Abstract
Pneumolysin (PLY), an important virulence factor of Streptococcus pneumoniae, is known to exert various effects on the host immune cells, including cytokine induction, in addition to its known cytolytic activity as a member of the thiol-activated cytolysins. It is of interest to determine whether cytolytic activity is involved in triggering the cytokine production. In this study, we constructed full-length recombinant PLY and noncytolytic truncated PLYs with C-terminal deletions to examine the response of spleen cells to these PLY preparations. When cytolytic activity was blocked by treatment with cholesterol, full-length PLY was capable of inducing gamma interferon (IFN-γ) production. Truncated PLYs that originally exhibited no cytolytic activity were also active in IFN-γ induction. Therefore, the IFN-γ-inducing ability of PLY appeared to be independent of the cytolytic activity. Furthermore, IFN-γ-inducing preparations were also capable of inducing nitric oxide synthase expression and nitric oxide (NO) production, and the addition of neutralizing antibody to IFN-γ abolished the NO production. These results clearly demonstrated that PLY is capable of inducing IFN-γ production in spleen cells by a mechanism different from pore formation and that the induced IFN-γ stimulates NO production. These findings were discussed with reference to the contribution of PLY to the virulence of S. pneumoniae in vivo.
Streptococcus pneumoniae is an important human pathogen that causes life-threatening diseases such as pneumonia and meningitis, as well as less serious but very prevalent diseases such as otitis media and sinusitis. Interest has been focused on the virulence mechanism of this bacterium because of the numerous number of deaths associated with pneumococcal disease (13, 21), the emergence of antibiotic resistance (16, 29, 38, 44), and the problems in efficacy of current vaccines, especially for children younger than 2 years of age and immunocompromised patients (2, 13, 18, 21, 30).
Pneumolysin (PLY), produced by all clinical isolates of S. pneumoniae, is a 53-kDa protein toxin consisting of 471 amino acids (43). PLY has been regarded as an essential virulence factor of this bacterium (1, 7–10, 34, 35) and one of the candidates for vaccine development against pneumococcal infection (2, 25, 31). PLY is a pore-forming, cytolytic toxin and a member of the family of structurally related thiol-activated cytolysins (TACYs), including listeriolysin O (LLO), streptolysin O (SLO), and perfringolysin O (PFO), with a common characteristic that the cytolytic activity is abrogated by cholesterol and is dependent on the presence of highly conserved undecapeptide at the C termini (3, 11). In addition to the direct damage of host cells, PLY has been reported to affect various host responses. At sublytic concentrations, several studies have indicated the inhibition by PLY of respiratory burst in human phagocytes (27, 32) and lymphokine production (17), which were blocked by treatment with cholesterol. PLY is also shown to activate phospholipase A in endothelial cells of the pulmonary artery through the pore-forming mechanism (36). Recent studies have shown that PLY potentiates the proinflammatory activity of neutrophils by a pore-forming mechanism resulting in Ca2+ influx (14, 15).
In contrast, there are an accumulating number of reports showing that PLY is capable of stimulating host response, including complement activation (26, 33), cytokine production from human monocytes (20), and nitric oxide (NO) production from murine macrophages (12). Thus, PLY appears to exhibit dual aspects of biological activity; however, little is known about the relationship between cytolytic and stimulating activities.
Among family members of the TACYs, SLO (19, 37) and LLO (23, 40) have been reported to exert various effects on host cells leading to the modulation of inflammatory response. We have previously reported that LLO from Listeria monocytogenes is a potent inducer for cytokines in spleen cells (28). Among various cytokines induced by LLO, a striking level of gamma interferon (IFN-γ) production was observed, and we have shown that IFN-γ plays an important role especially for inducing protective immunity to L. monocytogenes (45). Induction of protective TH1 cells could be achieved by immunization of mice with an LLO-negative strain of L. monocytogenes, along with liposome-encapsulated LLO (41). In addition, we have also shown that the IFN-γ-inducing ability of LLO was not affected by cholesterol treatment, although the hemolytic activity of LLO was blocked (28). It was suggested that LLO could induce IFN-γ production in spleen cells by a mechanism different from cytolytic activity.
