Abstract
Bivalent recombinant strains of Mycobacterium bovis BCG (rBCG) expressing the early regulatory nef and the structural gag(p26) genes from the simian immunodeficiency virus (SIV) SIVmac251 were engineered so that both genes were cotranscribed from a synthetic operon. The expression cassette was cloned into a multicopy-replicating vector, and the expression levels of both nef and gag in the bivalent rBCG(nef-gag) strain were found to be comparable to those of monovalent rBCG(nef) or rBCG(gag) strains. However, extrachromosomal cloning of the nef-gag operon into a replicative plasmid resulted in strains of low genetic stability that rapidly lost the plasmid in vivo. Thus, the nef-gag operon was inserted site specifically into the BCG chromosome by means of mycobacteriophage Ms6-derived vectors. The resulting integrative rBCG(nef-gag) strains showed very high genetic stability both in vitro and in vivo. The in vivo expression of the heterologous genes was much longer lived when the expression cassette was inserted into the BCG chromosome. In one of the strains obtained, integrative cloning did not reduce the expression levels of the genes even though a single copy was present. Accordingly, this strain induced cellular immune responses of the same magnitude as that of the replicative rBCG strain containing several copies of the genes.
Mycobacterium bovis BCG, an attenuated mycobacterial strain administered to protect against tuberculosis and leprosy, is a particularly attractive vector for the delivery of heterologous antigens. This live and persistent vaccine, which can be administered at birth, has been given to over three billion people with remarkably low levels of major side effects in immunocompetent individuals. Its excellent immunomodulating properties and the fact that it can be administered by mucosal routes make BCG one of the most attractive live vectors for the development of vaccines against other diseases. The development of expression vectors for mycobacteria allowed the construction of recombinant BCG (rBCG) vaccines expressing a variety of parasitic or bacterial genes that, in some instances, induce protective immune responses in the mouse model (1, 9, 25, 27, 30). rBCG strains expressing genes from immunodeficiency viruses have also been shown to induce specific immune responses both in mouse and primate models (23, 36, 39). Most of these studies were performed with monovalent rBCG strains expressing a single heterologous gene. However, an important challenge for modern vaccinology is the development of multivalent, single-dose vaccines that can protect against several pathogens. In the field of AIDS vaccinology, no consensus is presently available regarding which antigen should be targeted and it is widely accepted that valuable candidate vaccines should contain several human immunodeficiency virus antigens (22). An attractive possibility is thus to engineer rBCG multivalent vaccines to express several antigens from one or various pathogens.
Genetic systems for the manipulation and expression of heterologous genes in rBCG have mostly been developed from pAL5000, a Mycobacterium fortuitum cryptic plasmid (18). As five copies are present per transformed cell (28, 29), the expression levels of heterologous genes are high in these rBCG strains and they generally induce good levels of immune responses against heterologous antigens. However, some genes might be less stably maintained in rBCG, especially in the hostile macrophage environment. This could be detrimental to the obtainment of long-term immune memory. Integrative vectors, derived from temperate mycobacteriophages, such as L5 (reviewed in reference 15) or Ms6 (12), have also been developed. These integration-proficient vectors encode integrase functions that allow a recombination event between the phage attP and the bacterial homologous attB sites. Such vectors integrate site specifically into the bacterial chromosome, and when no excision functions are present, the vector can be stably maintained (21). Integrative rBCG strains have been obtained thanks to such vectors and have induced immune responses in mice (31). However, the disadvantage of such strains is the reduced expression level of heterologous genes compared to that of multicopy plasmids, which can be counterbalanced by the persistent synthesis of the foreign antigen in vivo. To date, no study has directly compared the immune responses induced by the same antigen expressed either from a replicative or an integrative vector. In particular, the influence of long-lasting production of the heterologous gene in vivo on the efficacy of induction of specific immunity is unknown.
In this report, we describe the construction of rBCG strains coexpressing the early regulatory nef and structural gag(p26) genes from SIVmac251, the simian immunodeficiency virus (SIV) homologue to human immunodeficiency virus type 2. nef and gag genes were organized as a synthetic operon and either cloned into pAL5000-derived replicative vectors or into Ms6-derived integrative vectors. The expression levels of the heterologous genes carried by the replicative or integrative rBCG strains were evaluated both in vitro and in vivo in the mouse model. We observed that the genetic stability, both in vitro and in vivo, of integrative rBCG strains was much higher than that of replicative strains. Moreover, the integrative vectors described here allowed the high and long-lived expression of heterologous genes.
MATERIALS AND METHODS
Animals and immunizations.
Female 6- to 8-week old BALB/c mice, purchased from the Centre d’Elevage Janvier, Le Genet-St.-Isle, France, were used to evaluate the in vivo stability and induction of the cellular and humoral immune responses by rBCG strains. For the stability study, mice were inoculated intravenously (i.v.) into the tail vein with 107 CFU of BCG. For the evaluation of the humoral immune responses, mice were immunized twice intraperitoneally (i.p.) with 106 CFU and were boosted by the same route once with 5 μg of recombinant Gag(p26). For the assessment of the cellular immune responses, mice were immunized i.v. with 5 × 106 CFU and boosted by the same route with 5 μg of recombinant Gag(p26).
Bacterial strains and growth conditions.
All cloning steps were performed in XL1 Blue Escherichia coli (7) grown in Luria-Bertani medium supplemented with ampicillin (100 μg ml−1) or kanamycin (20 μg ml−1). rBCG strains were derived from the BCG1173P2 Pasteur strain (13). Mycobacterial strains were electroporated as described previously (37). Recombinant BCG clones were first cultivated on Lowenstein-Jensen medium supplemented with 10 μg of kanamycin ml−1 and were then transferred into Sauton medium containing 10 μg of kanamycin ml−1. Vaccines were prepared and used to immunize the animals as described previously (14). The number of CFU and the expression of nef and gag genes were assayed in all vaccine preparations by Western blotting. All strains are described in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain, plasmid, or primer | Characteristics or sequence (5′ to 3′) | Source or reference |
|---|---|---|
| Bacterial strains | ||
| M. bovis BCG | Pasteur strain; 1173P2 isolate | 13 |
| E. coli XL1 Blue | F′::Tn10proA+B+laclqΔ(lacZ)M15/recA1endA1gyrA96(NalR)thihsdR17(rk−mk+)supE44relA1lac | 7 |
| Plasmids | ||
| pTG627 | E. coli vector containing full-length SIVmac251 gag(p55) and pol genes | Transgene |
| pSN24 | E. coli/mycobacterial shuttle vector containing full-length SIVmac251 nef gene | 36 |
| pJN30 | E. coli/mycobacterial replicative shuttle vector; contains the lacZ gene from E. coli under control of up-regulated pBlaF* promoter from M. fortuitum and the transcription terminator of bacteriophage T4; contains kanR gene from Tn903 | 33 |
| pAV6950 | E. coli replicative/mycobacterial integrative shuttle vector; contains the attachment site and the gene coding for integrase of mycobacteriophage Ms6; contains kanR gene from Tn5 | 12 |
| pOIP23 | pAV6950 derivative; contains two transcription terminators of bacteriophage T4 around the unique EcoRI cloning site | This study |
| pJL17 | pJN30 derivative; contains entire nef gene of SIVmac251 under the control of pBlaF* (pBlaF*-nef cassette) | This study |
| pJL24 | pJN30 derivative; contains entire gag(p26) gene of SIVmac251 under the control of pBlaF* (pBlaF*-gag cassette) | This study |
| pJLN29 | pJN30 derivative; contains entire nef and gag(p26) genes of SIVmac251 organized as an operon under the control of pBlaF* (pBlaF*-nef-gag cassette) | This study |
| pOIP4 | pAV6950 derivative; contains pBlaF*-nef-gag cassette; sense orientation | This study |
| pOIP12 | pAV6950 derivative; contains pBlaF*-nef-gag cassette; antisense orientation | This study |
| pOIP33 | pOIP23 derivative; contains pBlaF*-nef-gag cassette isolated by two transcription terminators | This study |
| Primers | ||
| Kmr gene Tn5 | ||
| K1 | AGGTCTGCCTCGTGAAGAAGG | |
| K2 | CGTTGTGTCTCAAAATCTCTGA | |
| Kmr gene Tn903 | ||
| K3 | GGGCGAAGAACTCCAGCATG | |
| K4 | CAAGAGACAGGATGAGGATCG | |
| SIVmac251 nef gene | ||
| N1 | CGGTCCAAGCCGGCTGGA | |
| N2 | AATCCCTTCCAGTCCCCCC | |
| N3 | CCAAGTGGGATGACCCTTGGG | |
| JYL6 | TAGAATTCGGTACCTTAGCCAGAAGGAGAAGTACCGATGGGTGGAGCTATTTCCATG | |
| JYL7 | CCCAAGCTTGATATCGCTAGCTTAGCCTTCTTCTAACCTCTT | |
| SIVmac251 gag gene | ||
| G1 | CCGAGAACATTAAATGCCTGG | |
| G2 | TGATCCTGACGGCTCCCT | |
| G3 | GGCACTACTTCTGCTCCAAATTT | |
| JYL8 | TAGAATTCGCTAGCTAAGGTACCAGAAGGAGAAGTACCGATGGAAACTATGCCAAAAACAAG | |
| JYL10 | CCCAAGCTTGATATCTTATCTAGCCTTCTGTCCTGG |
Construction of nef-gag(p26) expression vectors.
