Abstract
We studied the mechanisms underlying the severely impaired wound healing associated with human leukocyte-adhesion deficiency syndrome-1 (LAD1) using a murine disease model. In CD18−/− mice, healing of full-thickness wounds was severely delayed during granulation-tissue contraction, a phase where myofibroblasts play a major role. Interestingly, expression levels of myofibroblast markers α-smooth muscle actin and ED-A fibronectin were substantially reduced in wounds of CD18−/− mice, suggesting an impaired myofibroblast differentiation. TGF-β signalling was clearly involved since TGF-β1 and TGF-β receptor type-II protein levels were decreased, while TGF-β1 injections into wound margins fully re-established wound closure. Since, in CD18−/− mice, defective migration leads to a severe reduction of neutrophils in wounds, infiltrating macrophages might not phagocytose apoptotic CD18−/− neutrophils. Macrophages would thus be lacking their main stimulus to secrete TGF-β1. Indeed, in neutrophil–macrophage cocultures, lack of CD18 on either cell type leads to dramatically reduced TGF-β1 release by macrophages due to defective adhesion to, and subsequent impaired phagocytic clearance of, neutrophils. Our data demonstrates that the paracrine secretion of growth factors is essential for cellular differentiation in wound healing.
Keywords: cellular differentiation, growth factors, integrins, myofibroblast, wound healing
Introduction
Normal tissue repair follows a sequence of events involving clotting, inflammation, remodelling and re-epithelialisation (reviewed in: Martin, 1997; Singer and Clark, 1999). Within a few hours after injury, first polymorphonuclear neutrophils (PMN) and later macrophages (Mφ) invade the wound site. They produce proteinases and reactive oxygen species to combat contaminating microorganisms and phagocytose cellular debris. In addition, inflammatory cells are an important source of growth factors and cytokines, which initiate myofibroblast-dependent wound contraction (Martin, 1997; Hinz et al, 2001a) and other tissue-repair regulatory functions. Myofibroblasts are generally characterised by the expression of α-smooth muscle actin (α-SMA) (Tomasek et al, 2002), the actin isoform characteristic of vascular smooth muscle cells (Skalli et al, 1986; Serini and Gabbiani, 1999), which confers a high contractile activity to these cells (Hinz et al, 2001a). TGF-β1 is the major cytokine inducing α-SMA expression, myofibroblast differentiation (Desmoulière et al, 1993) and subsequent wound contraction (Montesano and Orci, 1988). This action of TGF-β1 depends on the presence of the fibronectin (FN) splice variant ED-A FN in the extracellular matrix (Serini et al, 1998). TGF-β1 is released by different cell types, among them Mφ (for a review, see Werner and Grose, 2003). These complex interrelated processes depend on a well-regulated influx of distinct blood-derived inflammatory cells at the wound site. A large number of leukocyte and endothelial adhesion molecules play a role in leukocyte trafficking during this inflammatory phase of wound healing (Springer and Anderson, 1986). The integrins CD11a/CD18 (LFA-1), CD11b/CD18 (Mac-1), CD11c/CD18 (gp150,95) and CD11d/CD18 are constitutively expressed on the surface of leukocytes; these heterodimers consist of a common β2 subunit (CD18) and a variable α subunit (CD11a, CD11b, CD11c, CD11d) (Arnaout, 1990). LFA-1 and Mac-1 support the firm adhesion to ICAM-1 and -2 (Dustin and Springer, 1988; Staunton et al, 1989; Diamond et al, 1990), which is a prerequisite for leukocyte emigration from the blood vessels to the interstitial connective tissue.
CD18 expression is defective in patients who suffer from leukocyte-adhesion deficiency-1 (LAD1), a recessively inherited disorder in which deletions, truncations, substitutions or frameshift mutations impair β2-integrin function. This leads to a severely reduced PMN recruitment to sites of infection and tissue damage, while monocyte emigration is largely normal (Bowen et al, 1982; Anderson et al, 1984; Kuijpers et al, 1997). LAD1 patients are typically subject to recurrent bacterial or fungal infections, severe periodontitis, peripheral leukocytosis and impaired wound healing (Anderson and Smith, 2001). The lack of PMN emigration explains recurrent infections in these patients; however, the cause for impaired tissue repair is less clear.
To carefully dissect the role of PMN in cutaneous wound healing, here we used our previously generated CD18−/− mice that share the essential characteristics of LAD1 patients (Mizgerd et al, 1997; Scharffetter-Kochanek et al, 1998; Walzog et al, 1999b; Kess et al, 2003), such as complete CD18-expression deficiency and virtually no recruitment of PMN to sites of toxic or allergic dermatitis (Mizgerd et al, 1997; Scharffetter-Kochanek et al, 1998; Grabbe et al, 2002). We show that wounds of CD18−/− mice remain almost devoid of PMN, whereas infiltration by Mφ is normal. Moreover, CD18−/− mice show delayed wound closure and impaired myofibroblast differentiation, which is characterised by reduced expression of α-SMA, ED-A FN and TGF-β receptor type II (TGF-βRII) in the granulation tissue. Immunohistological staining of CD18−/− wounds for TGF-β1 and TGF-β1-specific ELISAs of wound lysates suggest that impaired myofibroblast differentiation is caused by the deficient release of active TGF-β1 by Mφ. In fact, CD18−/− as well as WT Mφ secrete substantially less TGF-β1 when cultured alone, or when cocultured with CD18−/− PMN, as compared to cocultures with WT PMN. This is linked to decreased adhesion between PMN and Mφ, a prerequisite for the phagocytic clearance of CD18−/− PMN in vitro. Finally, reduced wound healing was fully rescued by repeatedly injecting recombinant TGF-β1 into the wound margins of CD18−/− mice.
As cutaneous wound healing in mice is comparable to that in humans (Fahey et al, 1991; Kondo and Ohshima, 1996), the results reported herein may have implications for the treatment of impaired healing of wounds in LAD1 patients.
Results
CD18−/− mice display delayed wound healing with absent PMN, but normal Mφ recruitment to the wound site
Full-thickness 5-mm punch biopsies were produced on both sites of the shaved back of CD18−/− mice and WT controls, and wound sizes were monitored. Wound closure in CD18−/− mice was significantly impaired between 5 and 14 days after wounding (Figure 1) as compared to WT.
Figure 1.
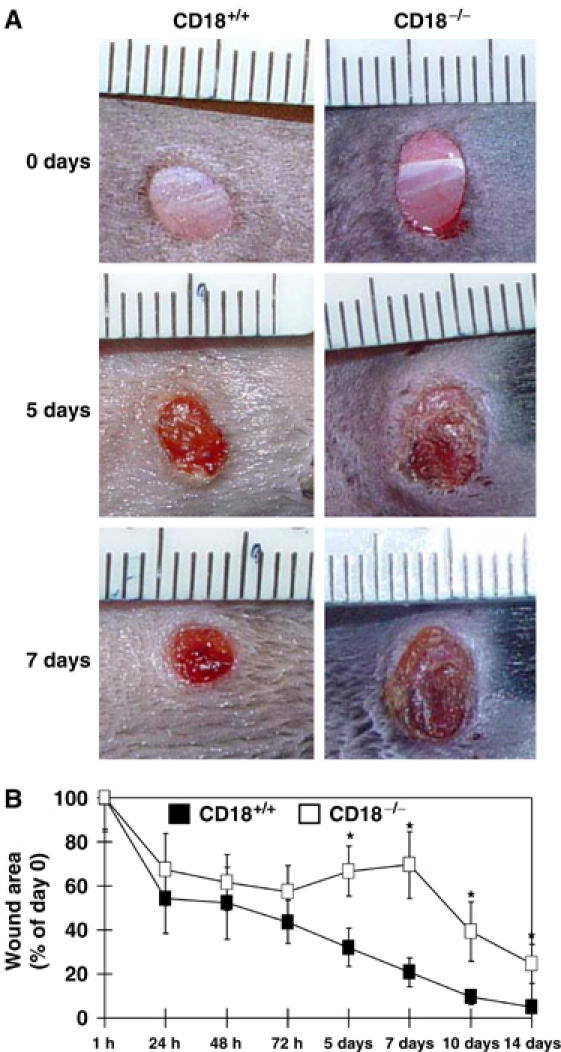
Wound closure of full-thickness wounds is delayed in CD18−/− mice. Full-thickness (including the panniculus carnosus) excisional wounds were punched at two sites in the middle of the dorsum using 5-mm biopsy round knives. Each wound region was digitally photographed at the indicated time points, and wound areas were calculated using Adobe Photoshop® software. (A) Macroscopic observation of wounds in CD18−/− and WT mice. Representative results of six wounds in each cohort are shown. (B) Wound sizes at any given time point after wounding were expressed as percentage of initial (day 0) wound area for CD18−/− and WT mice. Results are expressed as the mean±s.d. (n=6). *P<0.05.