Based on the findings described above, there was a possibility that PLY exerts IFN-γ-inducing ability even in the absence of cytolytic activity like that of LLO. In the present study, we have constructed a full-length PLY and two C-terminal truncated forms of PLY to determine whether PLY is capable of inducing IFN-γ independent of pore-forming activity. By using recombinant PLY preparations, we have examined the ability to induce IFN-γ production and NO production that highly depends on the IFN-γ produced.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
S.pneumoniae IID553 (serotype 2), one of the standard strains of the Japanese Society for Bacteriology, was obtained from the Laboratory Culture Collection (Institute of Medical Science, University of Tokyo, Tokyo, Japan) and grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) at 37°C for preparation of chromosomal DNA. Escherichia coli M15 (Qiagen, Hilden, Germany) harboring pREP4 plasmid, which contains lacI and kanamycin-resistant genes, was used as a host cell. Expression vector pQE-31 (Qiagen), which is designed to place a tag of six histidines (His6) at the N terminus of the protein of interest, was used. In order to express the recombinant protein, E. coli harboring recombinant plasmid was grown in tryptic soy broth (Difco) containing 100 μ g of ampicillin and 25 μ g of kanamycin/ml.
Production and purification of recombinant PLYs.
The full-length form of PLY, PLY471, consisting of 471 amino acids, and two truncated forms of PLY, PLY437 and PLY426, with deletion of C-terminal 34 and 45 amino acids including undecapeptide, respectively, were prepared by the procedure described previously (4). Briefly, chromosomal DNA extracted from S. pneumoniae IID553 was used as a template in PCR. The gene encoding full-length or truncated PLYs was amplified by PCR with the specific primer sets designed according to the sequence of the ply gene (43). The primers contained modifications to add appropriate restriction enzyme sites for insertion into the vector: a BamHI site in the forward primer and a KpnI site in the reverse primer. The sequences of all PCR products were confirmed by DNA sequencing with an ABI PRISM310 Genetic Analyzer (PE Applied Biosystems, Foster City, Calif.). Both PCR product and pQE-31 expression vector were cut with BamHI and KpnI enzymes (New England Biolabs, Beverly, Mass.) and ligated by using T4 ligase (New England Biolabs). The recombinant plasmid was transformed into E. coli M15 by electroporation by using the Gene Pulser II Electroporation System (Bio-Rad Laboratories, Hercules, Calif.). Transformants were cultured in tryptic soy broth (Difco) containing ampicillin and kanamycin, and isopropyl-β-d-thiogalactopyranoside (IPTG) was added to induce the expression of His-tagged recombinant protein at the middle of the log phase of the culture. The cells were harvested by centrifugation at 4°C after an appropriate period of the induction, resuspended in phosphate buffer (50 mM Na2HPO4, pH 8.0; 300 mM NaCl) containing 20 mM imidazole, and disrupted by homogenization with zirconia-silica beads (BioSpec Products, Inc., Bartlesville, Okla.) after treatment with 1 mg of lysozyme/ml on ice. Lysate was centrifuged at 10,000 × g for 30 min at 4°C. His-tagged recombinant protein was purified from the supernatant with an Ni-nitrilotriacetic acid (NTA) column (Qiagen) under native conditions according to the manufacturer’s instructions. Purified recombinant protein was desalted by a passage through a PD-10 column of Sephadex G-25 M (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Contaminating lipopolysaccharide (LPS) was extensively removed by using Detoxi-Gel Endotoxin Removing Gel (Pierce Chemical Company, Rockford, Ill.). The level of LPS was determined by Limulus Color KY Test (Wako Pure Chemical Industries, Osaka, Japan). The purity was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting by using anti-His-tag monoclonal antibody (Penta-His Antibody; Qiagen).