All restriction enzymes were purchased from Roche-Boehringer (Mannheim, Germany). The nef gene from SIVmac251 was PCR amplified with pfu polymerase (New England Biolabs, Hetfordshire, United Kingdom) using JYL6 and JYL7 primers and pSN24 as a template (36). The PCR product was digested with Asp718 and EcoRV and ligated to pJN30 (33) cut with the same enzymes. This gave rise to pJL17 containing the pBlaF*-nef expression cassette. The gag gene was PCR amplified from template pTG627 (kindly provided by Transgene, Strasbourg, France), a plasmid containing the gag and pol genes from SIVmac251, with primers JYL8 and JYL10. The PCR product was digested with Asp718 and EcoRV and ligated to pJN30 cut with the same enzymes. This gave rise to pJL24 containing the pBlaF*-gag expression cassette. Both JYL6 and JYL8 (forward primers) contained the MegaSD sequence AGAAGGAGAAG. To construct pJLN29 (pBlaF*-nef-gag expression cassette), the gag PCR product obtained from pTG627 with JYL8 and JYL10 was cut with NheI and EcoRV and was inserted into pJL17 digested with the same enzymes.
The pBlaf*-nef-gag cassette was then cut from pJLN29 with ScaI and EcoRV and inserted into the unique EcoRI site of pAV6950 (12), which had been blunt ended with T4 DNA polymerase (Roche-Boehringer) and dephosphorylated with Calf Intestinal Alkaline Phosphatase (Roche-Boehringer). The resulting integration-proficient vectors were called pOIP4 and pOIP12 according to the orientation of the pBlaF*-nef-gag expression cassette in comparison with the Ms6 int gene. Vector pOIP23 was obtained by cloning two transcription terminators from coliphage T4 (33) into the HindIII and SacI sites of pAV6950. The pBlaF*-nef-gag cassette from pJLN29 was cloned into the unique EcoRI site of pOIP23 to give rise to pOIP33. All vectors and primers described in this section are summarized in Table 1.
Preparation of bone marrow-derived macrophages (BMMP) and dendritic-cell cultures.
To obtain bone marrow-derived dendritic cells (BMDC), bone marrow was flushed from the femurs and tibias of 6-week-old BALB/c mice. White blood cells were plated in bacterial-grade 100- by 15-mm petri dishes (Falcon; Becton Dickinson, Paramus, N.J.) at 2 × 105 cells ml−1 in 10 ml of total volume in Iscove’s modified Dulbecco’s medium (IMDM) medium (Biowhittaker, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS) (Gibco BRL, Life Technologies, Paisley, United Kingdom), 1% supernatant from the J558 cell line (41) producing murine granulocyte-macrophage colony-stimulating factor, antibiotics (100 U of penicillin ml−1 and 100 μg of streptomycin ml−1) and 5 × 10−5 M 2-mercaptoethanol. On day 3, 10 ml of complete IMDM medium (supplemented as described above) was added. On days 6 and 10, loosely adherent cells were collected and replated in fresh medium. Alternatively, cells obtained on day 10 were frozen in liquid nitrogen. BMDC were thawed 4 days before the proliferative test and replated at 2 × 105 cells ml−1 in complete IMDM medium without antibiotics. Cells were phenotyped by fluorescence-activated cell sorter (Becton Dickinson) and were routinely 90 to 95% CD11c+, CD11b+, I-Ad int to hi, B7.1lo, B7.2lo, and CD40lo. This profile was characteristic of immature dendritic cells. Eighteen hours before the proliferative tests, either Gag-purified protein (10 μg ml−1) or BCG culture filtrate antigen (CFA) (10 μg ml−1) was added to the cells. Cells were then harvested, treated with 4 μg of mitomycin C (Sigma, St. Louis, Mo.) ml−1 for 30 min, and washed twice with fresh medium before being mixed with CD4+ T lymphocytes.
BMMP were prepared as previously described (16). Briefly, cells obtained from the bone marrow of 7- to 8-week-old BALB/c mice were cultured for 5 to 6 days in Dulbecco’s modified Eagle’s medium (Seromed, Berlin, Germany) supplemented with 10% FCS (Dominique Dutscher, Brumath, France), 10% L-cell-conditioned medium, 100 U of penicillin ml−1, and 100 μg of streptomycin ml−1 at a concentration of 5 × 105 cells ml−1. Eighteen hours prior to infection, cells were washed with Dulbecco’s modified Eagle’s medium without antibiotics and were incubated in medium with 10% FCS and 5% L-cell-conditioned medium.
RT-PCR analysis of BMMP.
Infection assays were performed with 107 BMMP infected with either rBCG(pJLN29), a mixture of rBCG(pJL17) plus rBCG(pJL24), or BCG1173P2 at a multiplicity of infection of 10 bacteria per cell. After 18 h of infection, cells were washed with fresh Dulbecco’s modified Eagle’s medium and were incubated for 48 h at 37°C in a 5% CO2 atmosphere. Cells were harvested, lysed with cell culture lysis reagent (Promega, Madison, Wis.), and washed twice with diethyl pyrocarbonate-treated water (Ambion, Austin, Tex.). Pellets were resuspended in 1 ml of Trizol reagent (Gibco BRL) and shaken with 0.1-mm-diameter glass beads in a bead-beater (Biospec Products) to disrupt the bacteria. Total RNA was extracted by phenol chloroform (5:1 [vol:vol]) and was stored at −80°C until use. DNA was digested at 37°C by 10 U of DNase I (Amersham, Uppsala, Sweden) for 2 h. DNase I was discarded by chloroform extraction prior to the reverse transcription-PCR (RT-PCR). Reverse primers derived from nef (N2) or gag (G2) (Table 1) were used to synthesize cDNA from RNA. RT-PCRs were performed with 50 U of Expand RTase (Roche-Boehringer) in a 20-μl volume reaction according to the manufacturer’s recommendations. Reactions were performed on 1 μg of RNA with 50 pmol of either N2 or G2, 100 mM dithiothreitol, 10 mM deoxynucleoside triphosphate, and 20 U of RNase inhibitor. Mixtures were incubated for 50 min at 42°C.
PCRs were performed using the AmpliTaq Gold kit (Perkin-Elmer, Norwalk, Conn.) in a final volume of 50 μl containing 2 μl of RT reaction, 25 mM MgCl2, 2.5 mM deoxynucleoside triphosphate, 25 pmol of each primer, and 2 U of polymerase. A 10-min cycle at 95°C was performed and followed by 30 cycles of 1 min at 95°C, 1 min at 58°C, and 1 min 30 s at 72°C. PCR products were analyzed on 1.5% agarose gels.
Western blot analysis of rBCG strains.
The expression of nef and gag in rBCG strains was evaluated with total protein extracts prepared as previously described (37). Protein concentrations were assayed by the bicinchoninic acid assay at 560 nm (BCA kit; Pierce, Rockford, Ill.). Protein extracts (20 μg) were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and were electrotransferred onto a nitrocellulose membrane (Hybond; Amersham). Membranes were saturated with 3% dried skim milk in phosphate-buffered saline (PBS). The presence of Nef was detected with 1:500 diluted serum from an SIVmac251-infected macaque (kindly provided by Roger Legrand, CEA, Fontenay-aux-Roses, France). The presence of Gag(p26) was detected with 1:1,000 diluted mouse monoclonal antibody (clone 26S-A; Hybridolab, Institut Pasteur, Paris, France). The immunoblots were revealed with anti-macaque immunoglobulin G (IgG) peroxidase-conjugated serum and anti-mouse IgG peroxidase-conjugated serum and were visualized with the enhanced-chemiluminescence kit (Amersham).
