Abstract
Pterins are essential for the growth of Leishmania species, and recent work has led to the isolation of the biopterin transporter BT1. In this study, we inactivated the Leishmania donovani biopterin transporter BT1 by gene disruption mediated by homologous recombination. No transport of biopterin was detected in this mutant. The L. donovani BT1 null mutant showed a much lesser capacity for inducing infection in mice than wild-type parasites and could elicit protective immunity in mice susceptible to infection against a L. donovani challenge. Splenocytes isolated from mice immunized with the BT1 null mutant parasites produced significant amounts of interferon gamma following stimulation with L. donovani promastigotes as measured by enzyme-linked immunosorbent assay and enzyme-linked immunospot assays. Overall, these results show that by genetically manipulating the pterin transport in L. donovani, it is possible to generate an attenuated organism that could be part of a vaccination strategy.
Leishmania is a protozoan parasite that is distributed worldwide, being endemic in 88 countries. Each year, 1.5 million new cases of cutaneous leishmaniasis and 500,000 new cases of visceral leishmaniasis are estimated (15). The latter, caused mainly by Leishmania donovani, can be fatal if left untreated. The treatment relies mainly on chemotherapy, and the mainstay consists in different formulations of pentavalent antimony, although alternatives, such as liposomal amphotericin B, are also effective (3, 15). Nonetheless, in some parts of the world, notably in the state of Bihar, India, more than 50% of the patients are unresponsive or relapse after conventional chemotherapy (42). Several approaches (killed parasites with or without BCG [a naturally attenuated form of Mycobacterium bovis]; subunit vaccines; DNA vaccines; attenuated organisms) have been used as vaccination strategies against Leishmania, but none has yet translated into an effective product (see references 14 and 31 for recent reviews).
With the advent of gene transfection technology with Leishmania, a number of innovative approaches were used in attempts to generate vaccines. These include the inactivation of genes encoding enzymes thought to be important for parasite intracellular survival, including the dihydrofolate reductase gene (43), the cysteine proteinase genes (2), the HSP100 heat shock protein (16), and the trypanothione reductase (10; also unpublished data). Other approaches relying on the expression of antisense RNA (45) or of cytotoxic genes (26) were also used. Most of this work was done with Leishmania species giving rise to cutaneous lesions, and with the exception of antisense RNA, no recombinant attenuated L. donovani organisms produced by gene inactivation have been reported. In order to generate attenuated L. donovani organisms for vaccination purposes, we have targeted genes thought to be important for intracellular survival. Our first attempt was to inactivate trypanothione reductase (10), a gene product essential for keeping thiols in a reduced form (11). Generation of a heterozygous mutant was readily achieved, but generation of a trypanothione reductase null mutant turned out not to be feasible, since a third allele was obtained by genomic rearrangements. The ability of Leishmania to rearrange its genome during attempts to disrupt genes thought to be essential has been frequently reported (7, 25) (10).
Pterins are known to be essential for growth of Leishmania and of other kinetoplastid parasites (44), and pterins, including biopterin, have been demonstrated to reduce the requirements for folates for Crithidia fasciculata (21). Work on the mechanisms of resistance to the antifolate methotrexate has contributed greatly to the isolation of gene products involved in pterin metabolism and transport (28, 30). A recent study has shown that the expression of genes involved in either pterin reduction (PTR1) or transport of biopterin (BT1) is modulated in Leishmania grown in pterin-limited medium (35). Pterins may be involved in the biosynthesis of folates, but other roles in Leishmania still remain to be elucidated (28, 30), although reduced pterins appear to be important for the process of metacyclogenesis (8). The biopterin transporter BT1 has recently been characterized (22, 24), and due to the central role of biopterin in Leishmania growth, we hypothesized that generating an L. donovani BT1 null mutant may lead to an attenuated strain useful for vaccination. In this study, we report on the generation of an L. donovani recombinant BT1 null mutant and the possibility of using this attenuated organism as part of a strategy of vaccination against visceral leishmaniasis.
MATERIALS AND METHODS
Leishmania growth and infection.
The Leishmania donovani donovani Sudanese 1S2D strain was grown in SDM-79 medium (5) supplemented with 10% heat-inactivated fetal bovine serum and 5 mg of hemin/ml. L. donovani promastigotes were transfected by electroporation as reported previously (33). Late-stationary-phase L. donovani promastigotes (5 × 107 cells) were injected in the tail veins of BALB/c mice (Charles River, St-Constant, Canada). At 4 weeks postinfection, impression smears of the livers and spleens were made and stained using Giemsa or Diff-Quick as described previously (29). Four weeks represents the time point for maximum infection rates of L. donovani in the BALB/c mouse model (9, 34). Alternatively, the infection was measured using recombinant parasites expressing the luciferase gene (34). For immunization and challenge experiments, stationary-phase L. donovani BT1 null mutants (5 × 107 promastigotes) were injected intravenously (i.v.) into the tail veins of BALB/c mice. After 6 weeks, the mice were challenged by i.v. injection of 5 × 107 stationary-phase L. donovani promastigotes expressing the luciferase (LUC) gene. Four weeks after the i.v. challenge, mice were sacrificed and livers and spleens were isolated, and the luciferase activity was measured as described previously (34).