H&E and immunostainings with a PMN-specific monoclonal antibody (mAb) (GR1) revealed virtually no PMN in the interstitial tissue at wound sites in CD18−/− mice (Figure 2A); PMN remained entrapped in vessels (Figure 2C), whereas a strong extravasation and recruitment of PMN to wound sites 24 h after wounding of WT (Figure 2B and D) were found. Immunostaining of day 5 wounds with an mAb directed against Mφ (F4/80) did not show any difference in Mφ recruitment to the wound sites between CD18−/− and WT mice (Figure 2E and F). These data were confirmed using a time-course analysis, counting PMN and Mφ, which egressed from the vessel into the interstitial tissue of the wound bed. Compared to high numbers of egressed PMN in WT wounds at 24 and 48 h, almost no PMN were detected in the granulation tissue of CD18−/− wounds at any time point (Figure 2G). However, no differences in the numbers of egressed Mφ at the wound sites of WT and CD18−/− mice were observed (Figure 2H).
Figure 2.
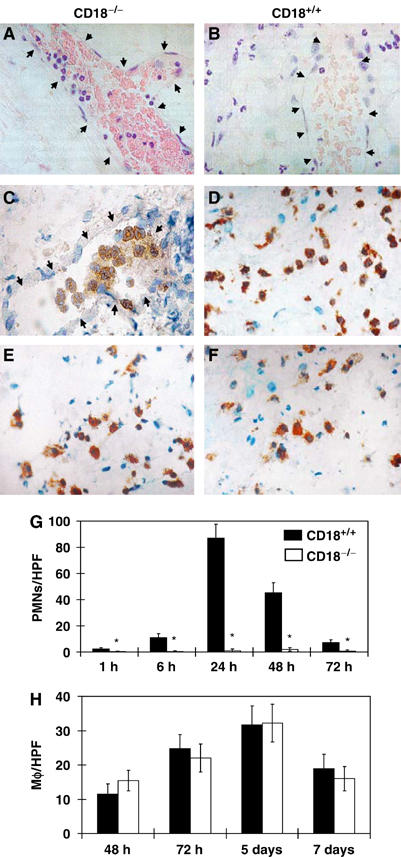
Impaired migration of PMN, but not Mφ, to wound beds of CD18−/− mice. H&E stainings (A, B) and immunostainings with a PMN-specific mAb (GR1) (C, D), as well as with an Mφ-specific mAb (F4/80) (E, F), were prepared from paraffin-embedded sections of CD18−/− (A, C, E) and WT mice (B, D, F) at 24 h (A–D) and 5 days (E, F) after full-thickness wounding. Arrows point at endothelial cells lining the vascular lumina. Subsequently, numbers of extravasated (G) GR1+ PMN and (H) F4/80+ Mφ within the dermis of the wound margins were counted in five high-power fields (HPF) at × 40 magnification using a light microscope. Results are given as the mean±s.d. (n=4). *P<0.05.
Wounds of CD18−/− mice exhibit reduced numbers of myofibroblasts
Since differences in wound size between WT and CD18−/− mice were most significant in the phase between day 5 and 7 post-wounding, which is characterised by myofibroblast-mediated wound contraction, we investigated expression of myofibroblast markers in CD18−/− and WT granulation tissue by immunohistochemistry. In 5-day-old wounds, α-SMA was abundant at the wound edges and less strongly expressed in the wound bed of WT animals, whereas it was virtually absent in the wounds of CD18−/− mice (Figure 3A). After 7 days, expression of α-SMA was increased throughout the wound and, in particular, beneath the hyperproliferative epidermis of wound edges in WT controls (Figure 3A). However, only a faint staining for α-SMA was detected at the edges of CD18−/− wounds (Figure 3A). To further characterise myofibroblast differentiation in vivo, in addition to α-SMA, we also assessed the expression of other myofibroblast markers such as ED-A FN and TGF-βRII at different stages of wound healing by semiquantitative Western blotting. Densitometric assessment of Western blots revealed lower expression levels of all myofibroblast markers in wounds of CD18−/− compared with those WT mice at any time point (Figure 3B and C). Vimentin served as a house-keeping protein to equilibrate loading for similar numbers of fibroblastic cells. These results suggested that lower numbers of myofibroblasts resided in wound beds of CD18−/− compared to WT mice.
Figure 3.
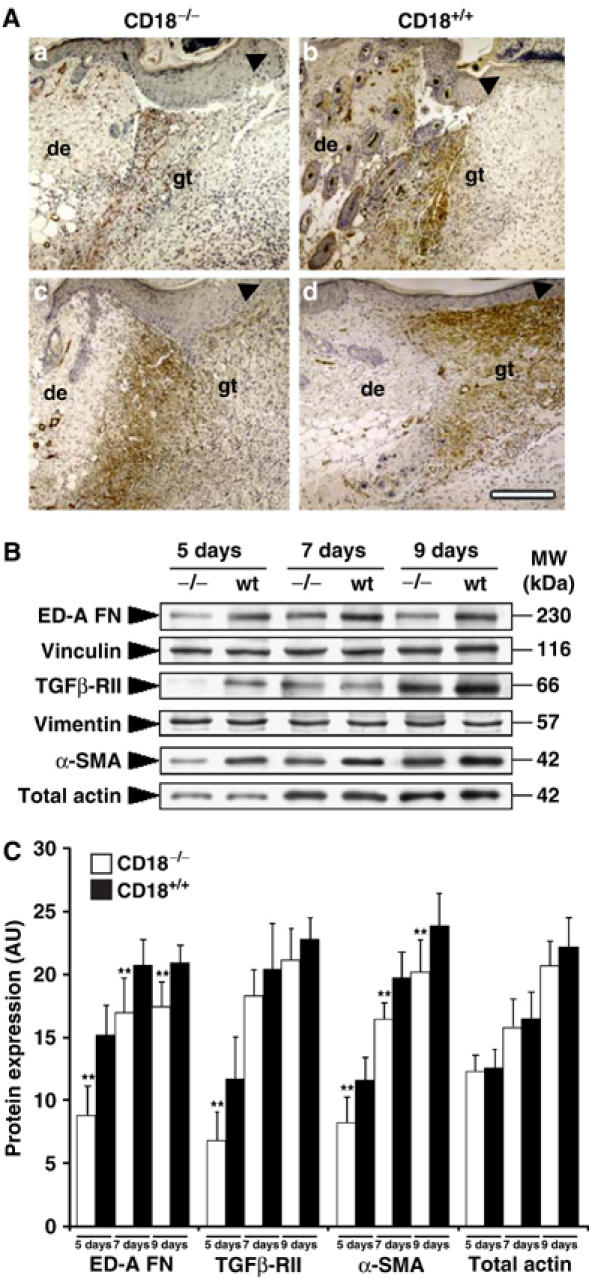
Reduced expression of myofibroblast-differentiation markers in wounds of CD18−/− mice. To detect key markers for myofibroblast differentiation and wound contraction, (A) paraffin-embedded granulation tissue of CD18−/− and WT mice was stained immunohistochemically for α-SMA, 5 (a, b) and 7 (c, d) days after wounding. The bar indicates 200 μm; de, adjacent dermis; gt, granulation tissue; arrows indicate epidermal leading edges. (B) Snap-frozen wound tissue was analysed by Western blotting, equilibrated to vimentin expression levels from Coomassie gels, to measure expression of myofibroblast markers including α-SMA, ED-A FN and TGFβ-RII at different time points. Panel B was assembled from separately performed blots for each indicated protein, using the same complete series of wound samples with identical loading between lanes (15 μg). (C) Semiquantitative balance analyses of Western blots were performed by densitometry of digitised Western blots. Data is given as the mean±s.d. **P<0.005.