Quantitative analysis of Gag(p26) production in rBCG strains by capture enzyme-linked immunosorbent assay (ELISA).
Ninety-six-well flat-bottomed plates (Nunc Maxisorp, Roskilde, Denmark) were coated with 0.05 μg of mouse monoclonal antibody per well at 4°C. After three washes with 0.1% PBS-Tween 20 (PBS-T), plates were blocked for 1 h with 3% bovine serum albumin (Sigma) in PBS-T. Plate contents were then incubated with dilutions of protein extracts of different rBCG strains in PBS-T-1% bovine serum albumin at 37°C for 2 h. Plates were washed three times with PBS-T and incubated for 1 h at 37°C with 1:1,000 diluted polyclonal anti-Gag(p26) serum obtained in the laboratory by hyperimmunization of rabbits with recombinant Gag(p26) protein. Following the washes, plate contents were incubated with alkaline phosphatase-conjugated anti-rabbit IgG (Bio-Rad, Hercules, Calif.) and alkaline phosphatase activity was revealed by adding para-nitrophenylphosphate (Sigma). A standard curve was constructed with several amounts ranging from 50 to 0.05 ng of Gag(p26) mixed with 10 μg of BCG1173P2 total protein. Optical density was measured at 405 nm.
In vitro stability analysis of rBCG strains.
Cultures of rBCG(pJLN29), rBCG::pOIP4, and rBCG::pOIP33 were grown in 7H9 medium supplemented with albumin-dextrose-catalase (ADC) (Becton Dickinson) without kanamycin. Serial dilutions of two different cultures were plated onto 7H11 medium supplemented with ADC (Becton Dickinson) with or without kanamycin after 14, 28, 50, 80, and 100 days of growth. After 21 days at 37°C, colonies were counted and the percentage of kanamycin-resistant colonies was calculated.
In vivo stability analysis of rBCG strains.
Four groups of 25 BALB/c mice were infected i.v. with rBCG(pJLN29), rBCG::pOIP4, rBCG::pOIP33, or BCG1173P2. On days 1, 28, 56, 70, and 100, the spleen, liver, and lungs from five individual mice per group were homogenized, serially diluted in Sauton medium, and plated onto 7H11 supplemented with OADC with or without kanamycin. After 21 days at 37°C, the colonies were counted and the percentage of kanamycin-resistant colonies was calculated.
In vivo analysis of expression of heterologous genes by immunohistochemical staining.
Four groups of four BALB/c mice were infected i.v. with rBCG(pJLN29), rBCG::pOIP4, rBCG::pOIP33, or BCG1173P2. Two additional mice were kept uninfected as controls. On days 14 and 77, the mice were sacrificed and their spleens were harvested and incubated for 3 days at 4°C in 10 ml of 0.1 M Tris, 0.05% calcium acetate, 0.5% ZnCl2, and 0.5% zinc acetate (pH 7.4). Organs were then dried by 15-min serial washes in baths ranging from 10 to 100% ethanol. A final wash was performed in ethanol mixed with paraffin (1:1 [vol:vol]) for 1 h at 37°C. The organs were fixed in paraffin overnight at 37°C. Sections of 5-μm thickness were transferred onto glass slides. The paraffin was removed by incubating the slides for 10 min in 100% ethanol. The slides were dried and rehydrated for 15 min in PBS without calcium and magnesium. Cell membranes were permeabilized by incubating slide contents for 8 min in 5 μg of proteinase K (Sigma) ml−1. After three washes in PBS, nonspecific binding was blocked for 15 min with Blocking Agent (DuPont NEN, Boston, Mass.). BCG was detected with a 1:750 diluted rabbit polyclonal anti-BCG serum (generously provided by Gilles Marchal, Institut Pasteur, Paris, France). The Gag protein was detected with 1:200 diluted rabbit polyclonal anti-Gag(p26) serum. The sera were diluted in PBS and were supplemented with PBS-0.05% saponin (PBSS) (Sigma) to allow access to the BCG antigens. Slide contents were incubated for 90 min at 20°C. After two washes in PBS and one wash in PBSS, endogenous peroxidases were inactivated by 15 min of incubation in PBS-1% H2O2. Following the washes in PBSS, slide contents were incubated with peroxidase-conjugated goat anti-rabbit IgG (Envision peroxidase kit; DAKO) according to the manufacturer’s instructions. After 30 min at 20°C and three washes in PBSS, peroxidase activity was revealed with aminoethyl carbazol (Sigma) diluted in PBS-3% H2O2. After color development, the reaction was stopped with distilled water. Cells were countercolored with hematoxylin (Shandon, Pittsburgh, Pa.). Slides were preserved in Aquamount (BDH, Poole Dorset, United Kingdom).
Cellular immune responses induced by rBCG strains in spleens.
On day 0, four groups of four BALB/c mice were i.v. immunized with rBCG(pJLN29), rBCG::pOIP4, rBCG::pOIP33, or BCG1173P2. A fifth group of four mice constituted an uninfected control. On day 55, the animals (including the noninfected control group) were injected with 5 μg of purified Gag(p26) administered by the same route. The day before the test, thawed BMDC were loaded with Gag(p26) or BCG CFA or were left unstimulated. Spleens were harvested from two mice per group on days 41 and 63. After red blood cell lysis, CD4+ T cells were purified by use of mouse anti-CD4-coated magnetic beads (Miltenyi Biotech, Bergisch Gladbrach, Germany) according to the manufacturer’s instructions. CD4+ T cells were cultured in RPMI 1640 (Gibco BRL) supplemented with 10% FCS (Dominique Dutscher), 5 × 10−5 M 2-mercaptoethanol, nonessential amino acids, 10 mM HEPES, 1 mM sodium pyruvate, 100 U of penicillin ml−1, and 100 μg of streptomycin ml−1 together with BMDC loaded as described above, at a ratio of 40 T cells per BMDC. After 72 h, culture supernatants were harvested, and gamma interferon (IFN-γ) production was estimated by ELISA using 0.1 μg of capture-purified anti-mouse IFN-γ (clone R4-6A2) (BD Pharmingen, San Diego, Calif.) and 0.05 μg of detection-biotinylated anti-mouse IFN-γ (clone XMG1.2) (BD Pharmingen) per well according to the manufacturer’s instructions.
Humoral immune responses induced in serum by rBCG strains.
On day 0, four groups of five mice were injected i.p. with rBCG(pJLN29), rBCG::pOIP4, rBCG::pOIP33, or BCG1173P2. One additional group was inoculated with PBS as a control. Mice received a second BCG immunization on day 28 and were inoculated with 5 μg of purified Gag(p26) by the same route on day 56. All mice, including the PBS-inoculated group, received this last dose. Blood was sampled on days 0, 20, 34, 48, 73, 94, and 115, and total anti-Gag and anti-CFA IgGs were measured in the serum of individual mice by ELISA (24) by using anti-mouse alkaline phosphatase-conjugated secondary antibodies. The negative control consisted of pooled normal sera. Titers were calculated as the dilution that gave an absorbance as high as twice the optical density obtained with the negative control.
RESULTS
Construction of rBCG strains coexpressing SIVmac251 nef and gag genes organized as an operon.
