Abstract
Leptospirosis is a spirochetal zoonosis that causes an acute febrile systemic illness in humans. Leptospira sp. hemolysins have been shown to be virulence factors for the pathogenesis of leptospirosis. Previously, we cloned a hemolysin SphH of Leptospira interrogans serovar lai, a homologue of L. borgpetersenii sphingomyelinase (SphA), from a genomic library (S. H. Lee, K. A. Kim, Y. K. Kim, I. W. Seong, M. J. Kim, and Y. J. Lee, Gene 254:19–28, 2000). Escherichia coli lysate harboring the sphH showed high hemolytic activities on sheep erythrocytes. However, it neither showed sphingomyelinase nor phospholipase activities, in contrast to SphA which was known to have sphingomyelinase activity. Interestingly, the SphH-mediated hemolysis on erythrocytes was osmotically protected by PEG 5000, suggesting that the SphH might have caused pore formation on the erythrocyte membrane. In the present study, we have prepared the Leptospira hemolysin SphH and investigated its hemolytic and cytotoxic activities on mammalian cells. SphH was shown to be a pore-forming protein on several mammalian cells: When treated with the SphH, the sheep erythrocyte membranes formed pores, which were morphologically confirmed by transmission electron microscopy. Furthermore, the SphH-mediated cytotoxicities on mammalian cells were demonstrated by the release of LDH and by inverted microscopic examinations. Finally, the immune serum against the full-length hemolysin could effectively neutralize the SphH-mediated hemolytic and cytotoxic activities. In conclusion, these results suggest that the virulence of Leptospira SphH was due to the pore formation on mammalian cell membranes.
Leptospirosis is a spirochetal zoonosis that causes an acute febrile and systemic illness in humans caused by pathogenic Leptospira species (12). The leptospirosis carriers are mainly wild or domestic animals, especially rodents, small marsupials, cattle, pigs, and dogs. The clinical syndromes include subclinical infection, self-limited anicteric febrile illness with or without meningitis, and severe and potentially fatal Weil’s syndrome that manifests hemorrhage, renal failure, and jaundice (10).
Although Leptospira virulence factors such as hemolysins (23, 47, 49), lipopolysaccharide (20), glycolipoprotein (1), peptidoglycan (8), heat shock proteins (42), flagellin (15), and others may contribute to the pathogenesis, their pathogenetic mechanisms have not been clearly understood. Leptospira hemolysins have been suggested to be phospholipases, which act on erythrocytes and other cell membranes containing the substrate phospholipids, leading to cytolysis (23, 47, 49). In 1986, Bernheimer and Bey (2) purified sphingomyelinase C with hemolytic activity from Leptospira interrogans serovar pomona, and subsequently, the sphingomyelinase C gene (sphA) of L. borgpetersenii serovar hardjo was cloned (7) and sequenced (41). However, its precise role in pathogenesis of leptospirosis has not been clearly understood yet.
In our previous study, the hemolysin gene sphH was cloned and sequenced from L. interrogans serovar lai strain HY-1 (27). It showed high similarity to the sphA of L. borgpetersenii serovar hardjo: 63.5% at the DNA level and 75% at the amino acid level. Interestingly, SphH did not have sphingomyelinase or other phospholipase activities, whereas SphA showed sphingomyelinase activity. Moreover, SphH-mediated hemolysis was protected reversibly by an osmotic protectant, polyethylene glycol 5000, indicating SphH as a putative pore-forming protein. Since the sphH was highly conserved among pathogenic leptospires, it appears to be one of the important virulence factors.
The pore formation of bacterial hemolysins on the target cell membrane has been shown in several pathogenic bacteria or protozoans, such as Bordetella pertussis (24), Staphylococcus aureus (22), Serratia marcescens (17, 39), and Leishmania species (32). In the case of Leptospira species, there are some reports on cytotoxicity of Leptospira virulence factors: Vinh et al. (50) have shown that the glycolipoprotein fraction from L. interrogans was cytotoxic to mouse fibroblast cultures, and Isogai et al. (19, 20) reported that administration of lipopolysaccharide from L. interrogans induced apoptosis of lymphocytes in mice. However, there have been no studies on the effects of a pore-forming protein of Leptospira species on mammalian cells.
In this study, with the partially purified recombinant hemolysin SphH from L. interrogans serovar lai and using a transmission electron microscope, we have clearly demonstrated that the cytotoxic mechanism of hemolysin SphH was due to pore formation on several mammalian cells, which can be reversed by SphH immune serum.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli XL-1 Blue (Stratagene, La Jolla, Calif.) was used as the host strain for transformation of the pHLK-2(4d) that contained the Leptospira hemolysin sphH in pBluescript (pBS) vector (27). For overexpression of the recombinant protein, the pET-14b expression vector (Novagen, Madison, Wis.) containing a hexahistidine tag and the Escherichia coli strain BL21(DE3)(pLysS) (43) which is lysogenic for a λ phage carrying the T7 RNA polymerase gene under control of the lacUV5 promoter were used. E. coli cells were grown in Luria-Bertani broth containing 1% tryptone (Difco Laboratories, Detroit, Mich.), 0.5% yeast extract, and 1% NaCl at pH 7.0, supplemented with ampicillin (100 μg/ml), chloramphenicol (34 μg/ml), or tetracycline (25 μg/ml) (Sigma, St. Louis, Mo.) where appropriate.
Purification of hemolysin SphH by ion-exchange chromatography.