Local injection of TGF-β1 restores myofibroblast differentiation and wound contraction in CD18−/− mice, but not PMN recruitment
TGF-β1 is the major growth factor inducing myofibroblast (Desmoulière et al, 1993) and vascular smooth muscle-cell differentiation (Majesky et al, 1991) during wound healing. To assess TGF-β1 levels in the wound tissue of CD18−/− versus WT mice, we performed immunostaining for TGF-β1 on sections of day 5 and day 7 wounds. A strong staining of the epidermis and hair follicles was noted in both CD18−/− and WT mice. These results fit with a previous report describing the TGF-β1 expression pattern during cutaneous tissue repair (Cowin et al, 2001). There was, however, distinctly less staining in the newly formed granulation tissue at day 5 after wounding in CD18−/− mice as compared to wounds of the WT (Figure 4A). Similar findings were made at day 7 after wounding (data not shown). This data was confirmed by measuring the levels of active TGF-β1 in wound tissue lysates using an ELISA specific for active TGF-β1 (Figure 4B). Our results suggest that reduced amounts of TGF-β1 may account for the decreased numbers of α-SMA-positive myofibroblasts and the impaired wound contraction in CD18−/− mice.
Figure 4.
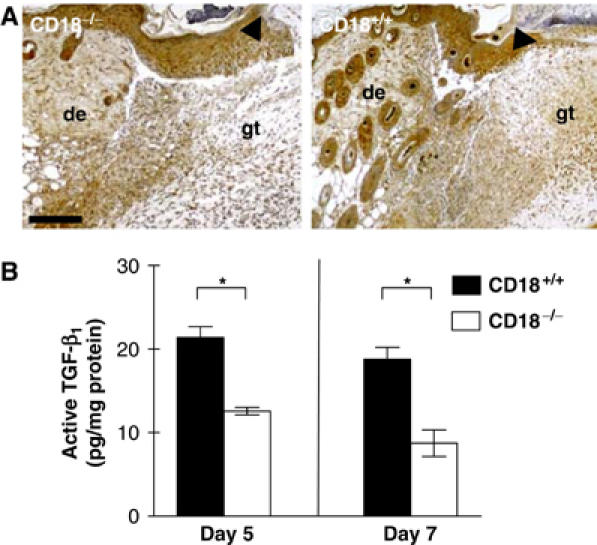
Reduced TGF-β1 in the granulation tissue of CD18−/− mice. (A) Paraffin-embedded sections of wound sites obtained from CD18−/− and WT mice at day 5 after injury were stained immunohistochemically for TGF-β1 (brown). Whereas the typical staining pattern for TGF-β1 was observed in WT wounds that apart from an epidermal staining gave a strong signal in the wound bed-replenishing granulation tissue, CD18−/− wounds showed only weak staining in the granulation tissue located directly underneath the wound. The bar indicates 200 μm; de, adjacent dermis; gt, granulation tissue; arrows indicate epidermal leading edges. (B) Lysates of snap-frozen wound tissues from CD18−/− and WT mice obtained at the indicated time points after wounding were also prepared and subjected to ELISA to detect active TGF-β1. Data is given as the mean±s.d. *P<0.05.
In an attempt to provide evidence for a causal role for TGF-β1 deficiency in reduced wound closure, we injected recombinant human TGF-β1 subcutaneously (s.c.) at four sites around the wound (Ashcroft et al, 2003); mock injections of solvent NaCl 0.9% only were taken as controls. Gross evaluation (Figure 5A) and digital measurement (Figure 5B) of wound sizes at days 5 and 7 demonstrated that impaired wound healing was fully restored in CD18−/− mice by local injection of recombinant TGF-β1. In contrast to wounds of mock-treated CD18−/− mice that did not show significant expression of myofibroblast markers in immunofluorescence, wounds that had received TGF-β1 injections showed high α-SMA expression levels, comparable to wounds from mock-treated WT mice (Figure 5C). Thus, in the absence of CD18, injection of TGF-β1 during the wound-healing process was sufficient to rescue myofibroblast differentiation and wound contraction. These data depict a causal role for the reduced secretion of active TGF-β1 in the retarded differentiation of myofibroblasts at wound sites of CD18−/− mice. As TGF-β1 has earlier been shown to stimulate PMN recruitment (Fava et al, 1991; Drake and Issekutz, 1993), a finding also confirmed by our experiments in CD18 WT mice (Supplementary data S1), we were interested whether TGF-β1 injection could stimulate PMN recruitment also in CD18−/− mice. In fact, this was not the case, suggesting that TGF-β1-induced recruitment of PMN depends on CD18 and does not activate alternate emigration pathways (Supplementary data S1).
Figure 5.
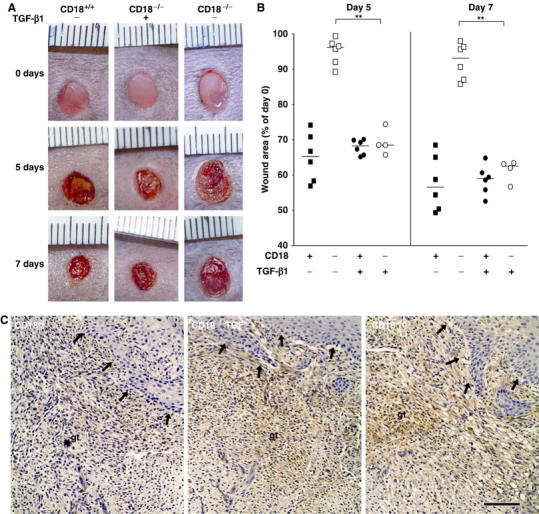
Injection of TGF-β1 in wound margins rescues wound closure and myofibroblast differentiation in CD18−/− mice. Recombinant human TGF-β1 (‘TGF') was injected s.c. at four sites around the wound, allowing to infiltrate the wound margins, at a total dose of 0.45 μg per wound. Mock injections were made using only the solvent NaCl 0.9%. First injections were carried out on day 1 after wounding, followed by further injections every second day until wounds were harvested. (A) Macroscopic observation of wounds in CD18−/−, WT mice with or without injection of TGF-β1. (B) Wound sizes were assessed at the indicated time points after wounding as previously. Bars depict the median of each cohort. **P<0.005. (C) Paraffin-embedded granulation tissue of CD18−/− and WT mice was stained immunohistochemically for α-SMA 5 days after wounding. The bar indicates 100 μm; de, adjacent dermis; gt, granulation tissue; arrows indicate the newly formed epidermal leading edges.