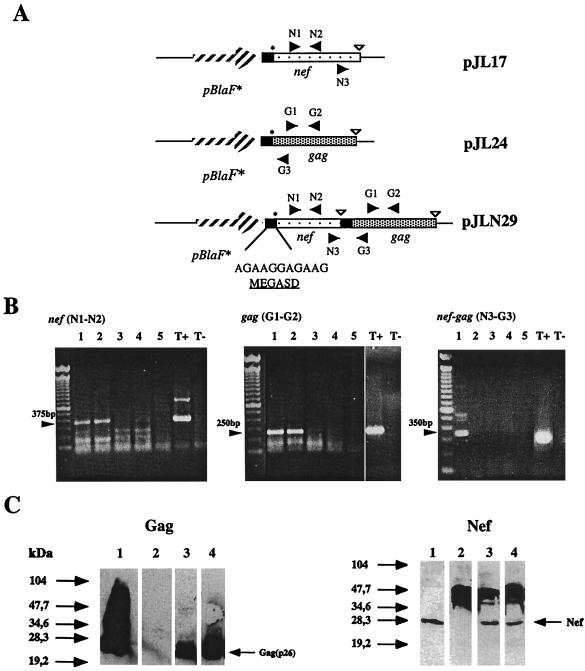
To coexpress the early regulatory SIVmac251 nef and the structural gag(p26) genes in the same rBCG strain, both genes were organized as a synthetic operon and placed under the control of pBlaF*, a strong M. fortuitum promoter, in the replicative plasmid pJN30 (34) (Fig. 1A). Nef and Gag translation was optimized by cloning a sequence derived from the Shine-Dalgarno consensus composed of a long stretch of 6 A and 5 G bases upstream of the ATG start codon of each protein. We previously showed that this MegaSD sequence allows the synthesis of a wide variety of heterologous proteins of bacterial, viral, and eukaryotic origin in rBCG (our unpublished results). The first construct, pJLN29, contained both nef and gag cloned extrachromosomally. In parallel, two other replicative plasmids, pJL17 and pJL24, carrying either nef or gag under the control of pBlaF* and the MegaSD sequence, were obtained. To check whether a bicistronic (nef-gag) mRNA could be transcribed from the pJLN29 expression cassette, BMMP were infected either with a mixture of rBCG(pJL17) and rBCG(pJL24) carrying one of the two genes or with rBCG(pJLN29) carrying the operon. After 2 days, total RNA was extracted from the infected macrophages and was amplified by RT-PCR using N1-N2 primers derived from the nef sequence, G1-G2 primers derived from the gag sequence, or N3-G3 primers that could only amplify a product derived from the bicistronic nef-gag mRNA. Although nef and gag single mRNAs amplified with N1-N2 or G1-G2 could be detected in macrophages infected with the three strains, bicistronic nef-gag mRNA could be detected only in macrophages infected with rBCG(pJLN29) after amplification with N3-G3 primers (Fig. 1B). The expression levels of nef and gag were then analyzed by Western blotting using anti-Nef polyclonal antiserum or anti-Gag monoclonal antibody, respectively. Comparable levels of production were obtained in rBCG strains transformed with single genes or carrying the operon (Fig. 1C). This indicated that two heterologous gene products could be synthesized in a bivalent rBCG strain without impairing in vitro growth and at expression levels comparable to those for the monovalent rBCG strains.
FIG. 1.
(A) Construction of monovalent and bivalent rBCG strains expressing SIVmac251 nef and gag genes: nef (light dots) and gag (heavy dots) genes from SIVmac251 were organized as an operon under the control of the M. fortuitum pBlaF* promoter (striped arrow). A sequence derived from the Shine-Dalgarno consensus (MegaSD) indicated as a black box was inserted upstream from the ATG start codon of Nef and Gag to facilitate translation. STOP codons were inserted downstream from each gene. The pBlaF*-nef-gag operon was cloned into a replicative vector to give rise to pJLN29. Two other plasmids carrying a single nef (pJL17) or gag (pJL24) gene under the control of pBlaF* plus MegaSD were also obtained. Primers used for RT-PCR of transcribed nef and gag mRNAs are indicated as black arrows. (B) RT-PCR products obtained from rBCG-infected BMMP. Macrophages were infected either with bivalent rBCG(pJLN29) expressing nef and gag genes or with a mixture of monovalent rBCG(pJL17) and rBCG(pJL24) strains expressing nef or gag, respectively. Total RNA was harvested from infected macrophages 48 h later and reverse transcribed. PCR amplification was performed with N1-N2 primers amplifying a 375-bp product from nef mRNA, G1-G2 primers amplifying a 250-bp product from gag mRNA, or N3-G3 primers that could only amplify a 350-bp product from a bicistronic nef-gag mRNA. Lane 1, rBCG(pJLN29); lane 2, rBCG(pJL17) plus rBCG(pJL24) mixture. Lanes for negative controls: 3, wild-type BCG; 4, noninfected macrophages; 5, total RNA from pJLN29-infected macrophage with no RT; T−, no RNA in the reaction. Lane for positive control: T+, pJLN29 plasmid DNA. (C) Western blot analysis of monovalent Nef or Gag and bivalent Nef-Gag-producing rBCG strains. Total protein was extracted from rBCG strains cultured in vitro. Western blots were performed on 20 μg of total protein with monoclonal antibody against SIVmac251 Gag(p26) (left panel) or with a serum from an SIVmac251-infected macaque recognizing the SIVmac251 Nef protein (right panel). Lanes for Gag panel: 1, recombinant Gag(p26) (1 μg); 2, wild-type BCG; 3, rBCG(pJL24); 4, rBCG(pJLN29). Lanes for Nef panel: 1, extract of insect cells infected with recombinant SIVmac251 nef baculovirus; 2, wild-type BCG; 3, rBCG(pJL17); 4, rBCG(pJLN29).
Site-specific integration of pBlaF*-nef-gag operon into BCG chromosome by means of mycobacteriophage Ms6-derived vectors.
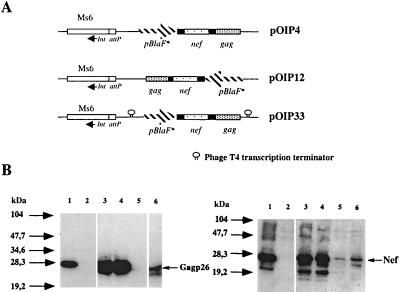
As plasmid loss may occur during in vivo multiplication of rBCG, we constructed genetically stable rBCG strains. A shuttle Ms6-derived vector that integrates into the mycobacterial chromosome was used for this purpose. Ms6 is a temperate mycobacteriophage able to integrate site specifically into tRNAAla loci in both Mycobacterium tuberculosis and Mycobacterium smegmatis (12). pAV6950, derived from Ms6, contains the gene for integrase (int) and the attP attachment site from Ms6 but no excision functions. The pBlaF*-nef-gag operon from pJLN29 was cloned into pAV6950 to give pOIP4 and pOIP12, depending on the orientation of the expression cassette in comparison with the int gene (Fig. 2A). To isolate pBlaF* from the promoters outside the expression cassette, another vector, pOIP33, containing pBlaF*-nef-gag surrounded by two transcription terminators from phage T4 (33) was constructed. The expression of nef and gag was analyzed by Western blotting in rBCG strains obtained after transformation with the four different vectors carrying the operon (Fig. 2B). nef and gag were expressed in all of the strains. As expected, the expression of both genes in rBCG(pJLN29) carrying the multicopy vector was higher than in rBCG::pOIP33, in which a single copy of the expression cassette was integrated into the tRNAAlaV gene in BCG and isolated from readthrough by two transcription terminators (Fig. 2B, compare lanes 3 and 6). Surprisingly, the expression of nef and gag in rBCG::pOIP4 was as high as in rBCG(pJLN29) (Fig. 2B, lanes 3 and 4) and was completely abrogated in rBCG::pOIP12, where the expression cassette was inserted head 2to tail in comparison to pOIP4 (Fig. 2B, lane 5). This showed that, in rBCG:: pOIP12, pBlaF* was unable to drive expression of the nef-gag operon from the expression cassette. Although several explanations can be envisaged to explain this phenomenon, the likeliest hypothesis is that a promoter is located outside the cassette and interferes with nef and gag transcription driven by pBlaF*. As regards the pBlaF*-nef-gag operon, this uncharacterized promoter oriented reverse to nef-gag transcription in pOIP12 would prevent Nef and Gag production in rBCG::pOIP12. Oriented upstream to the nef and gag genes in pOIP4, this promoter would allow high levels of antigen production in rBCG::pOIP4. This effect was prevented by the addition of transcription terminators upstream and downstream from the pBlaF*-nef-gag expression cassette in rBCG::pOIP33.
FIG. 2.
(A) Construction of integrative rBCG(pBlaF*nef-gag) strains by means of mycobacteriophage Ms6-derived vector pAV6950. The pBlaF*-nef-gag operon was isolated from pJLN29 and was cloned into the pAV6950 vector carrying the attP site and integrase gene from the Ms6 cassette. This gave rise to pOIP4 or pOIP12, according to the orientation of the expression cassette compared to the Ms6 int gene. In pOIP33, the pBlaF*-nef-gag operon was flanked by two T4 transcription terminators to prevent potential readthrough from outside promoters. (B) Western blot analysis of integrative rBCG pBlaF*-nef-gag strains. Western blotting was performed on 20 μg of total protein from rBCG strains cultured in vitro with anti-Gag(p26) monoclonal antibody (left panel) or with a serum from an SIVmac251-infected macaque reacting against the SIVmac251 Nef protein (right panel). On both gels positive controls were loaded on lane 1 and were either recombinant purified Gag(p26) (1 μg) or 10 μl of extract of insect cells infected with recombinant SIVmac251 nef baculovirus. Lanes for left and right panels: 2, rBCG(pJN30) (negative control); 3, rBCG(pJLN29); 4, rBCG::pOIP4; 5, rBCG::pOIP12; 6, rBCG::pOIP33. Comparable levels of Gag and Nef were produced by rBCG(pJLN29) and rBCG::pOIP4 strains. Approximately 10 times less Nef and Gag were produced by rBCG::pOIP33 than by rBCG::pOIP4. Nef and Gag proteins were barely detectable in rBCG::pOIP12.