Leptospira hemolysin fraction was prepared as follows. The 100-ml overnight culture of E. coli harboring pHLK-2(4d) was harvested by centrifugation (Supra 21k centrifuge; Hanil Science Industrial Co., Ltd., Incheon, Korea) at 600 × g for 20 min at 4°C. The pellets were washed twice with washing buffer (20 mM Tris-HCl, pH 7.2, containing 1 mM phenylmethylsulfonyl fluoride [PMSF]). The pellets were resuspended in 10 ml of the buffer, sonicated on ice by an ultrasonic dismembrator (Fisher Scientific, Pittsburgh, Pa.), and then centrifuged at 1,600 × g by Supra 21k for 20 min at 4°C. The cell-free lysate (supernatant fraction) was obtained and applied to a DEAE-cellulose column (Whatman, Springfield Mill, United Kingdom). The column was washed with 10 column volumes of the buffer and then eluted stepwise with 4 volumes of the buffer containing 0.1, 0.2, 0.3, 0.4, and 0.5 M NaCl. Only the 0.1 M NaCl eluate contained the hemolytic activity. The hemolytic activity was determined as described previously (27). Briefly, the reaction mixture containing 100 μl of 10× phosphate-buffered saline (PBS), pH 7.2; 50 μl of the eluate, 750 μl of distilled water; and 100 μl of 10% washed sheep erythrocyte suspension was incubated at 37°C for 1 h. After centrifugation (Micro 17R centrifuge; Hanil Science Industrial Co., Ltd.) at 10,000 × g for 30 s, the supernatant was then diluted 10-fold with PBS. The hemolytic activity was estimated by measuring the absorbance at 420 nm.
Mammalian cell cultures.
Mammalian cell lines were purchased from the Korean Cell Line Bank (KCLB) (Seoul, Korea). The Vero cells (KCLB 10081; monkey kidney epithelial cell line), A-549 cells (KCLB 10185; human lung epithelial carcinoma cell line), NCI-H1299 cells (KCLB 25803; human lung epithelial carcinoma large cell line), and L-132 cells (KCLB 10005; human lung epithelial cell line) were cultured in Dulbecco’s modified Eagle’s culture medium (DMEM) (Gibco-BRL, Rockville, Md.) which was buffered with sodium bicarbonate and supplemented with 10% fetal calf serum (FCS) (Gibco-BRL), 100 μg of penicillin per ml, and 100 μg of streptomycin (Gibco-BRL) per ml. The cells were grown in a 5% CO2 incubator at 37°C.
Expression and purification of the recombinant hemolysin in E. coli.
The pET-sphH containing the full-length sphH was constructed as described previously (27), and E. coli BL21(DE3)(pLysS) was transformed with pET-sphH and induced at an A600 of 0.6 by the addition of 0.6 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h. The IPTG-induced cells were lysed by vigorous sonication, and the insoluble pellet containing the recombinant His-SphH was dissolved in 6 M guanidine (20 mM Tris-HCl [pH 7.9], 0.5 M NaCl, 6 M guanidine-HCl, 1 mM PMSF, and 5 mM imidazole) on ice for 1 h. The dissolved His-SphH was then applied to Ni+-nitrilotriacetic acid agarose resin (Qiagen Inc., Chatsworth, Calif.) and purified according to the manufacturer’s procedure.
Production of Leptospira SphH immune serum.
Immune serum against recombinant hemolysin, His-SphH, was prepared as described (16). Briefly, after electrophoresis of the purified His-SphH, the band corresponding to the His-SphH was cut out and the protein was eluted with a model 422 Electro-Eluter (Bio-Rad, Richmond, Calif.). Approximately 150 μg of the His-SphH mixed with Freund’s complete adjuvant (Gibco-BRL) was injected subcutaneously into a New Zealand White female rabbit. At 4 and 8 weeks after the primary immunization, booster immunizations were given intramuscularly with approximately 150 μg of His-SphH mixed with Freund’s incomplete adjuvant, and the rabbit was bled 10 weeks after the primary immunization.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
The samples were electrophoresed on 8% polyacrylamide gel (26), and the proteins were then transferred to a nitrocellulose membrane (Amersham) via a SEMI-PHOR transfer system (Hoefer Scientific Instruments, San Francisco, Calif.) as described previously (18). The blot was then incubated with a blocking buffer (5% skim milk in TBST, which contained 10 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.05% Tween 20) for 1.5 h and reacted with the immune serum against the His-SphH (1:5,000 dilution in TBST) for 1 h. After washing with TBST, the blot was incubated for 30 min with peroxidase-conjugated donkey anti-rabbit immunoglobulin G antibody (Jackson ImmunoResearch Laboratory, West Grove, Pa.) diluted to 1:5,000 in TBST and then was placed in contact with ECL reagents (Amersham) for 1 min. Finally, the blot was exposed to Hyperfilm (Amersham).
Transmission electron microscopy (TEM).
For electron microscopic examination, the sheep erythrocytes were incubated with the 0.1 M NaCl SphH eluate for 20 min at 37°C. After supernatants were removed by centrifugation (in a Micro 17R centrifuge at 10,000 × g for 30 s), the sheep erythrocytes were prefixed with 1.5% glutaraldehyde containing 0.1 M phosphate buffer, pH 7.2, at 4°C for 2 h and postfixed with 1% osmium tetroxide solution containing 0.1 M phosphate buffer at room temperature for 1 h, and subsequent steps were prepared as described (46, 47). The samples were examined under an H-600 transmission electron microscope (Hitachi Scientific Instruments, Pleasanton, Calif.). PBS (pH 7.2) or E. coli lysate harboring pBS vector was used as a negative control.
Cytotoxicity assay of the SphH on mammalian cell lines.