Impaired release of TGF-β1 is due to an impaired adhesion with a subsequent defective phagocytic clearance of apoptotic PMN by Mφ in CD18−/− mice
After cleaning a wound from microbial contamination and debris, PMN undergo apoptosis in normal tissue repair. Apoptotic PMN are then cleared by Mφ by adhesion-dependent phagocytic engulfment, which stimulates them to release TGF-β1 in large amounts (Fadok et al, 1998; McDonald et al, 1999). To study whether TGF-β1 release and/or adhesion-mediated phagocytosis of apoptotic PMN by Mφ in CD18−/− mice differed from the WT controls, we rendered isolated CD18−/− and WT PMN apoptotic. Apoptotic CD18−/− or WT PMN were then cocultured with CD18−/− or WT Mφ at 37°C. After the indicated incubation times, supernatants were analysed by ELISA for TGF-β1. IL-1α, -1β and -6 have been shown to antagonise TGF-β1 in wound healing (Angele et al, 1999; Werner and Grose, 2003), and were also determined from Mφ-culture supernatants after stimulation with LPS. Whereas IL-1α, -1β and -6 were highly increased in the supernatants of CD18−/− Mφ when compared to WT (Supplementary data S2), only low amounts of active TGF-β1 were found in the supernatants from cocultures of viable PMN with either genotype of Mφ (data not shown). By contrast, a time-dependent (up to 20-fold) increase in active TGF-β1 release, with a maximum at 24 h, was observed in cocultures of WT apoptotic PMN and Mφ (Figure 6A). Only a minor (up to five-fold) increase in active TGF-β1 release was detected in supernatants from cocultures of WT PMN and CD18−/− Mφ. Interestingly, TGF-β1 release did not exceed control levels after 24 h in cocultures of CD18−/− PMN with WT Mφ. The same was true for cultures of CD18−/− Mφ with CD18−/− PMN. This coculture set-up most closely reflects the situation in our murine LAD1 model, where both PMN and Mφ are CD18 deficient. After 3 h of coculture, significantly elevated TGF-β1 levels were only observed in WT PMN–Mφ cocultures; in contrast, significant TGF-β1 release after 48 h occurred when at least PMN were WT. These results indicate that maximal release of TGF-β1 is dependent upon CD11/CD18 heterodimer expression on both PMN and Mφ. CD11/CD18 expression on apoptotic PMN in particular seems to be of great importance for sustained TGF-β1 release by Mφ.
Figure 6.
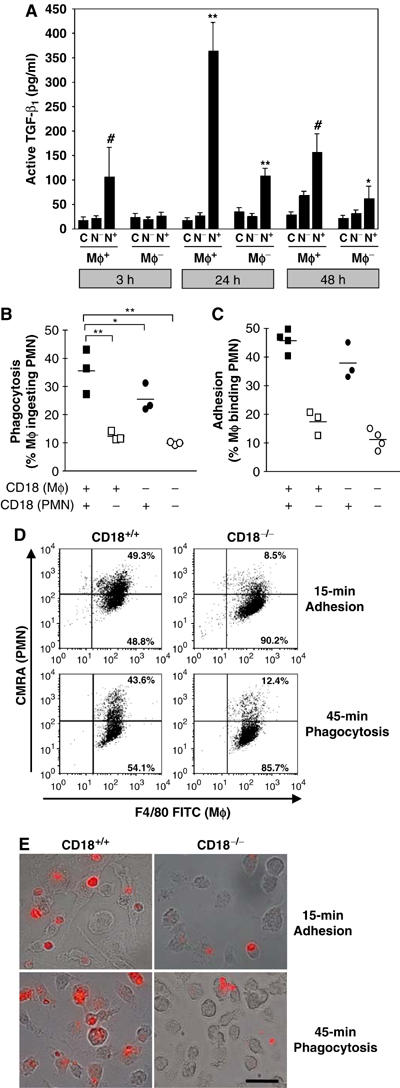
Impaired in-vitro adhesion/phagocytosis of apoptotic PMN and concurrent deficiency of TGF-β1 release by Mφ under CD18-deficient conditions. To measure the uptake of apoptotic PMN by Mφ and TGF-β1 release that physiologically accompanies phagocytosis of apoptotic PMN by Mφ in the wound bed, CD18−/− or WT Mφ were cocultured with apoptotic CD18−/− (N−) or WT (N+) PMN in an in vitro setting. As a control (C), Mφ and apoptotic PMN (not shown) were also incubated separately. (A) At the indicated time points, supernatants of cocultures were subjected to ELISA to detect active TGF-β1. Data is given as the mean±s.d. *,#P<0.05, **P<0.005. *,**Comparing CD18−/− and WT Mφ, #comparing CD18−/− and WT PMN. (B) In an identical setting, Mφ and PMN were coincubated, only for a shorter time. After 45 min, cells were collected from the well bottoms, stained and analysed by flow cytometry (for details, see Materials and methods in Supplementary data). Phagocytosis was assessed by calculating the percentage of Mφ phagocytosing PMN among the total Mφ counted (PMN-ingesting Mφ × 100/total number of Mφ). Bars indicate the median of each cohort. *P<0.05, **P<0.005. (C) Adhesion of apoptotic PMN preceding phagocytosis by Mφ depends on CD18. To investigate whether impaired phagocytosis in the absence of CD18 was due to an insufficient or absent adhesion between Mφ and PMN, we performed adhesion assays in which CD18−/− or WT Mφ were cocultured with apoptotic CD18−/− or WT PMN for 15 min. FACS-counted events were identified as single (i.e. nonadhering) apoptotic PMN (CMRA+ annexin-V+), single (nonadhering) Mφ (F4/80+) or as PMN–Mφ cell conjugates (CMRA+ F4/80+), stable enough to resist capillary shear forces during flow cytometry. The latter events were counted as Mφ-binding PMN (i.e. adhesion conjugates of Mφ with PMN), and were expressed as percentage of the total Mφ count (PMN-binding Mφ × 100/total number of Mφ). Each symbol indicates the median of a triplet analysis. *P<0.05, **P<0.005. (D) Representative flow-cytometric raw data of the adhesion and phagocytosis assays presented in (B, C) is provided. Mφ were stained using F4/80 FITC mAb; apoptotic PMN were loaded with CMRA prior to coculturing (for details see Materials and methods in Supplementary data). Cells appearing in the upper right quadrant of dot plots stain positive for both markers, thus representing either PMN adhering to Mφ after 15 min (‘Adhesion') or Mφ phagocytosing PMN after 45 min (‘Phagocytosis'), as also monitored by fluorescence microscopy. (E) Accordingly, Mφ phagocytosis of apoptotic PMN was assessed by immunofluorescence microscopy. For this purpose, coculturing of apoptotic PMN (labelled with CMRA, red/orange-fluorescing) with Mφ was performed using WT (left panel) and CD18−/− (right panel) PMN on Lab-Tek chamber slides (Nunc) for 15 min to detect adhesion, or for 45 min to assess phagocytosis. After cocultures, nonadherent PMN were washed away and microscopic pictures recorded digitally overlaying the differential interference contrast (DIC, using Nomarsky optics) with fluorescence pictures. The bars represent 40 μm.
To investigate whether the observed deficiency in TGF-β1 release coincided with reduced phagocytic clearance of apoptotic PMN by Mφ, apoptotic PMN were cocultured with Mφ in all possible combinations of WT or CD18−/− cells. To assess the adhesion of PMN to Mφ occurring prior to phagocytosis, Mφ–PMN conjugates were counted after 15 min and, to determine phagocytosis, Mφ phagocytosing PMN were detected after 45 min by flow cytometry (Figure 6B–D). Microscopic evaluation of adhesion versus phagocytosis was also performed to confirm flow-cytometric data (Figure 6E). Additional control experiments were also carried out using surface Ly-6G staining of adherent PMN (Supplementary data S7A) and lysosomal fluorescence tracking (Supplementary data S7B) to precisely distinguish between surface adhesion and completed engulfment of PMN. As shown by Figure 6B, after a 45-min period of coculturing, phagocytosis was severely impaired when either Mφ or PMN, or both, were CD18−/−.