Construction of an L. donovani BT1 null mutant.
The 2.3-kb BglII-NheI L. donovani fragment containing the BT1 gene was subcloned into pSP72 (Promega), and a hygromycin B phosphotransferase expression cassette (HYG) derived from pSPY-HYG (32) was introduced into the unique ApaI site of the BT1 gene. A 3.6-kb BglII-SalI HYG-containing fragment was used to disrupt one chromosomal BT1 allele by homologous recombination. A BT1 null mutant was obtained by selection for loss of heterozygosity (13) by increasing the hygromycin B selection pressure up to 600 μg/ml and cloning of the cell pool. The homozygous L. donovani BT1 mutant was characterized by Southern blot analysis as described previously (36).
Pteridine transport experiments.
Transport experiments were performed as described previously (22, 24). Tritium-labeled [3H]-biopterin (5.8 μCi/mmol)) was purchased from Movarek Biochemicals. Transport studies were carried out with 150 nM radioactive pteridines. To measure active biopterin transport, uptake in cells incubated on ice was subtracted from values obtained at room temperature.
Detection of secreted interferon-gamma by enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISPOT) assays.
Spleens of naive and vaccinated BALB/c mice were removed under aseptic conditions 1, 2, 4, 8 and 12 weeks postinfection, and splenocytes were cultured in triplicate in 96-well flat-bottom microplates (Costar). L. donovani promastigotes (4 × 105) were added to 5 × 106 splenocytes in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol. After 4 days, supernatants were collected and stored at −80°C. The production of interferon-gamma (IFN-γ) was first measured by sandwich ELISA using reagents from the R&D system (Minneapolis, Minn.). Briefly, 1 μg of anti-IFN-γ/ml was coated on 96-well plates (ImmunoPlate; Nunc, Naperville, Ill.) and incubated overnight at 4°C. The plates were blocked with 5% bovine serum albumin in phosphate-buffered saline, and culture supernatants were then added and incubated for 2 h at room temperature. Biotinylated anti-IFN-γ (50 ng/ml) was added and incubated for 1 h in 2% bovine serum albumin-phosphate-buffered saline. The bounded biotinylated antibody was detected by streptavidin-peroxidase conjugate (Research Diagnostics Inc., Flanders, N.J.). The absorbance was measured at 450 nm (Microwell system; Organon Teknika). ELISA permits the detection of bulk IFN-γ production. We also measured IFN-γ production by the ELISPOT assay, which permits the enumeration of individual cytokine-producing T cells. Plates (multiscreen; Millipore) were coated overnight at 4°C with the anti-IFN-γ (1 μg/ml) and then washed with RPMI and 10% fetal calf serum at 37°C. Freshly isolated splenocytes (7.5 × 106) were incubated with 4 × 105 Leishmania organisms for 3 h at 37°C, 5% CO2, in a 1.5-ml tube with complete RPMI medium. Then, 105 stimulated cells were added to the blocked plates and incubated at 37°C overnight. The cells were washed off, and bounded cytokines were detected with 100 ng of biotinylated anti-IFN-γ/ml, followed by streptavidin-alkaline phosphatase reaction. ELISPOT assays were developed using the substrate nitroblue tetrazolium (NBT)-5-bromo-4-chloro-3-indolyl phosphate (Bio-Rad, Mississauga, Ontario, Canada). The number of specific IFN-γ-secreting T cells was calculated by subtracting the negative control value from the established spot-forming cells (SFC) count. The count was calculated by averaging the numbers of spots for triplicate wells.
RESULTS
Generation of an L. donovani BT1 null mutant.