Cytotoxicity of the SphH was measured by lactate dehydrogenase (LDH) released from the cells as described previously (6, 31). Vero cells and A-549, NCI-H1299, and L-132 cell lines were grown up to 90% confluence. The cells were scraped, washed with DMEM supplemented with 10% FCS, and plated in a 96-well plate (Nunc) at a concentration of 104 cells per well a day before the experiment. A confluent monolayer of the cells in a 96-well plate was washed twice with PBS, and 180 μl of DMEM containing 2% FCS was added. The SphH eluate was diluted serially twofold in PBS. Twenty microliters of the samples was added to each well, and the plate was incubated under 5% CO2 at 37°C for 8 h. E. coli lysate harboring pBS vector was used as a control. Cytotoxicity was determined by the CytoTox 96 assay (Promega) measuring the released LDH activity into the medium. Cytotoxicity calculations were based on the following formula: % cytotoxicity = 100 × (Asample − Aspontaneous)/(Atotal − Aspontaneous), where Asample is the optical density (OD) of the treated cells, Aspontaneous is the OD of the untreated cells, and Atotal is the OD of cells lysed with 1% Triton X-100 at a final concentration for maximal LDH release. The background OD was measured with culture medium alone or the eluate in culture medium and subtracted from all other readings.
Neutralization assay.
The neutralization of SphH-mediated hemolytic or cytotoxic activities was tested as described earlier (6). For the neutralization assay, 50 μl of the SphH eluate was mixed with an equal volume of the immune serum against His-SphH and preincubated for 1 h at 37°C before addition to sheep erythrocytes or Vero cells. One hundred microliters of toxin-antitoxin mixtures was then added to 100 μl of 10% washed sheep erythrocyte suspension in 100 μl of 10× PBS and 700 μl of distilled water, and the mixture was incubated at 37°C for 1 h. In the case of Vero cells, 40 μl of toxin-antitoxin mixture was added to each well containing Vero cells in 160 μl of DMEM supplemented with 2% FCS. The plate was incubated under 5% CO2 for 8 h at 37°C, and then the hemolytic or cytotoxic activities were measured as described above.
RESULTS
Purification of Leptospira hemolysin SphH.
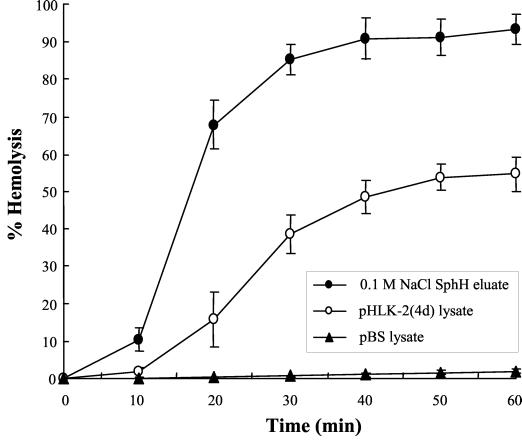
In our previous study, the recombinant SphH (His-SphH) was expressed as an insoluble form and did not show any hemolytic activity (27). To obtain a native form of the SphH containing hemolytic activity, Leptospira hemolysin SphH fraction was prepared from E. coli harboring pHLK-2(4d) that contained the sphH. The cell-free lysate (supernatant) was obtained from a culture of E. coli harboring pHLK-2(4d) and chromatographed on a DEAE-cellulose column. The hemolysin SphH was purified by eluting with 0.1 M NaCl containing 20 mM Tris-HCl and 1 mM PMSF. The hemolytic activity of the eluate and the cell-free lysate of E. coli harboring pHLK-2(4d) on sheep erythrocytes was measured (Fig. 1). The hemolytic activity of the eluate was about twofold higher than that of the cell-free lysate of E. coli harboring pHLK-2(4d). In addition, the specific activity of the eluate also increased about sixfold, compared to that of the cell-free lysate (data not shown). No hemolytic activity in cell-free lysate of E. coli harboring pBS vector was shown as a negative control which was essentially negative (Fig. 1).
FIG. 1.
Hemolytic activities of cell-free lysate and 0.1 M NaCl SphH eluate. Assays were performed on 1% sheep erythrocytes at 37°C for 1 h with 50 μl of cell-free lysate from E. coli harboring pHLK-2(4d) (○), 0.1 M NaCl SphH eluate (•), and cell-free lysate from E. coli harboring pBS vector (▴) as a negative control. The percent hemolysis was expressed in relation to 100% hemolysis of erythrocytes in distilled water. The data are the means ± standard deviations (error bars) of triplicates.
Purification of the recombinant hemolysin and production of rabbit immune serum.
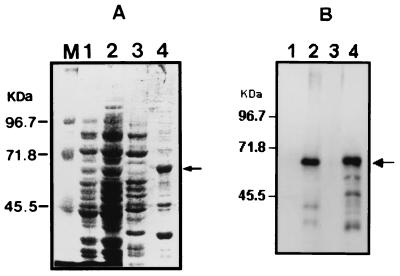
To produce the immune serum against the His-SphH, the recombinant His-SphH was expressed in E. coli and purified by affinity chromatography as described in Materials and Methods. The molecular mass of the His-SphH was estimated to be approximately 64 kDa by SDS-PAGE, which was slightly higher than that of the original protein due to the insertion of extra hexahistidines and 16 amino acid residues linked to the amino terminus of the SphH. Immune serum against His-SphH was raised in a rabbit, and immunoblot analysis was performed in order to detect whether the immune serum reacted against the SphH. As shown in Fig. 2B (lanes 2 and 4), the immune serum reacted to the approximate 64-kDa His-SphH clearly. However, there were few additional smaller molecular species reacting with the immune serum, which were most likely the degradation products of His-SphH, since the anti-six-His monoclonal antibody also reacted with these smaller bands (data not shown). These results indicated that the immune serum reacted the epitope against Leptospira hemolysin SphH.
FIG. 2.
Detection of Leptospira hemolysin SphH expressed in E. coli. (A) SDS-PAGE was performed in SDS-8% polyacrylamide gel and stained with Coomassie brilliant blue. (B) Immunoblot analysis was performed with rabbit immune serum against His-SphH fusion protein. E. coli harboring pET-sphH which contained full-length hemolysin was induced by IPTG for 3 h at 37°C, and sonic lysates were separated into soluble and insoluble fractions by centrifugation. Lanes: 1 to 2, whole-cell E. coli lysate harboring pET-sphH without IPTG induction (lane 1) and with IPTG induction (lane 2); 3 to 4, soluble fraction (lane 3) and insoluble fraction (lane 4) from E. coli lysate harboring pET-sphH induced by IPTG; M, molecular mass marker. The arrow indicates the approximately 64-kDa His-SphH fusion protein.