The expression of the gag gene was quantified by capture ELISA using monoclonal anti-Gag antibodies to capture the produced protein and polyclonal anti-Gag antibody to detect it (data not shown). The experiment was performed three times, and the percentage of Gag in the total protein was 1.11% ± 0.1% in rBCG(pJLN29) and fell to 0.14% ± 0.05% in rBCG::pOIP33, which correlated with the decrease in gag gene copies in rBCG::pOIP33 compared to in rBCG(pJLN29). Even though only one copy of the gag gene was present in rBCG::pOIP4, this strain produced Gag at levels identical (1.04 ± 0.07%) to those for the replicative rBCG(pJLN29) strain.
Integration of expression cassette into BCG chromosome greatly increases in vitro and in vivo genetic stability.
In vitro cultures of rBCG(pJLN29), rBCG::pOIP4, and rBCG::pOIP33 were grown with no antibiotic selection for various time periods, and the percentage of kanamycin-resistant colonies was calculated after plating onto medium with or without antibiotics. pJLN29 was not stably maintained in BCG, because, after 60 generations without selection pressure, only 45% of colonies were still resistant to kanamycin, indicating that more than half of the BCG progeny had lost the plasmid and genes of interest (Fig. 3A). Conversely, very few kanamycin-sensitive colonies were observed in rBCG::pOIP4 and rBCG::pOIP33 even after 100 generations without selection pressure.
FIG. 3.
(A) In vitro stability of replicative and integrative bivalent rBCG strains containing the pBlaF*-nef-gag operon. One colony of rBCG strains transformed either with plasmid pJLN29 or integration-proficient vectors pOIP4 and pOIP33 was inoculated into liquid medium without antibiotic and grown for 100 days. At different time points, aliquots of each culture were sampled and serially diluted before plating onto selective and nonselective medium. Ratios of kanamycin-resistant (rBCG) versus kanamycin-sensitive (total BCG) colonies were calculated for each of the three strains over cell divisions. Generations equal the number of days of culture, since the generation time for BCG is approximately 24 h. The most representative of two experiments is shown. (B) In vivo stability of replicative and integrative rBCG(pBlaF*-nef-gag) strains. On day 0 three groups of 25 BALB/c mice were inoculated i.v. with 5 × 106 CFU of rBCG(pJLN29), rBCG::pOIP4, or rBCG::pOIP33. At different times after initial inoculation, CFU recovered from spleen, liver, or lungs from five individual mice per group were numbered onto medium supplemented (rBCG colonies) or not supplemented (total BCG colonies) with kanamycin. Graph represents mean ratios of rBCG colonies/total BCG colonies ± standard deviations for the three mice groups.
The genetic stability of the three strains was also analyzed in vivo. After i.v. inoculation of BALB/c mice, rBCG growth was monitored in the spleen, lungs, and liver, which are the target organs for BCG replication. The percentage of kanamycin-resistant colonies was calculated at different time points after inoculation. Within 28 days the replicative plasmid had been lost from 40% of the colonies from rBCG(pJLN29) in the spleen of inoculated mice (Fig. 3B). Later (i.e., 100 days after inoculation), 75% of the recovered colonies had lost pJLN29. Conversely, in rBCG::pOIP4- and rBCG::pOIP33-inoculated mice, 85 and 98%, respectively, of the colonies recovered from the spleens at this time point were still resistant to kanamycin, indicating a very high in vivo genetic stability of these integrative strains. Twenty colonies recovered from infected spleens from each group were then randomly selected onto nonselective medium 70 days postinoculation. Their DNA content was analyzed by PCR using primers derived from the kanamycin resistance gene, as well as nef and gag. The expression levels of nef and gag were also tested by Western blotting. Of the 20 colonies tested from the rBCG(pJLN29)-inoculated mice, only five (25%) retained nef and gag gene expression. Conversely, in all of the colonies tested in rBCG::pOIP4 or rBCG::pOIP33 mouse groups, the gag and nef genes were expressed to comparable levels to those observed before inoculation. This indicates that no major rearrangement or mutation had occurred during growth in the animal (Table 2).
TABLE 2.
PCR and Western blot analysis of rBCG clones obtained from spleens of infected micea
| rBCG strain | PCR detection of
|
No. of colonies expressing nef and gag 70 days after in vivo growth | Conclusion | ||
|---|---|---|---|---|---|
| kanr | nef | gag | |||
| pJLN29 | 6/20 (+) | 5/6 (+) | 5/6 (+) | 5/6 (+) | Five clones kept entire plasmid |
| 1/6 (−) | 1/6 (−) | 1/6 (−) | One clone lost expression cassette | ||
| 14/20 (−) | 1/14 (+) | 1/14 (+) | 1/14 (+) | Thirteen of 14 clones lost entire plasmid | |
| 13/14 (−) | 13/14 (−) | 13/14 (−) | One clone lost kanamycin resistance gene but kept expression cassette | ||
| pOIP4 | 20/20 (+) | ND | ND | 20/20 (+) | Genetically stable in vivo |
| pOIP33 | 20/20 (+) | ND | ND | 20/20 (+) | Genetically stable in vivo |
Seventy days after i.v. inoculation with three rBCG strains, 20 colonies recovered from spleen were randomly picked on nonselective medium. The DNA content of each colony was analyzed by PCR using primers derived from the kanamycin resistance gene from Tn903 (K1-K2) or from Tn5 (K3-K4), as well as primers derived from nef (N1-N2) and gag (G1–G2). Expression of nef and gag was also analyzed by Western blotting. +, PCR product or gene expression detected; −, product or expression not detected; ND, not done.
In vivo expression of the heterologous gene is detected at a later stage in mice inoculated with integrative rBCG strains.
We subsequently checked whether high levels of heterologous-gene expression detected in vitro correlated with in vivo expression. For this purpose, we inoculated BALB/c mice i.v. with 5 × 106 CFU of wild-type BCG1173P2, rBCG(pJLN29), rBCG::pOIP4, or rBCG::pOIP33. Mice were sacrified 14 and 77 days postinoculation, and the in vivo expression of the gag gene was analyzed in spleens by immunohistochemistry using an anti-Gag polyclonal antibody. The bacillary load was performed on serial sections with an anti-BCG polyclonal antibody (data not shown). gag expression could be detected in animals inoculated with all rBCG strains at the early time point (14 days) (Fig. 4A to D). However, at the later time point (77 days), very few foci of Gag-positive bacilli were detected in mice inoculated with the rBCG(pJLN29) strain (Fig. 4F), whereas in mice inoculated with rBCG::pOIP4 (Fig. 4G) or rBCG::pOIP33 (Fig. 4H), Gag was detected in most foci of BCG infection, which correlated with the genetic stability of the integrative vectors. Gag staining was stronger in rBCG::pOIP4- than in rBCG::pOIP33-inoculated mice (compare Fig. 4G and H), suggesting that the high level of gag expression detected in vitro in rBCG::pOIP4 was maintained in vivo. No Gag labeling was observed in the spleens of mice inoculated with wild-type BCG1173P2 (Fig. 4A and E) or nonimmunized mice (Fig. 4I). When a polyclonal BCG antiserum was used, comparable levels of BCG infection were detected in all groups (data not shown). Representative anti-BCG staining is shown in mice infected with wild-type BCG1173P2 14 and 77 days postinfection (Fig. 4J and K).
FIG. 4.