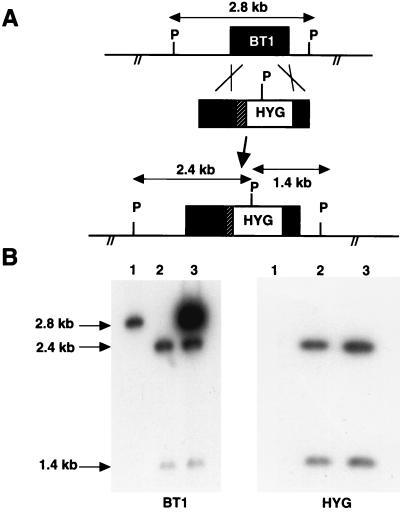
Disruption of the BT1 gene has already been achieved in a Leishmania tarentolae strain (22), in an L. donovani strain which has three BT1 alleles, one of which is translocated within the ribosomal locus (24), and more recently in one Leishmania major strain (8). No animal studies have been reported with any of these mutants. To study the role of biopterin transport in Leishmania infectivity and pathogenesis, we attempted to inactivate the BT1 gene in L. donovani. The L. donovani 1S strain has only two copies of BT1, and the parasites used were freshly isolated from animals and were highly infective. Experiments were done to disrupt the first BT1 allele using a hygromycin phosphotransferase (HYG) expression cassette (32) (Fig. 1A) which was electroporated into Leishmania. The analysis of a Southern blot indicated that a 2.8-kb PstI-PstI fragment harboring the BT1 gene hybridized to a BT1 probe in wild-type L. donovani (Fig. 1B, lane 1). Disruption of one copy of BT1 by the integration of the HYG cassette introduced an extra PstI restriction site, giving rise to two fragments, of 2.4 and 1.4 kb, hybridizing to both BT1 and HYG probes (Fig. 1A). Analysis of the initial hygromycin B-resistant cell pool showed the appearance of the two expected additional fragments in addition to one remaining intact BT1 allele (data not shown). The second BT1 allele was inactivated by loss of heterozygosity (13) by increasing the selection pressure with hygromycin B. This led to the disruption of the second BT1 allele by the HYG resistance marker, as indicated by Southern blot hybridization studies (Fig. 1B, lane 2). Biopterin transport in Leishmania appears nonetheless essential, since growth of the L. donovani BT1 null mutant was observed only when the medium was supplemented with biopterin.
FIG. 1.
BT1 gene inactivation in L. donovani. (A) The BT1 locus of L. donovani 1S, along with PstI sites, is shown, as well as the integration of the HYG expression cassette (32), which carries an extra PstI site. (B) Southern blots of L. donovani cells hybridized with a BT1 probe (left) and a HYG probe (right). Sizes of the bands were determined using the 1-kb ladder. Lane 1, L. donovani wild-type cell; lane 2, L. donovani BT1 null mutant; lane 3, L. donovani BT1 null mutant retransfected with a plasmid expressing the BT1 gene.
Transport properties of the BT1 null mutant.
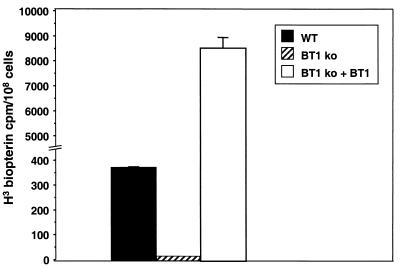
L. donovani 1S is capable of transporting biopterin (Fig. 2), although at a much lower level of efficiency than is described for L. tarentolae (22). Under our experimental conditions, no biopterin accumulation could be measured in the L. donovani BT1 null mutant (Fig. 2), suggesting that we have indeed disrupted the main biopterin transporter in this parasite. By introducing an expression vector carrying the BT1 gene into the BT1 null mutant (Fig. 1B, lane 3), we were able to rescue the transport phenotype of the mutant. In fact, overexpression of BT1 increases significantly the transport of biopterin compared to that observed in wild-type cells (Fig. 2).
FIG. 2.
Biopterin transport in L. donovani cells. Transport of [3H] biopterin was measured in L. donovani wild-type cells (black box), a BT1 null mutant (BT1 ko) (hatched box), and a BT1 null mutant retransfected with a plasmid expressing BT1 (white box). Results are averages of triplicate values, for which the uptake at 4°C was subtracted from the values at 25°C.
Reduced infectivity of the L. donovani BT1 null mutant in mice.
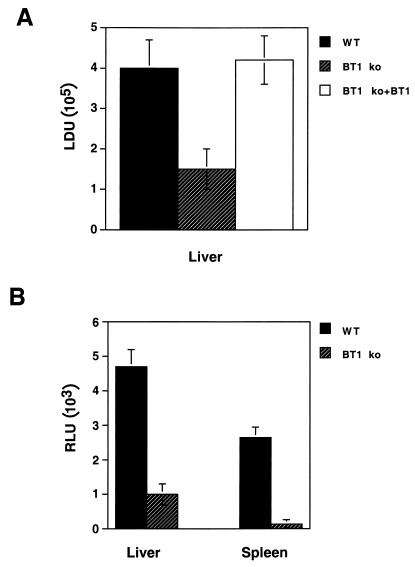
Given the known importance of pterins in Leishmania growth, we hypothesized that an L. donovani BT1 disruption mutant would be at a disadvantage during in vivo infection in the BALB/c model. Three groups of five BALB/c mice were each injected i.v. in the tail vein with late-stationary-phase promastigotes of either the L. donovani wild type or the L. donovani BT1 null mutant and/or the L. donovani BT1 null mutant expressing the BT1 gene as part of an episome (rescue transfectant). From previous data we know that in our infection model, the peak of L. donovani 1S infection in BALB/c mice is at 4 weeks (34). Consequently, the presence of L. donovani cells was measured at 4 weeks postinfection. A 65% lower infection load was observed in mice infected with the L. donovani BT1 null mutant than in wild-type cells when the Leishmania cells were analyzed microscopically (Fig. 3A). This reduced infectivity is a direct consequence of BT1 disruption, since mutants rescued with an episomal BT1 gene were equally as infective as wild-type cells (Fig. 3A). Expression of the luciferase reporter gene (LUC) allows a rapid, much simpler and yet accurate monitoring of Leishmania infections (34). In a separate set of experiments, and exploiting the LUC expression of the challenging parasites, we investigated the presence of L. donovani cells in the livers and spleens of infected mice 4 weeks postinfection and found an 80% reduction in infectivity of the BT1 null mutant in livers and 90% reduction in spleens (Fig. 3B). Thus, inactivation of BT1 reduces considerably the survival rate of L. donovani in mice. We could not detect attenuated parasites in either the liver or the spleen using impression smears or by measuring luciferase activity after 8 or 12 weeks postinfection (data not shown). Thus, the growth kinetic of the attenuated parasite was not delayed. We also isolated the livers and spleens of two mice 12 weeks postinfection. These organs were homogenized and put in culture. No Leishmania cells could be observed from the combined livers of two mice, but after 1 week we could detect growth of parasites in the culture of the combined spleens. From the generation time of the parasites, we estimated that they were fewer than 50 parasites/spleen, which is below the detection levels of our luciferase assay with amastigotes (38).