Firm adhesion of target cells to phagocytes markedly contributes to phagocytic efficacy (Fallman et al, 1993; Mevorach et al, 1998; Rosenkranz et al, 1998). We provide direct evidence that the defective phagocytosis and a reduced secretion of TGF-β1 in the absence of CD18 are, at least in part, caused by an impaired ‘physical' attachment of apoptotic PMN to Mφ (Figure 6C). Similar data concerning decrease in adhesion, phagocytosis and subsequent TGF-β1 release were found in coculture assays with human cells using either CD18-blocking mAb, or with monocyte-derived Mφ from CD18-deficient human LAD1 patients (Figure 7). The phagocytic machinery itself, as assessed by engulfment of latex beads, was functional (Supplementary data S3A). Furthermore, the intracellular pathway leading to TGF-β1 release following engulfment was functional, since latex beads engulfment also leads to TGF-β1 release (Supplementary data S3B). Although the absence of CD18 resulted in a reduction of active TGF-β1 and phagocytosis/adhesion, we could mostly rule out that this was due to a major impact of CD18 deficiency on maturation and differentiation of bone marrow (BM)-derived Mφ (Supplementary data S4).
Figure 7.
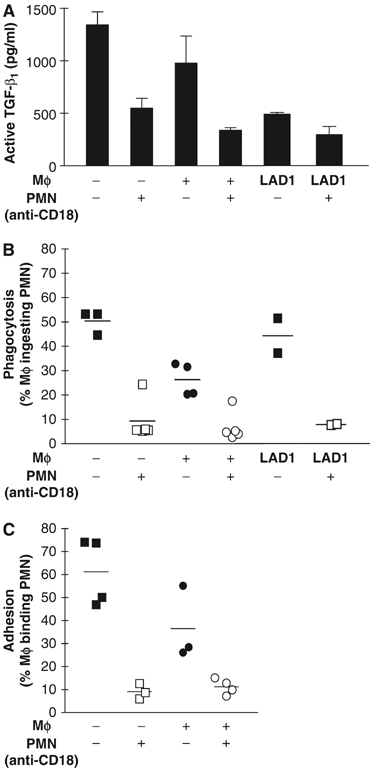
Ablation of CD18 function leads to an impaired adhesion and phagocytosis of apoptotic human PMN with a reduced TGF-β1 release by Mφ. To assess uptake of apoptotic PMN and TGF-β1 release by Mφ in the human setting, monocytes were obtained from LAD1 patients and healthy human donors and differentiated in vitro to Mφ. PMN were isolated from the peripheral blood of healthy donors and rendered apoptotic by culturing for 20 h. To disrupt function of CD18 on PMN surface, PMN were preincubated with blocking mAbs against CD18 (clones IB4 or TS1/18) before they were added to Mφ, whereas control PMN with functional CD18 were left untreated. PMN were cocultured with Mφ (A) for 24 h to detect release of active TGF-β1, or (B) for 45 min to measure phagocytosis. (C) Adhesion of human PMN to Mφ was also assessed performing identical cocultures, but only for 15 min.
Collectively, the observed defects in adhesion with subsequently impaired phagocytosis and TGF-β1 release seem to be causally related to a disturbed cell–cell contact/signalling between apoptotic PMN and Mφ in the absence of CD18 in vivo.
Discussion
The LAD1 syndrome results from a mutation of the CD18 gene that leads to a deficiency in β2 integrin expression on leukocytes. Patients with less than 1% residual expression of β2 integrins suffer from the severe form of LAD1, and show strongly impaired wound healing and atrophic scarring (Bowen et al, 1982; Anderson et al, 1984; Kuijpers et al, 1997; Anderson and Smith, 2001). They frequently die from septic complications due to reduced wound closure and the inability to effectively combat invading microorganisms. Using CD18−/− mice as a clinically relevant model for human LAD1 (Mizgerd et al, 1997; Scharffetter-Kochanek et al, 1998; Walzog et al, 1999b; Weinmann et al, 2003), we identified a mechanism that is most likely central to this complex pathophysiology. We demonstrate impaired PMN extravasation in CD18−/− mice after wounding leading to a decreased release of TGF-β1 by Mφ, which is usually stimulated by the engulfment of apoptotic PMN. Decreased TGF-β1 levels, in turn, result in reduced myofibroblast differentiation and impaired wound contraction. Hence, β2 integrins are essential for the coordinated cell–cell interactions and paracrine regulation occurring during the inflammatory and contraction phase of cutaneous wound healing.
In particular, around day 5–7 post-wounding, during the phase of granulation tissue contraction, wounds were significantly larger in CD18−/− mice as compared with WT. Simultaneously reduced expression levels of α-SMA and ED-A FN in CD18−/− granulation tissue suggest that decreased wound closure in CD18−/− mice results from impaired myofibroblast differentiation. Myofibroblast differentiation is characterised by the de novo expression of α-SMA in stress fibres, which mediates granulation tissue contraction in vivo (Serini and Gabbiani, 1999) and in vitro (Arora and McCulloch, 1994; Hinz et al, 2001b; Hinz et al, 2003). TGF-β1 is considered to be the major growth factor inducing α-SMA expression and myofibroblast differentiation (Desmoulière et al, 1993) through the specific binding to TGF-βRII (Derynck and Feng, 1997; Cowin et al, 2001). This TGF-β1 effect depends on the presence of the FN splice variant ED-A FN in the extracellular matrix (Serini et al, 1998). We could demonstrate that reduced levels of TGF-β1 in granulation tissue of CD18−/− mice is the major pathogenic event in impaired wound healing, since wound contraction was fully re-established upon local injection of recombinant TGF-β1.
Mφ are well-established sources for TGF-β1 in inflammation and wound healing. Since TGF-β1 release by Mφ is stimulated by the engulfment of apoptotic PMN at wound sites (Fadok et al, 1998; McDonald et al, 1999; Taylor et al, 2000), we assessed emigration patterns of both cell types during the inflammatory phase of cutaneous tissue repair. Emigration of PMN to the wound site was substantially suppressed in CD18−/− compared to WT mice, whereas Mφ emigration appeared to be normal. These results are consistent with the findings of previous studies in LAD1 patients (Anderson and Smith, 2001) and our mouse model for toxic dermatitis and thioglycolate-induced peritonitis (Mizgerd et al, 1997; Scharffetter-Kochanek et al, 1998; Walzog et al, 1999a; Grabbe et al, 2002). PMN extravasation depends on LFA-1 (CD11a/CD18), as shown in CD11b- (Mac-1) deficient mice (Lu et al, 1997). Here, the almost complete absence of β2-integrin-dependent PMN emigration from the vessels to the wound site resulted in a near disappearance of the induction of TGF-β1 release normally caused by PMN undergoing apoptosis at the wound site. In fact, very low numbers of PMN were found in the wounds of CD18−/− mice. These few PMN that entered the wound bed most likely did so via disrupted blood vessels, and the resulting microscopic bleeding into the tissue after wounding or via a β1-integrin-dependent emigration pathway (Sixt et al, 2001). TGF-β1 injection did not result in enhanced PMN recruitment into the wound beds of CD18−/− mice, in contrast to WT mice where increased PMN recruitment is seen, thus indicating that TGF-β1-induced PMN recruitment depends on CD18, and does not activate alternate emigration pathways.
We further evaluated whether decreased PMN numbers at the wound site may have resulted in reduced TGF-β1 release by Mφ, which is normally stimulated by phagocytic engulfment of apoptotic PMN. Moreover, we tested whether β2-integrin deficiency on PMN interfered with their release of TGF-β1. So far, only the lack of β2 integrins on Mφ had been studied (Wahl et al, 1990; Fallman et al, 1993; Mevorach et al, 1998; Ren et al, 2001). Since uptake of latex beads was unaltered in CD18−/− Mφ, the process of engulfment mediated by membrane receptor-independent phagocytosis appears to be normal. Nevertheless, phagocytosis of apoptotic PMN by Mφ and the concomitant physiologic release of TGF-β1 were significantly reduced in the absence of CD18 on either Mφ or PMN (or both). Since adhesion of target cells to phagocytes prior to the engulfment contributes to the efficacy of cell uptake (van Spriel et al, 2001), the observed impairment in attachment between PMN and Mφ may have accounted for the disturbed phagocytosis in the absence of CD18. In summary, CD18 is required for efficient cell–cell contact formation and thus promotes engulfment of apoptotic PMN by Mφ. The absence of CD18 leads to inefficient Mφ–PMN attachment, lack of engulfment, and thus to a failure to activate the downstream signalling pathway for TGF-β1 release by Mφ.