In vivo expression of the SIVmac251 gag gene in spleen of infected mice. BALB/c mice were inoculated i.v. with 5 × 106 CFU of wild-type BCG, rBCG(pJLN29), rBCG::pOIP4, or rBCG::pOIP33. Two animals were sacrificed on day 14 (A to D and J) and day 77 (E to H and K) postinoculation. Labeling of the spleen paraffin section was performed with anti-Gag polyclonal antibody (A to I) or anti-BCG polyclonal antibody (J and K), followed by secondary horseradish peroxidase-conjugated anti-rabbit IgG. Immunoperoxidase activity was revealed by redness, and cells were counterstained with hematoxylin. Spleen of nonimmunized control mouse (I) stained with anti-Gag is also shown.
T-cell and antibody immune responses induced against Gag antigen by rBCG strains.
The influence of the in vivo stability of rBCG strains on the induction of T-cell responses was then analyzed in BALB/c mice. Mice were inoculated i.v. with 5 × 106 CFU of rBCG(pJLN29), rBCG::pOIP4, or rBCG::pOIP33. Control mice were either infected with wild-type BCG1173P2 or left untreated. Fifty-six days after BCG inoculation, a protein boost of 5 μg of recombinant E. coli Gag(p26) was given by the same route. IFN-γ production was monitored by ELISA in CD4+ T lymphocytes purified from the spleen and restimulated in vitro in the presence of BMDC loaded with Gag or BCG CFA as antigen-presenting cells. Forty-one days after rBCG injection, IFN-γ production could be detected only in CD4+ T cells from rBCG::pOIP4-inoculated animals (Fig. 5, left panel). This indicates that the anti-Gag CD4+ memory T cells induced after rBCG immunization are only maintained in mice inoculated with strains that highly and persistently expressed the heterologous gene. However, 7 days after the Gag protein boost, IFN-γ production was detected in CD4+ T cells from both rBCG(pJLN29)- and rBCG::pOIP4-inoculated mice. This indicated that the priming of anti-Gag T cells was only efficient in rBCG strains expressing high levels of the gag gene in the first immunization and that memory cells could be recalled only by boosting with purified Gag protein in these mice groups. IFN-γ production was barely detectable in rBCG::pOIP33 mice even after the Gag booster. This indicates that the low level of gag expression in this strain was not enough to prime anti-Gag CD4+ T cells. IFN-γ production by CD4+ T cells stimulated with CFA-loaded dendritic cells was comparable in all mice groups immunized with rBCG or wild-type BCG strains. This shows that all strains have the same capacity to induce anti-BCG immune responses (Fig. 5B).
FIG. 5.
IFN-γ production by CD4+ T cells from replicative rBCG- and integrative rBCG-inoculated mice. BALB/c mice were inoculated once i.v. with 5 × 106 CFU of three rBCG strains; one group received the same dose of wild-type BCG (1173P2), and a last group was injected with PBS (Naives). Eight weeks later, all groups received 5 μg of recombinant Gag(p26) by the same route. On day 41 (white bars) or day 63—i.e., 1 week after boost (dotted bars)—splenocytes from two mice per group were pooled and CD4+ T cells were positively selected on magnetic beads. CD4+ T cells (106) were cocultured with 2.5 × 104 syngeneic BMDC (ratio, 40 to 1) that had been pulsed 18 h before with 10 μg of purified Gag protein ml−1 (left panel) or 10 μg of BCG CFA ml−1 (right panel). Culture supernatant was harvested 72 h later, and IFN-γ produced was monitored by ELISA.
Antibody responses against Gag were monitored in groups of five BALB/c mice inoculated i.p. with 106 CFU of rBCG(pJLN29), rBCG::pOIP4, or rBCG::pOIP33. Control mice were inoculated with wild-type BCG or left untreated. A booster with the same BCG dose was administered i.p. 4 weeks later, and 8 weeks after the initial immunization, a boost of 5 μg of purified nonadjuvanted Gag protein was administered by the same route. Anti-Gag antibody responses were monitored by ELISA. A stronger antibody response against Gag was detected in mice inoculated with integrative strains (Fig. 6). Surprisingly, the anti-Gag response was stronger in rBCG::pOIP33, despite the lower level of gag expression in this strain. The isotypes detected were mainly IgG2a and IgG3, indicating a Th1-oriented immune response (data not shown).
FIG. 6.
Antibody responses induced by rBCG strains. Groups of five BALB/c mice were inoculated i.p. with 106 CFU of rBCG(pJLN29), rBCG::pOIP4, rBCG::pOIP33, wild-type BCG1173P2, or PBS. A boost of the same dose of rBCG (two thicker arrows under x axis) was administered 4 weeks later by the same route. On week 8, all mice received 5 μg of purified Gag protein (light arrows) i.p. Total serum anti-IgG was followed by ELISA on each individual mouse. Results given in titers represent means for five individual animals.
DISCUSSION
BCG is the most widely used live bacterial vaccine in humans. It is one of the six vaccines of the Expanded Program for Immunization of the World Health Organization (11) and represents one of the most attractive live vectors for developing recombinant vaccines. Several approaches have been used to obtain multivalent recombinant BCG candidate vaccines. Bivalent rBCG strains expressing pertussis toxin-tetanus toxin hybrid proteins have been constructed (2). The administration of rBCG “cocktails” containing three rBCG strains that express nef, gag, and env from SIVmac251 proved to be efficient for the induction of the immune response in the mouse model (19). In this study, we engineered BCG to coexpress both SIVmac251 genes (nef and gag) in a single recombinant strain which presents interesting practical aspects. The genes were organized as a synthetic operon in a single expression cassette and placed under the control of a strong mycobacterial promoter, pBlaF* (34). This construct was designed to avoid hybrid proteins to preserve all potential epitopes that could naturally occur from native Nef and Gag antigen processing. Both genes were cotranscribed in a bicistronic mRNA, and the expression levels of each gene were comparable to those observed in rBCG strains carrying each single gene under the control of the same promoter.
The pBlaF*-nef-gag operon was first cloned into a multicopy plasmid carrying the pAL5000 origin of replication to give rise to rBCG(pJLN29). Due to the presence of the strong promoter and several copies of the heterologous genes in this strain, high levels of synthesis of Nef and Gag were detected in vitro. Western blot analysis indicated that the expression of gag was more efficient than that of nef. Although we cannot rule out that differences in anti-Gag and anti-Nef serum avidity might explain this observation, our inability to detect immune responses against Nef also indicates that limited quantities of Nef were produced in vivo. As Nef and Gag were translated from a single mRNA species in this strain, we do not believe that mRNA stability was responsible for the lower efficiency of Nef production. Alternatively, the Gag protein might be more stable than Nef.
When we analyzed plasmid stability in rBCG(pJLN29), we found that, during extracellular growth without selective antibiotics, the plasmid was lost from the progeny. Thus, in conditions in which no genes essential for BCG growth were carried by the plasmid, it segregated from rBCG. This could be due to the expression of nonmycobacterial genes that impair BCG fitness. In vivo, virulent mycobacteria grow intracellularly and are well equipped to resist the very hostile macrophage environment (8, 32, 38). However, deletion of certain M. tuberculosis genes, altering the intramacrophagic growth of the corresponding mutant strains, often renders them avirulent (5, 16, 26). We observed that, soon after in vivo replication of rBCG(pJLN29), the plasmid was lost from most of the recovered bacilli. This might be due to a counterselective pressure within the macrophage on strains expressing genes that are not part of the endogenous mycobacterial chromosome. However, the situation was clearly different when an integrative vector derived from the temperate mycobacteriophage Ms6 was used. The two strains, rBCG::pOIP4 and rBCG::pOIP33, in which the expression cassette was integrated into a specific site of the BCG chromosome showed impressive in vitro and in vivo genetic stability; after 100 days of in vivo growth in mice, up to 98% of colonies recovered from spleens had retained both nef and gag expression. This demonstrated that long-lasting expression of nonmycobacterial genes could be achieved by inserting the expression cassette into the BCG chromosome. This did not seem to impair in vivo rBCG fitness because no major differences were observed in BCG growth in mouse target organs between the wild-type and rBCG strains (data not shown). Thus, even though the expression of the heterologous genes does not seem to be toxic for rBCG strains harboring a plasmid, this genetic element can be easily lost from bacterial progeny in vivo. This confers a strong advantage for the long-lasting expression of heterologous genes to integrative vectors.