FIG. 3.
Infection rates of the L. donovani BT1 null mutant (BT1 ko) in BALB/c mice. Late-stationary-phase L. donovani promastigote cells (5 × 107), either wild-type (WT) (black box), BT1 null mutant (hatched box), or BT1 null mutant retransfected with an episomal BT1 gene (BT1 ko-BT1) (white box) were injected i.v. into the tail veins of BALB/c mice. (A) L. donovani cells were counted 4 weeks postinfection by impression smears of the liver and staining with Giemsa. Results are averages for three independent experiments with five mice per group. (B) Similar numbers of animals were infected with the L. donovani wild type and the BT1 null mutant expressing the firefly luciferase (LUC) gene. Mice were sacrificed 4 weeks postinfection, and luciferase activity was measured in the liver and the spleen. LDU, L. donovani units; RLU, relative luciferase units.
Protection against infectious challenge and correlates of immunity in mice.
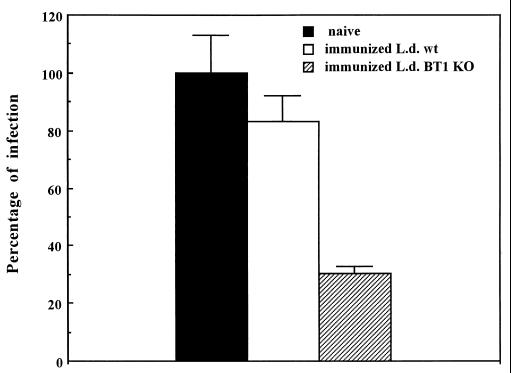
We have tested whether immunization with genetically attenuated L. donovani organisms in which the main biopterin transporter was inactivated could induce protective immunity in genetically susceptible mice. Groups of five naive and vaccinated BALB/c mice were challenged 6 weeks postimmunization i.v. with 5 × 107 luciferase-expressing virulent L. donovani promastigotes. Parasite loads in the liver were compared in naive and vaccinated mice 4 weeks postchallenge. Mice immunized with the L. donovani BT1 null mutant were significantly protected against infectious challenge, since infection rates in these animals were 75% lower than those for naive animals (Fig. 4). Since L. donovani infections are controlled in BALB/c mice, we compared the protection against reinfection in mice initially infected with the BT1 null mutant or with the wild-type strain. No significant protection was observed when mice were initially infected with the wild-type strain (Fig. 4).
FIG. 4.
Immunization with the L. donovani BT1 null mutant and infectious challenge in BALB/c mice. Late-stationary-phase L. donovani promastigote cells (5 × 107) expressing the LUC luciferase gene (34) were injected i.v. into the tail veins of either naive BALB/c animals (black box), mice previously immunized with the L. donovani BT1 null mutant (immunized L.d. BT1 KO) (hatched box), or mice previously infected with the wild-type parasites (immunized L.d. wt) (white box). Parasite loads in the liver were assessed by measuring luciferase activity as described previously (34). Results are averages for at least two independent experiments with five animals in each experiment.