Furthermore, in a recent publication, we reported that CD18−/− PMN are resistant to apoptosis-inducing signals due to upregulation of antiapoptotic bcl-xL and downregulation of proapoptotic bax-α, both of the bcl-2 family (Weinmann et al, 2003). Impaired PMN recruitment into the wound bed enhanced apoptotic resistance of PMN and reduced release of TGF-β1 from Mφ upon defective adhesion between Mφ and PMN, leading to impaired engulfment of apoptotic PMN, collectively contribute to the observed deficiency of TGF-β1 in wounds of CD18−/− mice. Similar data with a reduced release of active TGF-β1, impaired adhesion and phagocytosis were observed with CD18-deficient monocyte-derived Mφ from human LAD1 patients, indicating the relevance of our CD18−/− mouse model for human LAD1.
TGF-β1 perpetuates and amplifies its response via an autocrine mechanism with upregulation of TGF-βRII and enhanced release of TGF-β1 from myofibroblasts and inflammatory bystander cells. Therefore, a deficiency in the release of TGF-β1 by Mφ during the initial phases of wound healing will result in substantially amplified effects on subsequent events during tissue repair, such as myofibroblast differentiation. A human IL-8 orthologue (cCAF) has been demonstrated to induce myofibroblast differentiation and to participate in wound contraction in chicken (Feugate et al, 2002). Even though the murine homologues of human IL-8, KC and MIP-2, were decreased in early phases of wound healing in CD18−/− mice (Supplementary data S5B and D), probably caused by a reduced release during apoptotic PMN phagocytosis by Mφ (Supplementary data S5A and C), we did not find a major role of KC or MIP-2, neither for PMN recruitment (data not shown) nor for wound contraction (Supplementary data S5E) in the murine LAD1 model.
An alternative scenario where myofibroblasts may differentiate from hematopoietic precursor cells which require β2 integrins for being recruited to the wound site is less convincing; in this case, injection of recombinant TGF-β1 would not have restored wound-contraction efficacy. Two lines of evidence suggest that the normally occurring kick start of TGF-β1 release from platelets during the clotting phase of wound healing is not affected in CD18−/− mice. First, platelet counts as well as subaquatic bleeding times do not differ in CD18−/− versus WT mice (Supplementary data S6). Second, TGF-β1 concentrations measured in the serum post clotting did not show a significant difference between both genotypes (data not shown). Emigration of mast cells also depends on β2 integrins (Rosenkranz et al, 1998). As mast cells also contain and release TGF-β1 (Artuc et al, 1999), a lack of mast cells in wounds may potentially enhance the TGF-β1 deficiency in CD18−/− mice. However, mast-cell emigration into the wound site occurs around day 10, which is far later than the observed deficiency herein in wound contraction, which is already noticeable at around day 5.
Emigration from vessels to the interstitial tissue involves the coordinated interaction of selectins with their glycosylated sialyl Lewis x to slow down the velocity of PMN and Mφ in the process of rolling, thus preparing the adhesion between β2 integrins and ICAMs for PMN, and VLA-4 and VCAM-1 for Mφ (Issekutz et al, 1995). Thus, it is not surprising that (a) double P- and E-selectin-deficient mice (Subramaniam et al, 1997), (b) functional P- and E-selectin-deficient mice (mice deficient in β-1,4-galactosyltransferase, which glycosylates the P- and E-selectin ligands (Mori et al, 2004)) and (c) genetically deficient LAD2 patients suffering from a glycosylation deficiency due to the GDP–fucose transporter gene (Luhn et al, 2001) show altered emigration of PMN and Mφ, and impaired wound healing. Also, ICAM-1−/− mice lacking one of the multiple counter receptors of the β2 integrins reveal a subtle wound-healing defect (Nagaoka et al, 2000). None of these studies have analysed wound contraction. Similarly, myofibroblast differentiation and wound contraction have not been studied in several mice lines with genetic or functional deficiency for TGF-β1 (Crowe et al, 2000; Koch et al, 2000). Our data provides evidence that PMN play a central role in the induction of TGF-β1-dependent myofibroblast differentiation and wound closure. At first glance, our results are in contrast to the data by Simpson and Ross (1972), who supported the view that PMN are not necessary for an appropriate tissue repair. The obvious discrepancy can be resolved when considering that Simpson and Ross have studied an incisional model of injury in which wound contraction and myofibroblast differentiation are not absolutely mandatory for wound closure. As wound closure in incisional wounds does rather depend on re-epithelialisation but not on myofibroblast-dependent wound contraction, our results do not contradict Simpson's and Ross' findings. Apparently, it is of utmost importance to precisely define the parameter and wound model studied (primary versus secondary wound healing, re-epithelialisation versus wound contraction) for studies on tissue and wound repair.
Here, we demonstrated that the impairment in secondary wound healing in CD18−/− mice results from sequentially inter-related events starting with impaired PMN extravasation. Decreased numbers of PMN and the lack of adhesion of CD18−/− Mφ to CD18−/− PMN, as occurs in our LAD1 mouse model, cause low TGF-β1 levels in the wounds. CD18 deficiency has multiple effects on TGF-β1 availability: it reduces the total number of available PMN in the wound bed, inhibits PMN apoptosis and, in addition, by defective adhesion, impairs the phagocytic process of apoptotic PMN by Mφ, thus, severely reducing the TGF-β1 release by Mφ. The final outcome is, in any scenario, a reduction in TGF-β1 leading to reduced transition from fibroblasts to contractile myofibroblasts and thus impairing wound contraction. Given the fact that the defence against microbial infection is impaired in LAD1 patients, efficient wound closure is essential. Since TGF-β1 appears to be the central element in CD18−/− wound-healing deficiency, local injection of recombinant TGF-β1 may be a modality to effectively treat the defective wound healing in LAD1 patients.
Materials and methods
Mice
CD18−/− homozygotes (Scharffetter-Kochanek et al, 1998) and CD18+/+ WT controls were derived from heterozygote crosses on a mixed 129Sv × C57BL/6 background and constantly maintained under specific pathogen-free conditions. Wound-healing studies were performed using mice in experimental cohorts only of identical gender at an age of 8–12 weeks. All experiments were carried out in compliance with the German Law for Welfare of Laboratory Animals.
Wound-healing model
Prior to injury, mice were anaesthetised by intraperitoneal injection of a ketamine (10 g/l)/xylazine (8 g/l) solution (10 μl/g body weight). After shaving the dorsal hair and cleaning the exposed skin with 70% ethanol, full-thickness (including the panniculus carnosus) excisional wounds were punched at two sites in the middle of the dorsum using 5-mm biopsy stamps (STIEFEL, Offenbach, Germany). Each wound region was digitally photographed at indicated time points, and wound areas were calculated using Photoshop® software (version 7.0; Adobe Systems, San Jose, CA). Wound sizes at any given time point after wounding were expressed as percentage of initial (day 0) wound area. Wounds were left uncovered and harvested with an 8-mm biopsy punch in mice cohorts of n=3 at indicated time points. For expression analyses, two wounds from each animal were frozen in liquid nitrogen immediately after excision.
Subcutaneous injection of TGF-β1 at wound sites of mice
To locally supply additional TGF-β1 in the wound vicinity, carrier-free recombinant human TGF-β1 (R&D Systems, Wiesbaden, Germany) was injected s.c. at four sites around the wound, allowing to infiltrate the wound margins, at a total dose of 0.45 μg as described elsewhere (Ashcroft et al, 2003). Mock injections were made using only the solvent NaCl 0.9%. First injections were made on day 1 after wounding, followed by further injections every second day until wounds were harvested for snap freezing or paraffin-embedding at 5 or 7 days after wounding.
