed] [Google Scholar]
- 8.Drevets, D. A., P. J. Leenen, and P. A. Campbell. 1993. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J. Immunol. 151: 5431–5439. [PubMed] [Google Scholar]
- 9.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7: 419–432. [DOI] [PubMed] [Google Scholar]
- 10.Fehr, T., G. Schoedon, B. Odermatt, T. Holtschke, M. Schneemann, M. F. Bachmann, T. W. Mak, I. Horak, and R. M. Zinkernagel. 1997. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis, for protection against murine listeriosis. J. Exp. Med. 185: 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follenzi, A., S. Bakovic, P. Gual, M. C. Stella, P. Longati, and P. M. Comoglio. 2000. Cross-talk between the proto-oncogenes Met and Ron. Oncogene 19: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 12.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1997. Internalin B promotes the replication of Listeria monocytogenes in mouse hepatocytes. Infect. Immun. 65: 5137–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haworth, R., N. Platt, S. Keshav, D. Hughes, E. Darley, H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1997. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 186: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259: 1742–1745. [DOI] [PubMed] [Google Scholar]
- 15.Iwama, A., K. Okano, T. Sudo, Y. Matsuda, and T. Suda. 1994. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood 83: 3160–3169. [PubMed] [Google Scholar]
- 16.Iwama, A., M.-H. Wang, N. Yamaguchi, N. Ohno, K. Okano, T. Sudo, M. Takeya, F. Gervais, C. Morissette, E. J. Leonard, and T. Suda. 1995. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood 86: 3394–3403. [PubMed] [Google Scholar]
- 17.Liu, Q.-P., K. Fruit, J. Ward, and P. H. Correll. 1999. Negative regulation of macrophage activation in response to IFN-γ and lipopolysaccharide by the STK/RON receptor tyrosine kinase. J. Immunol. 163: 6606–6613. [PubMed] [Google Scholar]
- 18.Meraz, M. A., J. M. White, K. C. F. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84: 431–442. [DOI] [PubMed] [Google Scholar]
- 19.Muraoka, R., W. Sun, M. Colbert, S. Waltz, D. Witte, J. Degen, and S. Degen. 1999. The Ron/STK receptor tyrosine kinase is essential for peri-implantation development in the mouse. J. Clin. Investig. 103: 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Gen. 23: 159–165. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer, K., T. Matuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73: 457–467. [DOI] [PubMed] [Google Scholar]
- 22.Quantin, B., B. Schuhbaur, M.-C. Gesnel, P. Dolle, and R. Breathnach. 1995. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev. Dyn. 20: 383–390. [DOI] [PubMed] [Google Scholar]
- 23.Ronsin, C., F. Muscatelli, M.-G. Mattei, and R. Breathnach. 1992. A novel putative receptor protein tyrosine kinase of the met family. Oncogene 8: 1195–1202. [PubMed] [Google Scholar]
- 24.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364: 798–802. [DOI] [PubMed] [Google Scholar]
- 25.Sha, W. C., H.-C. Liou, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80: 321–330. [DOI] [PubMed] [Google Scholar]
- 26.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InlB-dependent internalization of Listeria is mediated by the met receptor tyrosine kinase. Cell 103: 501–510. [DOI] [PubMed] [Google Scholar]
- 27.Skeel, A., and E. J. Leonard. 1994. Action and target cell specificity of human macrophage-stimulating protein (MSP). J. Immunol. 152: 4618–4623. [PubMed] [Google Scholar]
- 28.Skeel, A., T. Yoshimura, S. D. Showalter, S. Tanaka, E. Appella, and E. J. Leonard. 1991. Macrophage stimulating protein: purification, partial amino acid sequence, and cellular activity. J. Exp. Med. 173: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waltz, S. E., L. Eaton, K. Toney-Earley, K. A. Hess, B. E. Peace, J. R. Ihlendorf, M. H. Wang, K. H. Kaestner, and S. J. Degen. 2001. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J. Clin. Investig. 108: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, M.-H., G. W. Cox, T. Yoshimura, L. A. Sheffler, A. Skeel, and E. J. Leonard. 1994. Macrophage-stimulating protein inhibits induction of nitric oxide production by endotoxin- or cytokine-stimulated mouse macrophages. J. Biol. Chem. 269: 14027–14031. [PubMed] [Google Scholar]
- 31.Wang, M.-H., C. Ronsin, M.-C. Gesnel, L. Coupey, A. Skeel, E. J. Leonard, and R. Breathnach. 1994. Identification of the ron gene product as the receptor for the human macrophage-stimulating protein. Science 266: 117–119. [DOI] [PubMed] [Google Scholar]
- 32.Wang, M.-H., T. Yoshimura, A. Skeel, and E. J. Leonard. 1994. Proteolytic conversion of single chain precursor macrophage-stimulating protein to a biologically active heterodimer by contact enzymes of the coagulation cascade. J. Biol. Chem. 269: 3436–3440. [PubMed] [Google Scholar]
- 33.Wei, X.-Q., I. G. Charles, A. Smith, J. Ure, G.-J. Feng, F.-P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375: 408–411. [DOI] [PubMed] [Google Scholar]