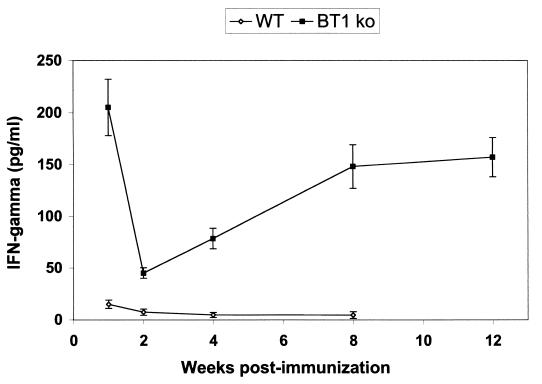
To assess immune correlates contributing to the significant protection seen with mice immunized with the L. donovani BT1 null mutant, we measured the release of IFN-γ by stimulated splenocytes. The production of IFN-γ is strongly associated with the control of L. donovani infection in mice (23, 40). Splenocytes from mice immunized with the attenuated recombinant parasites already at 1 week postimmunization produced much larger amounts of IFN-γ (210 versus 23 pg/ml) than mice infected with wild-type organisms, as shown by an ELISA assay (Fig. 5). In the second week of infection, the level of IFN-γ decreased in splenocytes isolated both from mice infected with the wild-type strain and from those infected with the mutant strain, but afterwards, the production of IFN-γ was consistently increasing upon stimulation in the splenocytes derived from the mice infected with the BT1 null mutant (Fig. 5). No production of IFN-γ was measured in splenocytes derived from mice vaccinated with the wild-type L. donovani cells (Fig. 5). Interestingly, upon stimulation, splenocytes from immunized mice were capable of specifically secreting IFN-γ even after 3 months postimmunization, suggesting the development of specific memory T cells. We have also looked for the production of the cytokine interleukin 4 (IL-4), which potentially inhibits the protective immune response in the mouse model, but we could not detect any measurable levels of IL-4 in stimulated splenocytes derived from the BT1 null-immunized mice at any time postimmunization (data not shown). To assess more quantitatively the IFN-γ response in the immunized mice, we have measured the ability of T cells to secrete IFN-γ at the second week postinfection, using the ELISPOT assay. A similar number of splenocytes (∼20/106 cells) isolated either from mice immunized with the BT1 null mutant or from naive animals secreted IFN-γ. The number of T cells derived from splenocytes of mice immunized with the BT1 null mutant that specifically secreted IFN-γ was increased very much above the background level upon stimulation with L. donovani promastigotes (Fig. 6), hence corroborating the ELISA results.
FIG. 5.
IFN-γ production by splenic T cells in immunized mice. Splenocytes of BALB/c mice immunized with either the L. donovani wild type or the L. donovani BT1 null mutant were isolated at 1, 2, 4, 8, and 12 weeks postinfection, stimulated with L. donovani promastigotes, and tested by ELISA for the production of IFN-γ as described in Materials and Methods. Results are representative of two independent experiments, each containing three replicate wells with pooled splenocytes from five mice.
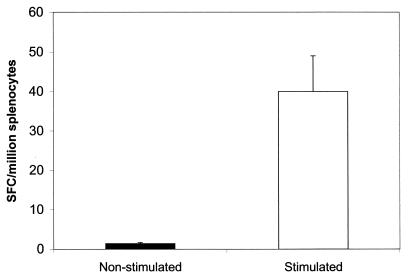
FIG. 6.
Frequency of IFN-γ-producing T cells measured by the ELISPOT assay. Splenocytes were isolated from mice immunized with the L. donovani BT1 null mutant 2 weeks postinfection and stimulated with L. donovani promastigotes (white box) or not stimulated (black box) as described in Materials and Methods. Individual IFN-γ-producing cells were detected by direct visualization and are expressed as SFC per 106 splenocytes. The SFC background determined from naive animals (∼20 SFC per million splenocytes) was subtracted.
DISCUSSION
The importance of pterins in Leishmania has been established for a long time, but it is only recently that gene products involved in pterin metabolism or transport were isolated. The biopterin transporter BT1 (22, 24) is likely to be important for Leishmania growth, and purified BT1 was shown to protect against experimental leishmaniasis (9). The BT1 gene (formerly known as ORFG [27]) is frequently amplified in various unselected Leishmania species (37), and it was recently shown to be overexpressed in cells grown in pterin-limited medium (35). The L. donovani BT1 null mutant does not demonstrate any biopterin transport activity (Fig. 2). However, the addition of biopterin in the culture medium was necessary for growth in vitro, suggesting that biopterin was entering by other routes, possibly by diffusion. The BT1 null mutant shows a marked reduction in infectivity in the BALB/c mouse model (Fig. 3), suggesting that the ability to transport pterins is important for parasite survival in vivo. Rescue of the attenuated phenotype was complete when the BT1 gene was retransfected into the mutant parasite (Fig. 3). An increased ability to transport pterins does not seem to increase parasitic load, suggesting that scavenging biopterin from the host is not a pathogenesis mechanism for L. donovani. This could have been the case, since reduced pterins are cofactors of nitric oxide synthase and the latter is important in the resolution of Leishmania infections (4). Metacyclogenesis was found to be increased in PTR1 and BT1 null mutants of L. major, suggesting that reduced pterin levels regulate metacyclogenesis (8). We have also observed an increase in metacyclogenesis with our L. donovani BT1 null mutant (unpublished observation). L. major PTR1 null mutants were unexpectedly more virulent than wild-type cells (8). Nonetheless, as shown in this study (Fig. 3), L. donovani BT1 disruption mutants are clearly less virulent. Hence, although low levels of reduced pterins are important for metacyclogenesis, the transport of pterins, at least for L. donovani, is important during in vivo infection.