Immunohistochemistry
Wound granulation tissues were fixed in 4% neutral buffered formalin and embedded in paraffin. Deparaffinised sections of 4 μm were immersed in methanol containing 0.5% H2O2 for 10 min. Sections were incubated with primary Ab either overnight at 4°C (anti-TGF-βRII, rbAb; Santa Cruz Biotechnology Inc., Heidelberg, Germany) or for 60 min at room temperature (RT) (anti-TGF-β1; rbAb, Santa Cruz), followed by 30-min incubation with a secondary biotinylated goat anti-rabbit Ab (Dako, Hamburg, Germany). α-SMA expression was probed with biotinylated anti-α-SM-1 (IgG2a mAb, (Skalli et al, 1986) for 60 min at RT. PMN were detected using biotinylated GR-1 (clone RB6-8C5; BD Pharmingen, Heidelberg, Germany), Mφ with biotinylated F4/80 (clone Cl:A3-1; Caltag, Hamburg, Germany) for 60 min at RT. Biotinylated Ab were detected by means of the streptavidin–biotin complex peroxidase method (Dako) and peroxidase activity was visualised with 3-amino-9-ethylcarbazole (Sigma, Taufkirchen, Germany). Slides were counterstained with haematoxylin and mounted in Eukit (O Kindler, Ochsenfurt, Germany). Pictures were acquired using a Zeiss Axiophot microscope (Carl Zeiss Inc., Oberkochen, Germany), with a digital colour camera and corresponding software (Axiocam®, Zeiss). All images were processed for printing using Adobe Photoshop® software.
Western blot analysis
Dissected tissues were frozen immediately in liquid nitrogen, crushed and dissolved in sample buffer, sonicated, boiled for 3 min and protein concentration was determined according to Bradford, as described previously (Hinz et al, 2001b). Equal amounts of total protein (5–20 μg) were loaded onto 10% SDS minigels (Bio-Rad Laboratories AG, Glattbrugg, Switzerland), separated by PAGE, and transferred to nitrocellulose membrane (Protran®; Schleicher & Schuell, Dassel, Germany). Membranes were then probed with primary Ab against α-SMA (anti-α-SM-1), ED-A FN (IST-9, IgG1 mAb; a kind gift from Dr L Zardi, Genoa, Italy) and TGF-βRII (Santa Cruz). Secondary goat anti-mouse and anti-rabbit Ab, conjugated with horseradish-peroxidase (Jackson ImmunoResearch, Cambridgeshire, UK), were used and signals were detected by ECL chemiluminescence (Amersham, Freiburg, Germany). Bands were digitised with a scanner (Arcus II; Agfa, Cologne, Germany) and the ratio between all band densities of one blot was calculated by commercial computer software (ImageQuant V3.3; Molecular Dynamics, Sunnyvale, CA). Relative protein expression was normalised to the respective values for vimentin, obtained from scanned coomassie blue-stained gels. Samples from four animals per experimental condition were tested and protein/vimentin mean ratio was calculated from three scanned Western blots per animal sample.
Isolation and culture of murine BM-derived Mφ
BM-derived Mφ were obtained from femurs of CD18−/− and WT mice, as described previously (Sunderkötter et al, 1993). Briefly, BM was flushed through with DMEM (Biochrom, Berlin, Germany). After osmotic shock, 3 × 106 cells/10 ml medium were grown in DMEM supplemented with 10% heat-inactivated FCS (PAA Laboratories, Pasching, Austria), 10% conditioned supernatant from L929 cells, 2% L-glutamine, penicillin/streptomycin 100 U/100 μg/ml and 1% nonessential amino acids (Biochrom, Berlin, Germany) in Teflon-coated bags (Heraeus, Hanau, Germany) in a 7% CO2 atmosphere at 37°C. After 6 days, cells were removed from Teflon bags, seeded into 24-well cell-culture plates (Nunc, Roskilde, Denmark) at a concentration of 2 × 105/well and cultured for an additional 1 day before being subjected to phagocytosis assays.
In vitro adhesion and phagocytosis in the murine model
A modified phagocytic assay for quantification of uptake of apoptotic PMN by Mφ, which has been extensively described and validated (Homburg et al, 1995; Fadok et al, 1998, 2001), was used in these studies. BM cells were flushed from femurs and granulocytes were separated by density gradient centrifugation using Histopaque 1077 and 1119 (Sigma). After washing of isolated PMN, apoptotic PMN were generated by 22-h incubation in Isecove's basal medium (Gibco, Karlsruhe, Germany) at 37°C, 5% CO2 in a humidified atmosphere as done elsewhere (Taylor et al, 2000). These conditions were tested optimal to obtain PMN apoptosis >50% and necrosis <5%, as confirmed by flow cytometry using annexin-V FITC and propidium iodide, respectively (BD Pharmingen). Apoptotic PMN were added to Mφ that had been previously plated out from Teflon bags onto 24-well plates or glass chamber slides, at a target/effector ratio of >10/1. After interaction times of either 15 min for adhesion or 45 min for phagocytosis, dishes were washed in cold (4°C) 0.9% saline to remove noningested PMN. Additional biological controls were also performed, and are given along with the extensive staining and assessment procedures for flow-cytometric and microscopic analyses of adhesion and phagocytosis in detail as Supplementary data.
Assessment of cytokine release during phagocytosis and from tissue lysates
Cocultures of CD18−/− and WT PMN and Mφ were carried out as described above. Supernatants were harvested at 3, 16, 24 h, and additionally at 48 and 72 h after starting of cocultures to observe time-shifted accumulation of cytokines. All supernatants were frozen at −80°C. To quantify TGF-β1 in wound tissues, lysates were prepared from wound tissue snap-frozen in liquid nitrogen. Frozen samples were ground using a micro-dismembrator (Braun Biotech International, Göttingen, Germany), and resuspended in homogenisation RIPA buffer (Boehringer, Mannheim, Germany) at 100 mg/ml. The homogenate was centrifuged at 14 000 r.p.m. for 20 min at 4°C. The supernatants were collected and stored at −80°C until ELISA analysis. ELISAs for murine TGF-β1 were then performed according to the manufacturer's protocols (all R&D Systems). Activation of the latent TGF-β complexes is an important step that regulates TGF-β1 functions in vivo. To assess the total TGF-β1 contained in the samples, acidification was carried out by adding 1 N HCl to cell culture supernatants, or 2.5 N acetic acid/10 M urea to tissue lysates. After incubation for 10 min at RT, samples were neutralised by adding 1.2 N NaOH/0.5 M HEPES or 2.7 N NaOH/1 M HEPES, respectively. In order to detect TGF-β1 already released from latent binding complexes and thus active at the time of sample collection, measurements were made without prior acidification.
Statistical analysis
Quantitative results are presented as mean values±standard deviation (s.d.). Mean values were tested by means of a two-tailed heteroscedastic Student's t-test, or, in case of a non-Gaussian distribution, Mann–Whitney U-test was used. Differences were considered to be statistically significant at values of P<0.05. In column charts, P-values <0.05 are indicated by *, or #, and P<0.005 by **, or ##.
Supplementary Material
Supplementary Data
Acknowledgments
We thank Dr Luciano Zardi (National Institute for Cancer Research, Laboratory of Cell Biology, Genoa, Italy) for providing IST-9 mAb, and P Henchoz and J Smith-Clerc for technical assistance. We are grateful to Dr Pierre Shephard (ALTANA Pharma, Konstanz, Germany) for critically reading this manuscript. The work of BH was supported by the Swiss National Science Foundation, grant 3100A0-102150/1, the work of BW by the Deutsche Forschungsgemeinschaft (DFG), grant WA 1048/1-1 and -2, the work of TK by the DFG grant SFB 589, and that of KS-K by the DFG grants 411/12-1 and SFB 497-C7.