One major drawback of single-copy integrative vectors such as those derived from mycobacteriophage L5 (3, 31) is the reduced level of expression of heterologous genes compared to that found in multicopy plasmids. Accordingly, in rBCG::pOIP33, where the pBlaF*-nef-gag expression cassette was inserted into the BCG chromosome and isolated by transcription terminators, gag expression was decreased 10-fold from that found in the replicative vector. Expression levels in the two other integrative strains where the operon was not isolated from readthrough showed discrepancies with nef and gag expression by rBCG::pOIP33. In rBCG::pOIP4, even though a single copy of the gag gene was inserted into the chromosome, the level of gag synthesis was identical to that of the replicative strain. This could be due to the topology of DNA in this construction, which could allow higher levels of nef-gag transcription driven by pBlaF* in this strain or more stable mRNA. Another and likelier hypothesis is that a promoter competing with pBlaF* is located outside the cassette, either in the BCG chromosome or in Ms6 DNA sequences downstream of the attP site. Depending on the orientation of this uncharacterized promoter in comparison with the nef and gag genes, several copies of sense or antisense nef-gag mRNA are transcribed from this promoter, leading either to Nef and Gag production comparable to that of replicative strains or barely detectable translation. Further characterization of this potential promoter will be needed to confirm this hypothesis.
Interestingly, expression of the gag gene was observed in highly productive strains in vivo, at early time points, as revealed by immunohistochemistry on the spleens of infected mice. Although the immunohistochemistry that we performed was mostly qualitative, we observed constantly stronger staining of Gag in mice inoculated with the two rBCG strains producing high quantities of Gag than in rBCG::pOIP33-immunized mice. This could indicate that the strong phage promoter could also be active in vivo. The adaptation of mycobacteria to the hostile macrophage environment may modify the pattern of bacterial gene expression. For instance, two studies using the gene encoding the Aequorea victoria green fluorescent protein as a reporter identified promoters from M. tuberculosis (35) or Mycobacterium marinum (4) that are highly up-regulated after macrophage infection. Alternatively, it is conceivable that promoters that are highly active in vitro are shut down when the mycobacterium grows intracellularly under harsher conditions. As no such information is available for pBlaF* or Ms6 promoters, our immunohistochemistry analysis indicated that the strong levels of expression observed in vitro correlated with the in vivo expression pattern. We believe that this is an important point, and we recommend that this method should be used whenever possible to ensure that the heterologous-gene expression observed in vitro can be extrapolated to in vivo conditions. This technique did not allow us to detect lower levels of protein synthesis, as we were not able to detect nef expression even in animals infected with rBCG strains that are highly expressive in vitro. However, as nef and gag mRNA is cotranscribed and as we were able to detect Gag synthesis in intracellular rBCG(pJLN29) and rBCG::pOIP4, we are confident that nef was also expressed in vivo, albeit at a lower level.
As BCG can persist within macrophages or dendritic cells, which are the most professional antigen-presenting cells (6), it is believed to release antigens continuously to boost the immune response. The three recombinant BCG strains developed in this study allowed us to assess directly the influence of heterologous-gene expression levels and stability on the duration of the immune response against Gag. Six weeks following a single rBCG inoculation, the anti-Gag IFN-γ producing CD4+ T cells could only be detected in rBCG(pOIP4)-inoculated mice, suggesting that both a high level and persistence of gag gene expression were required for the detection of memory T cells. However, after the administration of a purified Gag protein booster, similar levels of IFN-γ production were detected in splenocytes from mice inoculated with the replicative rBCG(pJLN29) and the integrative rBCG(pOIP4) strains. Both before and after the Gag booster immunization, the expression level of the gag gene in rBCG::pOIP33 was too low to prime the cellular immune response against Gag and was not compensated for by persistent expression. This suggested that only the initial high level of gag expression in rBCG was critical for the detection of the cellular immune response. This is consistent with previous studies (2, 17) which found that a threshold level of heterologous-gene expression by rBCG must be reached to prime specific T cells. This was further confirmed by the fact that no cellular immune response against Nef could be detected after inoculation of rBCG strains, probably because in vivo Nef antigen production was too low. It is not known whether this critical threshold differs for various antigens and whether it can be efficiently reached by appropriate rBCG strains in every case. We observed a different situation in the humoral response against Gag. Despite a low level of expression of the gag gene in rBCG::pOIP33, this strain induced significantly higher levels of anti-Gag antibodies than the two other strains. However, the BCG CFA antibody response was also higher in rBCG::pOIP33 mice than in the other groups (data not shown), indicating that a higher dose of BCG organisms had been administered to these mice despite similar injection of viable bacilli in all groups. This could be explained by a higher load of dead BCG organisms in the rBCG::pOIP33 vaccine preparation. As dead bacilli still contain the Gag protein, we think that this strongly adjuvanted extra dose of Gag induced higher levels of anti-Gag antibodies in rBCG::pOIP33-vaccinated mice. Again, the less genetically stable rBCG(pJLN29) strain induced the lowest levels of anti-Gag antibodies.
In conclusion we have demonstrated the feasibility of constructing multivalent rBCG strains that are highly genetically stable in vivo by use of integrative vectors. Moreover, the single-copy integrative strains described allowed comparable in vitro and in vivo levels of expression of heterologous genes to those observed in multicopy-replicative strains but induced more sustained immune responses. A number of studies have demonstrated the protective efficacy of rBCG against parasitic or bacterial challenge in small-animal models (1, 9, 20, 25, 27, 30). In primate models, rBCG strains were shown to induce humoral and cellular immune responses against simian AIDS (23, 40) and some degree of protection against measles (42). However, the first phase I clinical trial of a rBCG vaccine expressing the OspA antigen from Borrelia burgdorferi in humans was disappointing, as no specific immune response against OspA was detected in the vaccinees (10). One possibility for the failure of this rBCG strain to induce specific antibodies was that a replicative plasmid was used to express the heterologous gene in BCG and that plasmid loss might have occurred during BCG replication in vivo. We think that integrative rBCG strains are preferential for vaccine development (i) because heterologous genes were expressed at high levels and because this expression was long lived after a single rBCG injection that induced specific T-cell immune responses in the mouse model and (ii) because the use of such strains avoids the microbiological risk of vector transfer to other bacterial pathogens. This should help the future development of effective BCG-derived vaccines for humans.
Acknowledgments
We are very grateful to Patricia Charles for excellent technical assistance, Catherine Raynaud for help with BMMP cultures and RT-PCR experiments, and Micheline Lagranderie for help with rBCG in vivo stability studies. We thank Jean-Marc Reyrat for valuable discussion and critical reading of the manuscript. We also thank Elisabeth Pivert and Patrick Ave for help with histology and Gilles Marchal for providing the anti-BCG antibody.
This work was supported by Agence Nationale de Recherches contre le SIDA and European Community contracts IC18-CT97-0252 and ICA4-1999-40006. Isabelle Bourguin receives an ANRS fellowship.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abdelhak, S., H. Louzir, J. Timm, L. Blel, Z. Benlasfar, M. Lagranderie, M. Gheorghiu, K. Dellagi, and B. Gicquel. 1995. Recombinant BCG expressing the Leishmania surface antigen Gp63 induces protective immunity against Leishmania major infection in BALB/c mice. Microbiology 141: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 2.Abomoelak, B., K. Huygen, L. Kremer, M. Turneer, and C. Locht. 1999. Humoral and cellular immune responses in mice immunized with recombinant Mycobacterium bovis Bacillus Calmette-Guérin producing pertussis toxin-tetanus toxin hybrid protein. Infect. Immun. 67: 5100–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351: 479–482. [DOI] [PubMed] [Google Scholar]
- 4.Barker, L. P., D. M. Brooks, and P. L. C. Small. 1998. The identification of Mycobacterium marinum genes differentially expressed in macrophage phagosomes using promoter fusions to green fluorescent protein. Mol. Microbiol. 29: 1167–1177. [DOI] [PubMed] [Google Scholar]
- 5.Berthet, F. X., M. Lagranderie, P. Gounon, C. Laurent-Winter, D. Ensergueix, P. Chavarot, F. Thouron, E. Maranghi, V. Pelicic, D. Portnoi, G. Marchal, and B. Gicquel. 1998. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science 282: 759–762. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar, K., N. Serbina, and J. L. Flynn. 2001. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect. Immun. 69: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullock, W., J. Fernandez, and J. Short. 1987. A high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5: 376–380. [Google Scholar]
- 8.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell, N. D., E. Medina-Acosta, W. R. McMaster, B. R. Bloom, and D. G. Russell. 1993. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guérin expressing the Leishmania surface proteinase gp63. Proc. Natl. Acad. Sci. USA 90: 11473–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman, R., K. Palmer, K. G. Russ, H. P. Secrest, J. A. L. Becker, S. A. Bodison, J. G. Perry, A. R. Sills, A. G. Barbour, C. J. Luke, M. S. Hanson, C. K. Stover, J. E. Burlein, G. P. Bansal, E. M. Connor, and S. Koenig. 1998. Safety and immunogenicity of recombinant Bacille Calmette-Guérin (rBCG) expressing Borrelia burgdorferi outer surface protein A (OspA) lipoprotein in adult volunteers: a candidate Lyme disease vaccine. Vaccine 17: 904–914. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P., I. Carneiro, J. Milstein, and C. Clements. 1999. Issues relating to the use of BCG in immunization programmes. World Health Organization, Geneva, Switzerland.