SphH-mediated pore formation on sheep erythrocyte membrane by transmission electron microscopy.
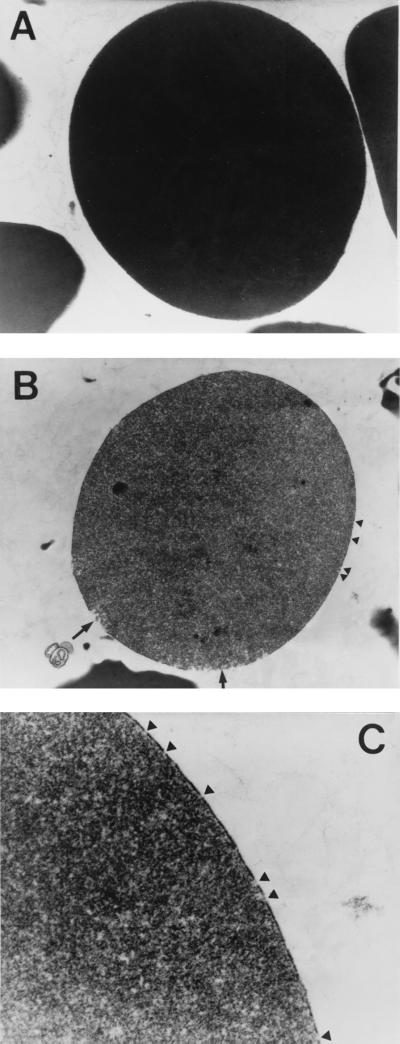
To identify pore formation caused by the SphH, sheep erythrocytes were mixed with the SphH eluate for 20 min at 37°C. PBS (pH 7.2) or lysate of E. coli harboring pBS vector was used as negative controls. The supernatant was removed after centrifugation, and sheep erythrocytes were examined under a transmission electron microscope. The SphH-treated erythrocyte membrane exhibited electron-faint interrupted sections (Fig. 3B and C), consisting of pores on the membrane, while the controls treated with the PBS or lysate of E. coli harboring pBS vector did not (Fig. 3A). In addition, the cytoplasm of SphH-treated erythrocytes was shown to be electron-faint, but that of control erythrocytes was electron-dense, suggesting that cellular contents were most likely released by osmotic lysis of the membrane. Moreover, the large amount of cellular debris appeared in the background of the SphH-treated erythrocytes, but these were absent in the controls. These findings further supported the colloidal osmotic nature of the SphH-mediated hemolysis, indicating the Leptospira hemolysin SphH to be a pore-forming protein.
FIG. 3.
Transmission electron micrographs of sheep erythrocyte membranes treated with the SphH. Sheep erythrocytes were mixed with 0.1 M NaCl SphH eluate and reacted at 37°C for 20 min. (A) Control sheep erythrocyte mixed with PBS (pH 7.2) or lysate of E. coli harboring pBS vector. (B and C) Sheep erythrocytes treated with SphH eluate. Arrowheads indicate the membrane pores induced by SphH, and arrows indicate the burst membrane regions caused by water influx through the SphH-induced pores by osmotic pressure. Magnification, ×16,000 (A and B) and ×40,000 (C).
The SphH induces cytotoxicities in mammalian cells.
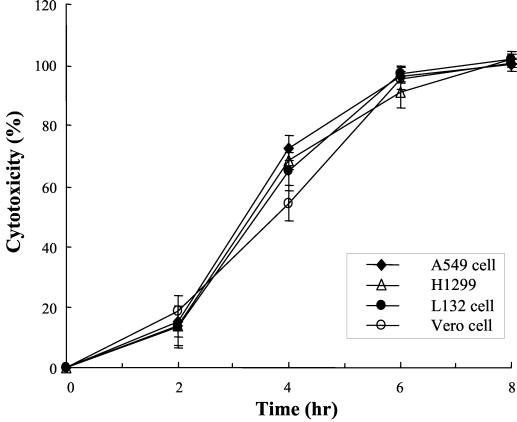
To verify cytotoxicity of the SphH on mammalian cells, we measured the release of the cytosolic LDH into the culture media from the damaged mammalian cells. The kinetics of the cytotoxic effect induced by the SphH eluate was determined in a microtiter plate assay (Fig. 4). Release of LDH from the cytoplasm was shown in the SphH-treated Vero cells and A549, H1299, and L132 cell lines. Cells began to release LDH from 2 h after addition of the SphH, and total lysis occurred between 6 and 8 h. E. coli lysate containing pBS vector added as a negative control did not release LDH from all the cells tested up to 8 h (data not shown). Furthermore, when exposed to serial twofold dilutions of 20 μl of the eluate, the mammalian cells showed LDH release in a concentration-dependent manner (Fig. 5). However, the eluate diluted more than 8-fold did not show any LDH release beyond the baseline. These findings indicated that mammalian cell injury was dependent on both Leptospira hemolysin SphH concentration and the period of incubation.
FIG. 4.
Kinetics of the SphH-mediated cytotoxic effect on mammalian cells. Kinetics of cytotoxicity induced by the 0.1 M NaCl SphH eluate on Vero, A549, H1299, and L132 cells were determined. Cells began to die 2 h after addition of the SphH, and total cell lysis occurred after 6 to 8 h. Cytotoxicity was not induced by E. coli lysate harboring pBS vector which was used as a negative control (data not shown). Cells were cultured as described in Materials and Methods. Cytotoxicity of mammalian cell lines was assayed by measuring the LDH released from the lysed cells and is expressed as a percentage of cell lysis, as indicated in Materials and Methods. Results represent the means ± standard deviations (error bars) of triplicates.