Mice susceptible to L. donovani infection were immunized using genetically engineered L. donovani cells that are deficient in biopterin transport. These vaccinated mice were partially protected from further parasite challenge, while mice initially infected with L. donovani wild-type cells were not (Fig. 4). Splenic T cells derived from mice infected with the BT1 null mutant, but not from mice infected with the wild-type parasites, are primed to produce IFN-γ upon stimulation with Leishmania cells (Fig. 5 and 6). It is likely that the ability of the L. donovani BT1 null mutant to protect mice from further challenge is due, at least in part, to the capacity of the splenic T cells of the vaccinated animals to produce IFN-γ. From these results, it would appear that the BT1 null mutant is both attenuated and more immunogenic than wild-type parasites. Previous reports of L. donovani immune protective correlates in mice showed that IFN-γ production is important for the control and acquired resistance to L. donovani (40) and that the capacity to produce IFN-γ determines the efficacy of the immune response in susceptible mice (23). Successful immunization with L. donovani recombinant proteins in BALB/c mice led to IFN-γ-producing splenocytes (39, 41). The produced IFN-γ may induce type 2 nitric oxide synthase, generating nitric oxide that exerts antileishmanial activity (reviewed in reference 4). The outcome of L. donovani infection in mice is highly dependent on the cytokines produced by T cells upon stimulation. Generally, the production of Th1 cytokines, such as IFN-γ, is protective and determines the efficacy of the immune response in susceptible mice, while production of Th2 cytokines, such as IL-4, results in progressive disease (reviewed in reference 17). The Th1/Th2 polarization in human visceral leishmaniasis is not as clear-cut, but numbers of Leishmania-specific T cells producing IFN-γ have been increased in patients who have recovered from visceral leishmaniasis (6, 18), although mixed Th1/Th2 Leishmania antigen-specific cells can be observed with infected or cured patients (17, 19). At 1 week postinfection we measured high levels of IFN-γ secretion in splenocytes derived from the immunized animals, and this level rapidly decreased at 2 weeks (Fig. 5). Possibly the L. donovani BT1 null mutant is eliminated rapidly, which leads to massive recruitment of cells involved in innate response, which may lead to secretion of IFN-γ. Interestingly, IL-12, which stimulates the production of IFN-γ from natural killer cells, was found to be produced early following L. donovani infections (12). This may explain the early massive production of IFN-γ.
In this study, we investigated whether live parasites that are genetically attenuated could induce a protective immunity against L. donovani infection. Although live attenuated parasites have been shown to be useful against experimental cutaneous leishmaniasis (43), this is the first report demonstrating their usefulness against visceral leishmaniasis. Given that a T-cell-mediated immunity is required for a protective immune response against Leishmania infections, live attenuated vaccines should be good candidates for use against visceral leishmaniasis. Although safety issues will need to be investigated in great detail, live attenuated vaccines are already in extensive use against several viral and bacterial diseases, and BCG, a naturally attenuated form of M. bovis, although of varying efficacy, is still being administered to millions of children every year. In fact, one of the largest clinical trial for Leishmania vaccines included BCG as an adjuvant combined with killed parasites (20). A complete attenuation of the parasite may be achieved by disrupting additional genes, since some growth of the BT1 null mutant is observed (Fig. 3A and B). Indeed, 3 months postinfection we could detect a few living attenuated parasites in the spleens by culturing them, although we could not detect them either by impression smears or by measuring luciferase activity. This residual growth may be due to the expression of other proteins capable of transporting in vivo low levels of pterins or to the entry of pterins by other means, such as diffusion. Some parasite replication is likely to be required for generating a T-cell-mediated response; this may not occur, however, if the parasites are too attenuated and hence do not replicate. Splenocytes derived from mice immunized with the BT1 null mutant still produce, upon stimulation, IFN-γ 3 months postimmunization (Fig. 5), suggesting the presence of specific memory T cells, and this may indeed require few rounds of parasite replication. We cannot exclude, however, the possibility that part of the stimulation might be due to the few persisting parasites, but this is unlikely, since splenocytes isolated form mice infected with wild-type parasites, which are also persisting, do not respond to stimulation (Fig. 5). To further boost the immunological response, it may be appropriate either to have a second round of immunization with the BT1 null strain or to coinject cytokines, such as IL-12 or other adjuvants (1), in addition to the attenuated parasites. Clearly, the generation of attenuated strains of L. donovani is a valid approach for vaccination strategies against visceral leishmaniasis, and further work is warranted to improve the efficacy of these vectors.