- 12.Freitas-Vieira, A., E. Anes, and J. Moniz-Pereira. 1998. The site-specific recombination locus of mycobacteriophage Ms6 determines DNA integration at the tRNAAla gene of Mycobacterium spp. Microbiology 144: 3397–3406. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiu, M., J. Augier, and P. Lagrange. 1983. Maintenance and control of the French strain 1173-P2 (primary and secondary seed-lots). Bull. Inst. Pasteur 81: 281–288. [Google Scholar]
- 14.Gheorghiu, M., P. Lagrange, and C. Fillastre. 1988. The stability and immunogenicity of a dispersed-grown freeze-dried Pasteur BCG vaccine. J. Biol. Stand. 16: 15–26. [DOI] [PubMed] [Google Scholar]
- 15.Hatfull, G. F. 2000. Molecular genetics of mycobacteriophages, p. 37–54. In G. F. Hatfull and W. R. Jacobs, Jr. (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, D.C.
- 16.Jackson, M., S. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67: 2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremer, L., G. Riveau, A. Baulard, A. Capron, and C. Locht. 1996. Neutralizing antibody responses elicited in mice immunized with recombinant bacillus Calmette-Guerin producing the Schistosoma mansoni glutathione S-transferase. J. Immunol. 156: 4309–4317. [PubMed] [Google Scholar]
- 18.Labidi, A., H. L. David, and D. Roulland-Dussoix. 1985. Restriction endonuclease mapping and cloning of Mycobacterium fortuitum var. fortuitum plasmid pAL5000. Ann. Inst. Pasteur Microbiol. 136B: 209–215. [DOI] [PubMed] [Google Scholar]
- 19.Lagranderie, M., N. Winter, A. Balazuc, B. Gicquel, and M. Gheorghiu. 1998. A cocktail of Mycobacterium bovis BCG recombinants expressing the SIV Nef, Env and Gag antigens induces antibody and cytotoxic responses in mice vaccinated by different mucosal routes. AIDS Res. Hum. Retrovir. 14: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 20.Langermann, S., S. R. Palaszynski, J. E. Burlein, S. Koenig, M. S. Hanson, D. E. Briles, and C. K. Stover. 1994. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guérin vaccines expressing pneumococcal surface protein A. J. Exp. Med. 180: 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, M. H., L. Pascopella, W. R. Jacobs, and G. F. Hatfull. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guérin. Proc. Natl. Acad. Sci. USA 88: 3111–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letvin, N. L. 1998. Progress in the development of an HIV-1 vaccine. Science 280: 1875–1880. [DOI] [PubMed] [Google Scholar]
- 23.Leung, N. J., A. Aldovini, R. Young, M. A. Jarvis, J. M. Smith, D. Meyer, D. E. Anderson, M. P. Carlos, M. B. Gardner, and J. V. Torres. 2000. The kinetics of specific immune responses in rhesus monkeys inoculated with live recombinant BCG expressing SIV Gag, Pol, Env and Nef proteins. Virology 268: 94–103. [DOI] [PubMed] [Google Scholar]
- 24.Lim, E. M., M. Lagranderie, R. Le Grand, J. Rauzier, M. Gheorghiu, B. Gicquel, and N. Winter. 1997. Recombinant Mycobacterium bovis-BCG producing the N-terminal half of SIVmac251 Env antigen induces neutralizing antibodies and CTL responses in mice and guinea pigs. AIDS Res. Hum. Retrovir. 13: 1573–1581. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto, S., H. Yukitake, H. Kanbara, and T. Yamada. 1998. Recombinant Mycobacterium bovis bacillus Calmette-Guérin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon γ and parasite-specific antibodies. J. Exp. Med. 188: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinney, J. D., K. Höner zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406: 735–738. [DOI] [PubMed] [Google Scholar]
- 27.Nascimento, I. P., W. O. Dias, R. P. Mazzantini, E. N. Miyaji, M. Gamberini, W. Quintillo, V. C. Gebara, D. F. Cardoso, P. L. Ho, I. Raw, N. Winter, B. Gicquel, R. Rappuoli, and L. C. C. Leite. 2000. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 68: 4877–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranes, M. G., J. Rauzier, M. Lagranderie, M. Gheorghiu, and B. Gicquel. 1990. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a “mini” mycobacterium-Escherichia coli shuttle vector. J. Bacteriol. 72: 2793–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolt, P., and N. Stocker. 1996. Functional definition of regions necessary for replication and incompatibility in the Mycobacterium fortuitum plasmid pAL5000. Microbiology 142: 2795–2802. [DOI] [PubMed] [Google Scholar]
- 30.Stover, C. K., G. P. Bansal, M. S. Hanson, S. R. Burlein, S. R. Palaszynski, J. F. Young, S. Koenig, D. B. Young, A. Sadziene, and A. G. Barbour. 1993. Protective immunity elicited by recombinant Bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J. Exp. Med. 178: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, S. B. Snapper, R. G. Barletta, W. R. Jacobs, and B. R. Bloom. 1991. New use of BCG for recombinant vaccines. Nature 351: 456–460. [DOI] [PubMed] [Google Scholar]
- 32.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263: 678–681. [DOI] [PubMed] [Google Scholar]
- 33.Timm, J., E. M. Lim, and B. Gicquel. 1994. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J. Bacteriol. 176: 6749–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timm, J., M. G. Perilli, C. Duez, J. Trias, G. Orefici, L. Fattorini, G. Amicosante, A. Oratore, B. Boris, J. M. Frere, A. P. Pugsley, and B. Gicquel. 1994. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitum β-lactamase genes cloned from a natural isolate and a high-level β-lactamase producer. Mol. Microbiol. 12: 491–504. [DOI] [PubMed] [Google Scholar]
- 35.Triccas, J. A., F. X. Berthet, V. Pelicic, and B. Gicquel. 1999. Use of fluorescence induction and sucrose counterselection to identify Mycobacterium tuberculosis genes expressed within host cells. Microbiology 145: 2923–2930. [DOI] [PubMed] [Google Scholar]
- 36.Winter, N., M. Lagranderie, S. Gangloff, C. Leclerc, M. Gheorghiu, and B. Gicquel. 1995. Recombinant BCG strains expressing the SIVmac251nef gene induce proliferative and CTL responses against nef synthetic peptides in mice. Vaccine 13: 471–478. [DOI] [PubMed] [Google Scholar]
- 37.Winter, N., M. Lagranderie, J. Rauzier, J. Timm, C. Leclerc, B. Guy, M. P. Kieny, M. Gheorghiu, and B. Gicquel. 1991. Expression of heterologous genes in Mycobacterium bovis BCG: induction of a cellular response against HIV-1 Nef protein. Gene 109: 47–54. [DOI] [PubMed] [Google Scholar]
- 38.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153: 2568–2578. [PubMed] [Google Scholar]
- 39.Yasutomi, Y., S. Koenig, S. S. Haun, K. Stover, R. K. Jackson, P. Conard, A. J. Conley, E. A. Emini, T. R. Fuerst, and N. L. Letvin. 1993. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 150: 3101–3107. [PubMed] [Google Scholar]
- 40.Yasutomi, Y., S. Koenig, R. M. Woods, J. Madsen, N. M. Wassef, C. R. Alving, H. J. Klein, T. E. Nolan, L. J. Boots, J. A. Kessler, and N. L. Levy. 1995. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J. Virol. 69: 2279–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zal, T., A. Volkmann, and B. Stockinger. 1994. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 180: 2089–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, Y. D., G. J. Fennelly, C. Miller, R. Tarara, I. Saxe, B. R. Bloom, and M. McChesney. 1997. Recombinant bacille Calmette-Guérin expressing the measles virus nucleoprotein protects infant rhesus macaques from measles virus pneumonia. J. Infect. Dis. 176: 1445–1453. [DOI] [PubMed] [Google Scholar]