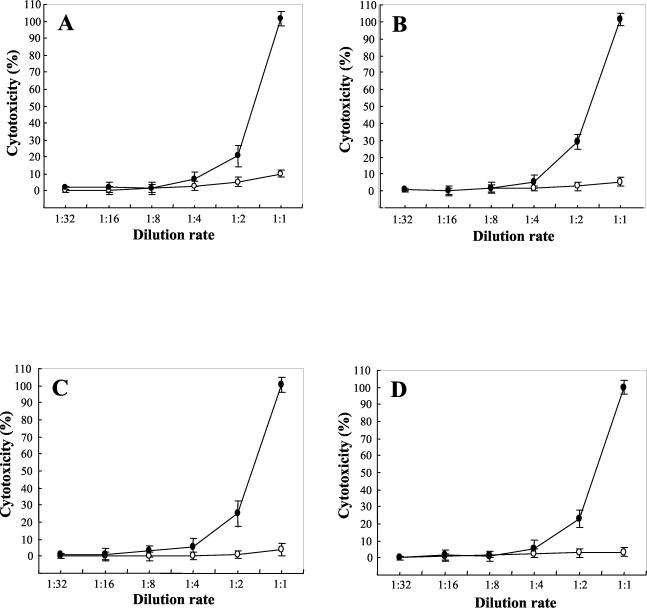
FIG. 5.
Dose-dependent cytotoxicity of SphH on mammalian cells. Vero cells (A), A549 cells (B), H1299 cells (C), and L132 cells (D) were incubated with twofold serially diluted 0.1 M NaCl SphH eluate under 5% CO2 at 37°C for 8 h. Increasing concentrations of the SphH (•) proportionally induced a rapid injury of cells. E. coli lysate harboring pBS vector (○) was used as a negative control. Membrane damage of mammalian cells was evaluated by the measuring LDH release, as indicated in Material and Methods. Results represent the means ± standard deviations (error bars) of triplicates.
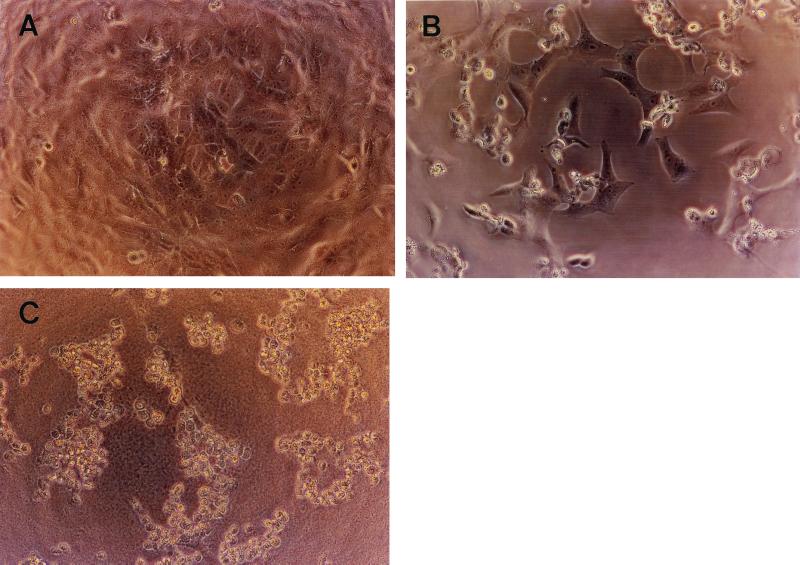
The morphological changes were also examined in the Vero cells 8 h after treatment with the SphH eluate by an inverted microscope equipped with a 35-mm camera. PBS or E. coli lysate harboring pBS vector was used as a negative control (Fig. 6). A remarkable difference in the morphology between the SphH-treated cells and the control Vero cells was evident: The former showed rounded and shrunken cells (Fig. 6B and C), while the latter continued to display normal morphology (Fig. 6A). These results clearly demonstrated that cell membrane damage by the SphH was morphologically evident, and thus, the comparable changes might possibly occur in the mammalian hosts.
FIG. 6.
The inverted photomicrographs of SphH-induced morphological changes on Vero cells. Vero cells were cultured in a 96-well plate until they reached 90 to ∼100% confluence. A 20-μl aliquot of 0.1 M NaCl SphH eluate was added to each well. After incubation under 5% CO2 at 37°C for 4 or 8 h, the cells were examined with an inverted microscope. (A) Vero cells treated with lysate of E. coli harboring pBS vector as a negative control. (B and C) Vero cells treated with the eluate for 4 or 8 h, respectively. Magnification, ×140.
Neutralization of the SphH-medicated hemolytic and cytotoxic activities by rabbit immune serum.
To further evaluate the hemolytic and cytotoxic activities on mammalian cells caused by the SphH, a neutralization assay was performed with rabbit immune serum raised against His-SphH. The SphH eluate was preincubated with the immune serum and added to sheep erythrocytes or Vero cells. As shown in Table 1, the immune serum neutralized approximately 90 and 70% of the SphH-mediated hemolysis on sheep erythrocytes and cytotoxicity on Vero cells, respectively. On the other hand, the preimmune serum neither inhibited the hemolytic nor cytotoxic activities of the SphH. These observations provided additional evidence that the SphH is a mediator of cytotoxicity in mammalian cells in vitro.
TABLE 1.