Cell culture.
C3H/HeN mice (SLC Japan, Hamamatsu, Japan) were used for experiments at 7 to 9 weeks of age. Normal spleen cells were aseptically removed from mice and teased between two sterile glass slides. After erythrocytes were lysed by treatment with 0.83% ammonium chloride in 0.17 mM Tris-HCl (pH 7.6), spleen cells were washed three times with Hanks balanced salt solution and suspended in RPMI 1640 medium (Gibco-BRL/Life Technologies, Inc., Rockville, Md.) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Gibco) and 5 μ g of gentamicin/ml. The cells were plated at 2.5 × 106 per well in a 48-well flat-bottomed tissue culture plate and stimulated with various concentrations of recombinant PLYs at 37°C in 5% CO2 for 24 or 48 h. The culture supernatant was collected by centrifugation for the measurement of the level of IFN-γ or NO produced from spleen cells. To determine the participation of IFN-γ in NO production, spleen cells were stimulated with recombinant PLYs in the presence of 2 μ g of rat anti-mouse IFN-γ monoclonal antibody (R&D Systems, Inc., Minneapolis, Minn.)/ml for 48 h, and NO production from spleen cells was measured.
IFN-γ and NO assay.
The IFN-γ level was measured by enzyme immunoassay (EIA) as follows. The wells of an EIA plate (Nalge Nunc International, Rochester, N.Y.) were precoated with rat anti-mouse IFN-γ antibody (Endogen, Inc., Woburn, Mass.) diluted at 1 μ g/ml with coating buffer (0.03 M sodium carbonate, 0.068 M sodium bicarbonate; pH 9.6). After incubation overnight at 4°C, the plate was washed with distilled water and then treated with 25% Block Ace (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) for 60 min to inhibit nonspecific binding. After a wash with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-Tween), 50 μ l of the test supernatants or standard murine IFN-γ was added to each well. After incubation for 60 min at room temperature, 50 μ l of 0.5 μ g of biotin-conjugated anti-mouse IFN-γ monoclonal antibody (Endogen)/ml was added to each well without washing, and the plate was incubated further for 60 min. After a wash with PBS-Tween, 0.125 μ g of horseradish peroxidase-conjugated streptavidin (Endogen)/ml was added. After incubation for 30 min, wells were washed, and then 100 μ l of 3,3′,5,5′-tetramethylbenzidine dihydrochloride dihydrate (TMB) (50 μ g/ml) in phosphate-citrate buffer (pH 5.0) containing 0.01% H2O2 was added as a substrate solution. The absorbance was measured at 450 nm after termination of the reaction with 100 μ l of 0.18 M H2SO4. For quantification of NO production, the concentration of nitrite in the culture supernatant was measured by Griess reaction by using modified Griess reagent (Sigma-Aldrich, Inc., St. Louis, Mo.). A total of 100 μ l of Griess reagent was mixed with an equal volume of the test supernatant or standard in a 96-well flat-bottom plate. After incubation at room temperature for 15 min, the absorbance was measured at 540 nm. Nitrite was quantitated with sodium nitrite as a standard.
Cytotoxicity assay.
The cytotoxic activity of recombinant PLYs for spleen cells was determined by measuring the level of lactate dehydrogenase (LDH) in culture supernatant released from spleen cells. Cells were plated at 2.5 × 106 per well in a 48-well flat-bottom tissue culture plate and incubated in the presence of various doses of recombinant PLYs for 6 h. The culture supernatant was collected, and the level of LDH was measured by using an LDH Cytotoxicity Detection Kit (Takara Shuzo Co., Ltd., Otsu, Japan) according to the manufacturer’s instructions. Samples treated with 1% Triton X-100 or those with culture medium alone were defined as positive (100% LDH release) and negative (0% LDH release) controls, respectively, and then the relative LDH release (%) for each well was calculated.