Acknowledgments
This work was supported in part by the Canadian Institutes of Health Research (CIHR) to M. Ouellette and by an CIHR-Industry grant and a grant from the CURP program of Pasteur-Merieux-Connaught to B.P., M. Ouellette, and M. Olivier and more recently by the CANVAC Center of Excellence. C.K. is a postdoctoral fellow of the Schweizerischer Nationalfonds. B.P. is a senior FRSQ Scholar, M. Ouellette and M. Olivier are CIHR Investigators, and M.B. holds a CIHR studentship. B.P. and M. Olivier are Burroughs Wellcome Fund New Investigators in Molecular Parasitology, and M. Ouellette is a Burroughs Wellcome Fund Scholar in Molecular Parasitology.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aebischer, T., M. Wolfram, S. I. Patzer, T. Ilg, M. Wiese, and P. Overath. 2000. Subunit vaccination of mice against New World cutaneous leishmaniasis: comparison of three proteins expressed in amastigotes and six adjuvants. Infect. Immun. 68: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 161: 6794–6801. [PubMed] [Google Scholar]
- 3.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24: 684–703. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173: 17–26. [DOI] [PubMed] [Google Scholar]
- 5.Brun, R., and Schonenberger. 1979. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 36: 289–292. [PubMed] [Google Scholar]
- 6.Carvalho, E. M., A. Barral, D. Pedral-Sampaio, M. Barral-Netto, R. Badaro, H. Rocha, and W. D. Johnson, Jr. 1992. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 165: 535–540. [DOI] [PubMed] [Google Scholar]
- 7.Cruz, A. K., R. Titus, and S. M. Beverley. 1993. Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc. Natl. Acad. Sci. USA 90: 1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham, M. L., R. G. Titus, S. J. Turco, and S. M. Beverley. 2001. Regulation of differentiation to the infective stage of the protozoan parasite Leishmania major by tetrahydrobiopterin. Science 292: 285–287. [DOI] [PubMed] [Google Scholar]
- 9.Dole, V. S., V. S. Raj, A. Ghosh, R. Madhubala, P. J. Myler, and K. D. Stuart. 2000. Immunization with recombinant LD1 antigens protects against experimental leishmaniasis. Vaccine 19: 423–430. [DOI] [PubMed] [Google Scholar]
- 10.Dumas, C., M. Ouellette, J. Tovar, M. L. Cunningham, A. H. Fairlamb, S. Tamar, M. Olivier, and B. Papadopoulou. 1997. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 16: 2590–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46: 695–729. [DOI] [PubMed] [Google Scholar]
- 12.Gorak, P. M, C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28: 687–695. [DOI] [PubMed] [Google Scholar]
- 13.Gueiros-Filho, F. J., and S. M. Beverley. 1996. Selection against the dihydrofolate reductase-thymidylate synthase (DHFR-TS) locus as a probe of genetic alterations in Leishmania major. Mol. Cell. Biol. 16: 5655–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 16.Hubel, A., S. Krobitsch, A. Horauf, and J. Clos. 1997. Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol. Cell. Biol. 17: 5987–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp, K. 2000. Cytokine-producing T cell subsets in human leishmaniasis. Arch. Immunol. Ther. Exp. (Warsaw) 48: 173–176. [PubMed] [Google Scholar]
- 18.Kemp, K., M. Kemp, A. Kharazmi, A. Ismail, J. A. Kurtzhals, L. Hviid, and T. G. Theander. 1999. Leishmania-specific T cells expressing interferon-gamma (IFN-gamma) and IL-10 upon activation are expanded in individuals cured of visceral leishmaniasis. Clin. Exp. Immunol. 116: 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenney, R. T., D. L. Sacks, A. A. Gam, H. W. Murray, and S. Sundar. 1998. Splenic cytokine responses in Indian kala-azar before and after treatment. J. Infect. Dis. 177: 815–818. [DOI] [PubMed] [Google Scholar]
- 20.Khalil, E. A., A. M. El Hassan, E. E. Zijlstra, M. M. Mukhtar, H. W. Ghalib, B. Musa, M. E. Ibrahim, A. A. Kamil, M. Elsheikh, A. Babiker, and F. Modabber. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356: 1565–1569. [DOI] [PubMed] [Google Scholar]
- 21.Kidder, G., and B. Dutta. 1958. The growth and nutrition of Crithidia fasciculata. J. Gen. Microbiol. 18: 621–638. [DOI] [PubMed] [Google Scholar]
- 22.Kündig, C., A. Haimeur, D. Légaré, B. Papadopoulou, and M. Ouellette. 1999. Increased transport of pteridines compensates for mutations in the high affinity folate transporter and contributes to methotrexate resistance in the protozoan parasite Leishmania tarentolae. EMBO J. 18: 2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann, J., K. H. Enssle, I. Lehmann, A. Emmendorfer, and M. L. Lohmann-Matthes. 2000. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. J. Interferon Cytokine Res. 20: 63–77. [DOI] [PubMed] [Google Scholar]