Neutralization of SphH-mediated sheep erythrocyte hemolysis and Vero cell cytotoxicity by rabbit immune serum against His-SphH
| Sample | Mean % RBC hemolysisa ± SD | Mean % Vero cell cytotoxicitya ± SD |
|---|---|---|
| 0.1 M NaCl-eluted SphH | 96.9 ± 2.1 | 101 ± 1.1 |
| Preimmune serum + 0.1 M NaCl SphH eluate | 93.1 ± 7.1 | 97 ± 3.5 |
| Anti-His-SphH serum + 0.1 M NaCl SphH eluate | 9.6 ± 3.2 | 25 ± 4.3 |
Hemolysis or cytotoxicity was expressed as a percentage of the hemolytic or cytolytic activities relative to those of 0.1 M NaCl SphH eluate without preincubation. RBC, sheep erythrocyte.
DISCUSSION
The pathogenic Leptospira enters the hosts through small cuts or abrasions, and possibly through sodden skin, or by inhalation of aerosols of urine. Although the organisms are phagocytosed by macrophages fixed in the liver and lung, they spread immediately and are circulated in the bloodstream. The primary lesion was found to be at the endothelium of small blood vessels, which leads to localized ischemia in organs, resulting in renal tubular necrosis, hepatocellular damage, meningitis, myositis, and placentitis (11).
Leptospira hemolysins have been implicated in the pathogenesis of leptospirosis; however, its role as a virulence factor and/or the mechanism of action has not been fully understood. Thompson (46) has proposed that Leptospira hemolysin as a phospholipase may be responsible for yielding the holes in the erythrocyte of calves infected with serovar pomona. It has also been speculated that the pathogenesis of hepatic and renal lesions or lung hemorrhage in some patients with leptospirosis may arise from the actions of unspecified toxins, probably, distinguished from the action of phospholipases (48, 30).
Hemolysins are cytolytic toxins found in a wide spectrum of organisms and can be classified into three categories based on the mechanism of action on target cell membranes: enzymatic, pore formation, or surfactant (37). Hemolysins causing cytolysis by enzymatic disruption of target cell membranes include phospholipases such as alpha-toxin from Clostridium perfringens (25), beta-toxin (sphingomyelinase) of S. aureus (36), and phospholipases from other gram-negative or -positive bacteria (48). These phospholipases have shown a high degree of substrate specificity and have reacted with multiple target cells (48). The delta-toxin of S. aureus (13) and the heat-stable hemolysin from Pseudomonas aeruginosa (28) are known to be surfactants. These toxins are highly hydrophobic and act like detergent, causing cytolysis by solubilization of the target cell membrane.
A number of proteins forming stable transmembrane pores have been isolated both from prokaryotic and eukaryotic organisms. Well-known members of these proteins are the pore-forming cytolysins such as the alpha-toxin from S. aureus (14), streptolysin O (35), E. coli hemolysin (3), hemolysin from Tethya lyncurium (29), and Leishmania cytolysin (32). The mechanism of membrane damage by the pore-forming proteins involves the binding of the toxin to the target cell membrane and penetration followed by disruption of the membrane and alteration of membrane permeability, leading to cytolysis (37). Any pores created on the cell membrane are assumed to pass ions and small molecules freely across the bilayer. The high concentration of macromolecules inside the cell generates osmotic pressure, causing a water influx that leads the cell to burst. This process, known as colloid-osmotic lysis, can be prevented by the addition of macromolecules, such as PEG, to the extracellular compartment to compensate for the osmotic imbalance (32, 33).
In our previous study (27), a 7.3-kb Leptospira DNA fragment containing a hemolysin gene (sphH) was isolated from the genomic library by plaque hybridization using the sequence derived from the sphingomyelinase C gene (sphA) of L. borgpetersenii serovar hardjo. Although the isolated clone harboring the sphH showed distinct hemolytic activities on sheep erythrocytes and the sequence of the sphH showed significantly high similarity to that of the sphA at the DNA and amino acid levels, the clone neither hydrolyzed sphingomyelin nor any other phospholipids tested. This functional discrepancy between the SphH and SphA could be further explained by the fact of difference in their secondary structures, suggesting sphH as a novel hemolysin of the Leptospira species. We have also shown that the SphH-mediated hemolysis was completely protected by the osmotic protectant polyethylene glycol 5000 (27), indicative of the colloid-osmotic nature of the hemolysis (32, 33). These findings strongly suggested that the hemolysin SphH might be a potential pore-forming protein with a diameter between 3.8 and 5.2 nm according to its Einstein-Stokes hydrodynamic radius (38).
In the present study, the Leptospira hemolysin SphH was confirmed as a novel pore-forming protein by transmission electron microscopy for the first time: the SphH-induced hemolysis on sheep erythrocytes showed discrete membrane disruptions, cellular swelling, and loss of the cytoplasmic density, which are all consistent with entry of water into the cell and hypo-osmotic damage, quite analogous to the changes seen with S. aureus alpha-toxin (4) and/or the terminal membrane attack complex (C5b-9) of human complement, which is known to be a pore-forming protein (34). Pores can be visualized as an electron-faint disrupted area of membrane at the margin of erythrocyte.
We further showed that the Leptospira hemolysin SphH could damage membranes of several mammalian epithelial cells, including Vero cells, A-549 cells, H1299 cells, and L-132 cells. Based on these findings, the SphH appears to exhibit a broader range of host cell specificity and thus can be classified as a membrane-disrupting cytolysin rather than hemolysin. We also observed that approximately 50% cell lysis occurred in sheep erythrocytes 15 min after treatment with the SphH, but in the case of the mammalian cells, it took 3 to 4 h. This time difference between sheep erythrocytes and the mammalian cells might be due to different sensitivities of the cell membranes against the hemolysin SphH. In addition, we demonstrated a remarkable difference in morphology between the SphH-treated cells and the control Vero cells. The SphH-treated Vero cells contained rounded and shrunken shapes by membrane damage evidenced by microscopic examination, suggesting pore formation.