RT-PCR.
Spleen cells were plated at 5.0 × 106 per well in a 24-well flat-bottom tissue culture plate and stimulated with recombinant PLYs at 37°C in 5% CO2 for 12 h. Total cellular RNA was extracted by RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions, and then cDNA was produced by reverse transcription (RT) as follows. The total RNA extracted (100 ng) was mixed with 10 μ l of 5× RT buffer, 5 μ l of 100 mM dithiothreitol, 1.25 μ l of RNasin (Promega, Madison, Wis.), 2.5 μ l of 10 mM deoxynucleoside triphosphates (dNTPs; Amersham), 1.75 μ l of random primer (Gibco), 1.25 μ l of reverse transcriptase (SuperScript II RNase H− Reverse Transcriptase; Gibco), and distilled water to give a total volume of 50 μ l. The RT reaction was carried out by four steps consisting of 42°C for 1 min, 30°C for 10 min, 42°C for 25 min, and 94°C for 3 min for inactivation. The samples were kept at −20°C until used. The PCR mixture consisted of 2 μ l of sample cDNA, 2 μ l of 10× PCR buffer, and 0.8 μ l of 25 mM Mg2SO4, 2 μ l of 2 mM dNTPs, 0.3 μ l of 20 μ M forward primer, 0.3 μ l of 20 μ M reverse primer, 0.4 μ l of DNA polymerase (KOD-Plus; Toyobo, Osaka, Japan), and distilled water to give a total volume of 20 μ l. The sequences of the oligonucleotide primers used were as follows: 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ for inducible nitric oxide synthase (iNOS) and 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ for β-actin. The predicted sizes of the amplified products for iNOS and β-actin were 497 and 349 bp, respectively. PCR amplification was performed by a thermal cycler (GeneAmp PCR System 9700; PE Applied Biosystems). The PCR program consisted of one cycle of 94°C for 15 s, 55°C for 30 s, and 68°C for 60 s. Samples were amplified for 31 cycles for iNOS and 23 cycles for β-actin, followed by incubation at 68°C for 7 min, and the products were kept at 4°C in the cycler. Analysis of the PCR products was performed by agarose gel electrophoresis with a 2% low-melting-point agarose gel in 0.5× Tris-acetate-EDTA buffer. PCR products were electrophoresed on 2% agarose gel and the gel was stained with 0.005% ethidium bromide. The bands were visualized by a UV transilluminator.
RESULTS
IFN-γ-inducing ability and cytotoxicity of full-length PLY.
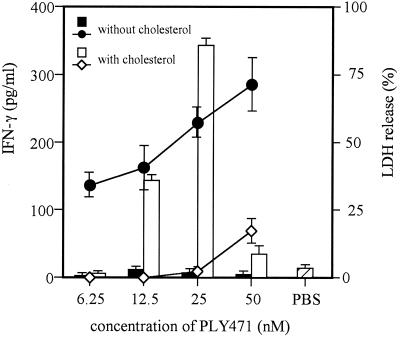
Normal spleen cells were stimulated for 24 h with full-length PLY (PLY471) that had been treated with or without cholesterol, and then the level of IFN-γ in the culture supernatant was assayed by EIA. We also measured the level of LDH in culture supernatant released from spleen cells to determine the cytotoxicity. According to the results of preliminary experiments, we determined the LDH release 6 h after the addition of PLY because the LDH level at this time points represented the real cytotoxicity without high background due to spontaneous release. In the range of 6.25 to 50 nM, full-length PLY471 exhibited cytotoxicity to spleen cells if not treated with cholesterol. IFN-γ production was not induced at all by various concentrations of cytolytic PLY (Fig. 1). Though there are several reports showing that the sublytic concentration of PLY induced inflammatory cytokines from human mononuclear phagocytes (20) and superoxide, elastase, prostaglandin E2, and leukotriene B4 from human neutrophils (14, 15), our preparation of PLY471 did not induce IFN-γ production even at much lower, sublytic concentrations (data not shown). In contrast, when PLY471 was treated with 10 μ g of cholesterol/ml to inhibit its cytolytic activity, it became capable of inducing IFN-γ production at concentrations of 12.5 and 25 nM, a dose range that never causes LDH release from the cells. At higher concentrations, the level of IFN-γ production became lower probably because of the incomplete blockade of cytotoxicity due to the limit in the amount of cholesterol solubilized for pretreatment (Fig. 1). These data implied that PLY471 could induce IFN-γ production by a mechanism different from cytolytic, pore-forming activity, and the cytotoxicity of PLY471 seemed to interfere with IFN-γ-inducing ability.