References
- Anderson DC, Schmalstieg FC, Arnaout MA, Kohl S, Tosi MF, Dana N, Buffone GJ, Hughes BJ, Brinkley BR, Dickey WD, Abramson JS, Springer T, Boxer LA, Hollers JM, Smith CW (1984) Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest 74: 536–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC, Smith CW (2001) Leukocyte Adhesion Deficiencies. New York, NY: McGraw-Hill [Google Scholar]
- Angele MK, Knoferl MW, Ayala A, Albina JE, Cioffi WG, Bland KI, Chaudry IH (1999) Trauma-hemorrhage delays wound healing potentially by increasing pro-inflammatory cytokines at the wound site. Surgery 126: 279–285 [PubMed] [Google Scholar]
- Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75: 1037–1050 [PubMed] [Google Scholar]
- Arora PD, McCulloch CA (1994) Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 159: 161–175 [DOI] [PubMed] [Google Scholar]
- Artuc M, Hermes B, Steckelings UM, Grutzkau A, Henz BM (1999) Mast cells and their mediators in cutaneous wound healing—active participants or innocent bystanders? Exp Dermatol 8: 1–16 [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T (2003) Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest 111: 1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen TJ, Ochs HD, Altman LC, Price TH, Van Epps DE, Brautigan DL, Rosin RE, Perkins WD, Babior BM, Klebanoff SJ, Wedgwood RJ (1982) Severe recurrent bacterial infections associated with defective adherence and chemotaxis in two patients with neutrophils deficient in a cell-associated glycoprotein. J Pediatr 101: 932–940 [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Holmes TM, Brosnan P, Ferguson MW (2001) Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol 11: 424–431 [PubMed] [Google Scholar]
- Crowe MJ, Doetschman T, Greenhalgh DG (2000) Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol 115: 3–11 [DOI] [PubMed] [Google Scholar]
- Derynck R, Feng XH (1997) TGF-beta receptor signaling. Biochim Biophys Acta 1333: F105–F150 [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122: 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, Springer TA (1990) ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol 111: 3129–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake WT, Issekutz AC (1993) Transforming growth factor-beta 1 enhances polymorphonuclear leucocyte accumulation in dermal inflammation and transendothelial migration by a priming action. Immunology 78: 197–204 [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Springer TA (1988) Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol 107: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem 276: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Fahey TJ III, Sadaty A, Jones WG II, Barber A, Smoller B, Shires GT (1991) Diabetes impairs the late inflammatory response to wound healing. J Surg Res 50: 308–313 [DOI] [PubMed] [Google Scholar]
- Fallman M, Andersson R, Andersson T (1993) Signaling properties of CR3 (CD11b/CD18) and CR1 (CD35) in relation to phagocytosis of complement-opsonized particles. J Immunol 151: 330–338 [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, Lucas C, Townes AS (1991) Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med 173: 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feugate JE, Li Q, Wong L, Martins-Green M (2002) The cxc chemokine cCAF stimulates differentiation of fibroblasts into myofibroblasts and accelerates wound closure. J Cell Biol 156: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S, Varga G, Beissert S, Steinert M, Pendl G, Seeliger S, Bloch W, Peters T, Schwarz T, Sunderkötter C, Scharffetter-Kochanek K (2002) Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J Clin Invest 109: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001a) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C (2003) Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14: 2508–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G (2001b) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159: 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D (1995) Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood 85: 532–540 [PubMed] [Google Scholar]
- Issekutz AC, Chuluyan HE, Lopes N (1995) CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol 57: 553–561 [DOI] [PubMed] [Google Scholar]
- Kess D, Peters T, Zamek J, Wickenhauser C, Tawadros S, Loser K, Varga G, Grabbe S, Nischt R, Sunderkötter C, Muller W, Krieg T, Scharffetter-Kochanek K (2003) CD4+ T cell-associated pathophysiology critically depends on CD18 gene dose effects in a murine model of psoriasis. J Immunol 171: 5697–5706 [DOI] [PubMed] [Google Scholar]
- Koch RM, Roche NS, Parks WT, Ashcroft GS, Letterio JJ, Roberts AB (2000) Incisional wound healing in transforming growth factor-beta1 null mice. Wound Repair Regen 8: 179–191 [DOI] [PubMed] [Google Scholar]
- Kondo T, Ohshima T (1996) The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med 108: 231–236 [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, Weening RS, Verhoeven AJ, Roos D (1997) Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest 100: 1725–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM (1997) LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest 99: 1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D (2001) The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28: 69–72 [DOI] [PubMed] [Google Scholar]
- Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA (1991) Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest 88: 904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276: 75–81 [DOI] [PubMed] [Google Scholar]
- McDonald PP, Fadok VA, Bratton D, Henson PM (1999) Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 163: 6164–6172 [PubMed] [Google Scholar]
- Mevorach D, Mascarenhas JO, Gershov D, Elkon KB (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med 188: 2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM (1997) Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med 186: 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Orci L (1988) Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 85: 4894–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Kondo T, Nishie T, Ohshima T, Asano M (2004) Impairment of skin wound healing in beta-1, 4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am J Pathol 164: 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S (2000) Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol 157: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Stuart L, Lindberg FP, Rosenkranz AR, Chen Y, Mayadas TN, Savill J (2001) Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. J Immunol 166: 4743–4750 [DOI] [PubMed] [Google Scholar]
- Rosenkranz AR, Coxon A, Maurer M, Gurish MF, Austen KF, Friend DS, Galli SJ, Mayadas TN (1998) Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J Immunol 161: 6463–6467 [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL (1998) Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med 188: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G (1998) The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 142: 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Gabbiani G (1999) Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 250: 273–283 [DOI] [PubMed] [Google Scholar]
- Simpson DM, Ross R (1972) The neutrophilic leukocyte in wound repair a study with anti-neutrophil serum. J Clin Invest 51: 2009–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341: 738–746 [DOI] [PubMed] [Google Scholar]
- Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM (2001) Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J Biol Chem 276: 18878–18887 [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103: 2787–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA, Anderson DC (1986) The importance of the Mac-1, LFA-1 glycoprotein family in monocyte and granulocyte adherence, chemotaxis, and migration into inflammatory sites: insights from an experiment of nature. Ciba Found Symp 118: 102–126 [DOI] [PubMed] [Google Scholar]
- Staunton DE, Dustin ML, Springer TA (1989) Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature 339: 61–64 [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, Wagner DD (1997) Role of endothelial selectins in wound repair. Am J Pathol 150: 1701–1709 [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C, Kunz M, Steinbrink K, Meinardus-Hager G, Goebeler M, Bildau H, Sorg C (1993) Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol 151: 4891–4901 [PubMed] [Google Scholar]
- Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ (2000) A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 192: 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363 [DOI] [PubMed] [Google Scholar]
- van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG (2001) Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 97: 2478–2486 [DOI] [PubMed] [Google Scholar]
- Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF (1990) Macrophage production of TGF-beta and regulation by TGF-beta. Ann NY Acad Sci 593: 188–196 [DOI] [PubMed] [Google Scholar]
- Walzog B, Scharffetter-Kochanek K, Gaehtgens P (1999a) Impairment of neutrophil emigration in CD18-null mice. Am J Physiol 276: G1125–G1130 [DOI] [PubMed] [Google Scholar]
- Walzog B, Weinmann P, Jeblonski F, Scharffetter-Kochanek K, Bommert K, Gaehtgens P (1999b) A role for beta(2) integrins (CD11/CD18) in the regulation of cytokine gene expression of polymorphonuclear neutrophils during the inflammatory response. FASEB J 13: 1855–1865 [DOI] [PubMed] [Google Scholar]
- Weinmann P, Scharffetter-Kochanek K, Forlow SB, Peters T, Walzog B (2003) A role for apoptosis in the control of neutrophil homeostasis in the circulation: insights from CD18-deficient mice. Blood 101: 739–746 [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83: 835–870 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data