There have been some reports on mammalian epithelial cell injuries by bacterial hemolysins, e.g., the alpha-toxin of S. aureus (45), streptolysin O of Streptococcus pyogenes (5), and the plasmid pAD1-encoded hemolysin-bacteriocin of Enterococcus faecalis (21). There are also precedent examples of pore-forming bacterial hemolysin-cytolysins as virulence factors producing lung injury. S. aureus alpha-toxin (40) and E. coli hemolysin (Hly) (9) produce thromboxane-mediated vasoconstriction and edema formation in the isolated and perfused rabbit lungs and thus are implicated in the development of septic lung failure. In addition, two RTX family hemolysins of the gram-negative bacterium Actinobacillus pleuropneumoniae were shown to be important virulence factors in production of hemorrhagic and necrotic lung infections in swine (44).
In the present study, we demonstrated that the hemolytic and cytotoxic activities mediated by the SphH were neutralized by rabbit immune serum against the SphH, representing the Leptospira hemolysin SphH as the primary mediator of cytotoxicity in vitro. Injury of mammalian cells by the SphH in vitro suggests a potential pathogenic role of this molecule in leptospirosis. Direct damage to host cell membranes could contribute to the hemorrhage or renal failure characteristic of Weil’s syndrome, a severe leptospirosis. Disruption of the epithelial cell barrier might also facilitate the organism to enter into the bloodstream easily and result in systemic spread.
In conclusion, our findings strongly suggest for the first time that the SphH is a novel Leptospira hemolysin identified as a pore-forming protein among the pathogenic Leptospira. Direct membrane damage of sheep erythrocytes and mammalian cells caused by the SphH in vitro indicated its potential role in pathogenesis of leptospirosis. Further studies are needed to confirm the pathogenic role of the Leptospira hemolysin SphH in an experimental animal model.
Acknowledgments
This work was supported by a grant from the Korea University Medical Science Research Center and the Brain Korea 21 Project.
Editor: J. D. Clements
REFERENCES
- 1.Alves, V. A., L. C. Gayotto, T. De Brito, R. T. Santos, A. Wakamatsu, M. R. Vianna, and E. E. Sakata. 1992. Leptospiral antigens in the liver of experimentally infected guinea pig and their relation to the morphogenesis of liver damage. Exp. Toxicol. Pathol. 44: 425–434. [DOI] [PubMed] [Google Scholar]
- 2.Bernheimer, A. W., and R. F. Bey. 1986. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect. Immun. 54: 262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi, S., N. Mackman, J. M. Nicaud, and I. B. Holland. 1986. Escherichia coli hemolysin may damage target cell membrane by generating transmembrane pores. Infect. Immun. 52: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi, S., and J. Tranum-Jensen. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55: 733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi, S., J. Tranum-Jensen, and A. Sziegoleit. 1985. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 47: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billson, F. M., C. Harbour, W. P. Michalski, J. M. Tennent, J. R. Egerton, and J. L. Hodgson. 2000. Characterization of hemolysin of Moraxella bovis using a hemolysis-neutralizing monoclonal antibody. Infect. Immun. 68: 3469–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Real, G., R. P. A. M. Segers, B. A. M. Van del Zeijst, and W. Gaastra. 1989. Cloning of a hemolysin gene from Leptospira interrogans serovar hardjo. Infect. Immun. 59: 2588–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrina, A., E. Nardon, E. Vecile, M. Cinco, and P. Patriarca. 1995. Leptospira icterohaemorrhagiae and leptospire peptidoglycans induce endothelial cell adhesiveness for polymorphonuclear leukocytes. Infect. Immun. 63: 2995–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermert, L., S. Rousseau, H. Schutte, R. G. Birkemeyer, F. Grimminger, S. Bhakdi, H. R. Duncker, and W. Seeger. 1992. Induction of severe vascular leakage by low doses of Escherichia coli hemolysin in perfused rabbit lungs. Lab. Investig. 66: 362–369. [PubMed] [Google Scholar]
- 10.Faine, S. 1994. Leptospira and leptospirosis, 1st ed. CRC press, Boca Raton, Fla.
- 11.Faine, S. 1991. Leptospirosis, p. 367–393. In A. S. Evans and P. S. Brachman (ed.), Bacterial infections of humans. Epidemiology and control, 2nd ed. Plenum Medical, New York, N.Y.