- 24.Lemley, C., S. Yan, V. S. Dole, R. Madhubala, M. L. Cunningham, S. M. Beverley, P. J. Myler, and K. D. Stuart. 1999. The leishmania donovani LD1 locus gene ORFG encodes a biopterin transporter (BT1). Mol. Biochem. Parasitol. 104: 93–105. [DOI] [PubMed] [Google Scholar]
- 25.Mottram, J. C., B. P. McCready, K. G. Brown, and K. M. Grant. 1996. Gene disruptions indicate an essential function for the LmmCRK1 cdc2- related kinase of Leishmania mexicana. Mol. Microbiol. 22: 573–583. [DOI] [PubMed] [Google Scholar]
- 26.Muyombwe, A., M. Olivier, P. Harvie, M. G. Bergeron, M. Ouellette, and B. Papadopoulou. 1998. Protection against Leishmania major challenge infection in mice vaccinated with live recombinant parasites expressing a cytotoxic gene. J. Infect. Dis. 177: 188–195. [DOI] [PubMed] [Google Scholar]
- 27.Myler, P. J., M. J. Lodes, G. Merlin, T. de Vos, and K. D. Stuart. 1994. An amplified DNA element in Leishmania encodes potential integral membrane and nucleotide-binding proteins. Mol. Biochem. Parasitol. 66: 11–20. [DOI] [PubMed] [Google Scholar]
- 28.Nare, B., J. Luba, L. W. Hardy, and S. Beverley. 1997. New approaches to Leishmania chemotherapy: pteridine reductase 1 (PTR1) as a target and modulator of antifolate sensitivity. Parasitology 114(Suppl.): S101–S110. [PubMed] [Google Scholar]
- 29.Olivier, M., C. Proulx, and C. E. Tanner. 1989. Importance of lymphokines in the control of multiplication and dispersion of Leishmania donovani within liver macrophages of resistant and susceptible mice. J. Parasitol. 75: 720–727. [PubMed] [Google Scholar]
- 30.Ouellette, M., E. Leblanc, C. Kundig, and B. Papadopoulou. 1998. Antifolate resistance mechanisms from bacteria to cancer cells with emphasis on parasites. Adv. Exp. Med. Biol. 456: 99–113. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulou, B., M. Olivier, and M. Ouellette. 1999. Recent progress in vaccine development against Leishmania species infections. Can. J. Infect. Dis. 10(Suppl.C): 9C–15C. [Google Scholar]
- 32.Papadopoulou, B., G. Roy, and M. Ouellette. 1994. Autonomous replication of bacterial DNA plasmid oligomers in Leishmania. Mol. Biochem. Parasitol. 65: 39–49. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulou, B., G. Roy, and M. Ouellette. 1992. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 11: 3601–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy, G., C. Dumas, D. Sereno, Y. Wu, A. K. Singh, M. J. Tremblay, M. Ouellette, M. Olivier, and B. Papadopoulou. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110: 195–206. [DOI] [PubMed] [Google Scholar]
- 35.Roy, G., C. Kundig, M. Olivier, B. Papadopoulou, and M. Ouellette. 2001. Adaptation of leishmania cells to in vitro culture results in a more efficient reduction and transport of biopterin. Exp. Parasitol. 97: 161–168. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Segovia, M., and G. Ortiz. 1997. LD1 amplifications in Leishmania. Parasitol. Today 13: 342–348. [DOI] [PubMed] [Google Scholar]
- 38.Sereno, D., G. Roy, J. L. Lemesre, B. Papadopoulou, and M. Ouellette. 2001. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob. Agents Chemother. 45: 1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto, M., C. Alonso, and J. M. Requena. 2000. The Leishmania infantum acidic ribosomal protein LiP2a induces a prominent humoral response in vivo and stimulates cell proliferation in vitro and interferon-gamma (IFN-gamma) production by murine splenocytes. Clin. Exp. Immunol. 122: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squires, K. E., R. D. Schreiber, M. J. McElrath, B. Y. Rubin, S. L. Anderson, and H. W. Murray. 1989. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J. Immunol. 143: 4244–4249. [PubMed] [Google Scholar]
- 41.Stager, S., D. F. Smith, and P. M. Kaye. 2000. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 165: 7064–7071. [DOI] [PubMed] [Google Scholar]
- 42.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 43.Titus, R. G., F. J. Gueiros-Filho, L. A. de Freitas, and S. M. Beverley. 1995. Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. USA 92: 10267–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trager, W. 1969. Pteridine requirement of the hemoflagellate Leishmania tarentolae. J. Protozool. 16: 372–375. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, W. W., and G. Matlashewski. 1997. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc. Natl. Acad. Sci. USA 94: 8807–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]