- 12.Farr, R. W. 1995. Leptospirosis. Clin. Infect. Dis. 21: 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Freer, J. H., and J. P. Arbuthnott. 1982. Toxins of Staphylococcus aureus. Pharmacol. Ther. 19: 55–106. [DOI] [PubMed] [Google Scholar]
- 14.Fussle, R., S. Bhakdi, A. Sziegoleit, J. Tranum-Jensen, T. Kranz, and H. J. Wellensiek. 1981. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J. Cell Biol. 91: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein, S. F., and N. W. Charon. 1990. Multiple exposure photographic analysis of motile spirochetes. Proc. Natl. Acad. Sci. USA 87: 4895–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68: 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertle, R., M. Hilger, S. W. Kocher, and I. Walev. 1999. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect. Immun. 67: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikigai, H., A. Akatsuka, H. Tsujiyama, T. Nakae, and T. Shimamura. 1996. Mechanism of membrane damage by El Tor hemolysin of Vibrio cholerae O1. Infect. Immun. 64: 2968–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isogai, E., H. Isogai, T. Kubota, N. Fujii, S. Hayashi, T. Incoh, S. Takagi, H. Miura, and K. Kimura. 1998. Apoptosis of lymphocytes in mice administered lipopolysaccharide from Leptospira interrogans. J. Vet. Med. B 45: 529–537. [DOI] [PubMed] [Google Scholar]
- 20.Isogai, E., H. Isogai, Y. Kurebayashi, and N. Ito. 1986. Biological activities of leptospiral lipopolysaccharide. Zentbl. Bakteriol. Mikrobiol. Hyg. 261: 53–64. [DOI] [PubMed] [Google Scholar]
- 21.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60: 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas, D., I. Walev, T. Berger, M. Liebetrau, M. Palmer, and S. Bhakdi. 1994. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 62: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasarov, L. B. 1970. Degradation of erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J. Med. Microbiol. 3: 29–37. [DOI] [PubMed] [Google Scholar]
- 24.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 61: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krug, E. L., and C. Kent. 1984. Phospholipase C from Clostridium perfringens: preparation and characterization of homogenous enzyme. Arch. Biochem. Biophys. 231: 400–410. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. H., K. A. Kim, Y. K. Kim, I. W. Seong, M. J. Kim, and Y. J. Lee. 2000. Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar lai. Gene 254: 19–28. [DOI] [PubMed] [Google Scholar]
- 28.Liu, P. V. 1974. Extracellular toxins of Pseudomonas aeruginosa. J. Infect. Dis. 130(Suppl.): S94–99. [DOI] [PubMed] [Google Scholar]
- 29.Mangel, A., J. M. Leitao, R. Batel, H. Zimmermann, W. E. G. Muller, and H. C. Schoder. 1992. Purification and characterization of a pore-forming protein from the marine sponge Tethya lyncurium. Eur. J. Biochem. 210: 499–507. [DOI] [PubMed] [Google Scholar]
- 30.Miller, N. G., J. E. Allen, and R. B. Wilson. 1974. The pathogenesis of hemorrhage in the lung of the hamster during acute leptospirosis. Med. Microbiol. Immunol. 160: 269–278. [DOI] [PubMed] [Google Scholar]
- 31.Nadia, K., and G. Nicole. 1995. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS. Microbiol. Lett. 134: 27–32. [DOI] [PubMed] [Google Scholar]
- 32.Noronha, F. S. M., F. J. Ramalho-Pinto, and M. F. Horta. 1996. Cytolytic activity in the genus Leishmania: involvement of a putative pore-forming protein. Infect. Immun. 64: 3975–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojcius, D. M., and J. D. E. Young. 1991. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem. Sci. 16: 225–229. [DOI] [PubMed] [Google Scholar]
- 34.Papadimitrious, J. C., C. B. Drachenberg, M. L. Shin, and B. F. Trump. 1994. Ultrastructural studies of complement mediated cell death: a biological reaction model to plasma membrane injury. Virchows Arch. 424: 677–685. [DOI] [PubMed] [Google Scholar]
- 35.Pinkney, M., E. Beachey, and M. Kehoe. 1989. The thio-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect. Immun. 57: 2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Projan, S. J., J. Kornblum, B. Kreiswirth, S. L. Moghazeh, W. Eiser, and R. P. Novick. 1989. Nucleotide sequence of the β-hemolysin gene of Staphylococcus aureus. Nucleic Acids Res. 17: 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe, G. E., and R. A. Welch. 1994. Assays of hemolytic toxins. Methods Enzymol. 235: 657–667. [DOI] [PubMed] [Google Scholar]
- 38.Scherrer, R., and P. Gerhardt. 1971. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 107: 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonherr, R., M. Hilger, S. Broer, R. Benz, and V. Braun. 1994. Interaction of Serratia marcescens hemolysin (ShlA) with artificial and erythrocyte membranes: demonstration of the formation of aqueous multistate channels. 223: 655–663. [DOI] [PubMed]
- 40.Seeger, W., R. G. Birkemeyer, L. Ermert, N. Suttorp, S. Bhakdi, and H. R. Duncker. 1990. Staphylococcal alpha-toxin-induced vascular leakage in isolated perfused rabbit lungs. Lab. Investig. 63: 341–349. [PubMed] [Google Scholar]
- 41.Segers, R. P., A. M. A van der Drift, A. De Nijs, P. Corcione, B. A. M. van der Zeijst, and W. Gaastra. 1990. Molecular analysis of sphingomyelinase C gene from Leptospira interrogans serovar hardjo. Infect. Immun. 58: 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamm, L. V., F. C. Gherardini, E. A. Parrish, and C. R. Moomaw. 1991. Heat shock response of spirochetes. Infect. Immun. 59: 1572–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189: 113–130. [DOI] [PubMed] [Google Scholar]
- 44.Tascon, R. I., J. A. Vazquez-Boland, C. B. Gutierrez-Martin, I. Rodriguez-Barbosa, and E. F. Rodriguez-Ferri. 1994. The RTX haemolysins ApxI and ApxII major virulence factors of the swine pathogen Actinobacillus pleuropneumoniae: evidence from mutation analysis. Mol. Microbiol. 14: 207–216. [DOI] [PubMed] [Google Scholar]
- 45.Thelestam, M., and L. Blomqvist. 1988. Staphylococcal alpha toxin—recent advances. Toxicon 26: 51–65. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, J. C. 1986. Morphological changes in red blood cells of calves caused by Leptospira interrogans serovar pomona. J. Comp. Pathol. 96: 512–527. [DOI] [PubMed] [Google Scholar]
- 47.Thompson, J. C., and B. W. Manktelow. 1986. Pathogenesis and red blood cell destruction in haemoglobinaemic leptospirosis. J. Comp. Pathol. 96: 529–540. [DOI] [PubMed] [Google Scholar]
- 48.Titball, R. W. 1993. Bacterial phospholipase C. Microbiol. Rev. 57: 347–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trowbridge, A. A., J. B. Green, J. D. Bonnet, S. B. Shohet, B. D. Ponnappa, and W. B. Mccombs. 1981. Hemolytic anemia associated with leptospirosis morphologic and lipid studies. Am. J. Clin. Pathol. 76: 493–498. [DOI] [PubMed] [Google Scholar]
- 50.Vinh, T., B. Adler, and S. Faine. 1986. Glycolipoprotein cytotoxin from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 13: 2111–2123. [DOI] [PubMed] [Google Scholar]