FIG. 1.
IFN-γ-inducing ability and cytotoxicity of full-length PLY with or without cholesterol treatment. To determine IFN-γ-inducing ability, normal spleen cells were stimulated for 24 h with PLY471 (full-length PLY) that had been treated with or without 10 μ g of cholesterol/ml and the level of IFN-γ in the culture supernatant was assayed by EIA. To determine cytotoxicity for spleen cells, cells were incubated with the same PLY471 preparations for 6 h, and the level of LDH released in the culture supernatant from the cells was measured. Each column represents the level of IFN-γ induced by PLY471 with (open column) or without (closed column) cholesterol, and each point connected by a line represents the cytotoxicity of cholesterol-treated (open squares) or nontreated (closed circles) PLY471. Data are shown as the mean of triplicate determinations ± the standard deviation (SD). The result shown here is the representative of three similar experiments.
IFN-γ-inducing ability and cytotoxicity of truncated PLYs.
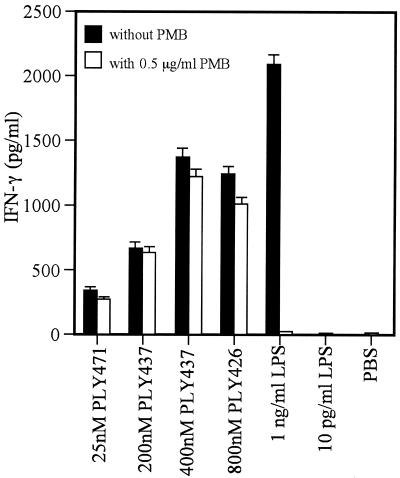
In our previous report, we constructed truncated PLYs with the deletions of C-terminal 34 amino acids (PLY437) and 45 amino acids (PLY426), neither of which showed the ability to bind to membrane cholesterol or hemolytic activity (4). In order to confirm that PLY is able to induce IFN-γ production independently of cytolytic, pore-forming activity, we examined the IFN-γ-inducing ability of these nonhemolytic recombinant PLYs with C-terminal truncations. We examined first whether both of these truncated PLYs had no cytotoxicity against spleen cells as well as erythrocytes. As shown in Fig. 2, two truncated recombinant PLYs exhibited no cytotoxicity at all against spleen cells even at higher concentrations. After confirmation of the absence of cytotoxicity, the IFN-γ-inducing ability of these noncytolytic preparations, PLY437 and PLY426, was examined. PLY437 was capable of inducing IFN-γ production at doses of >200 nM (Fig. 2A), although the dose required was higher than that for cholesterol-treated PLY471. PLY426 was also able to induce IFN-γ production at a much higher concentration, 800 nM (Fig. 2B). In order to rule out the possibility that IFN-γ production depends on contaminating LPS, recombinant proteins were passed extensively through an LPS-removing column. The amount of LPS in the working concentration of PLY preparations was less than 10 pg/ml. As shown in Fig. 3, IFN-γ production was not induced in the spleen cells by 10 pg of exogenously added LPS/ml. In addition, the IFN-γ-inducing ability of recombinant PLYs was not affected by polymyxin B, whereas the response to 1 ng of LPS/ml was completely inhibited by the same treatment, indicating that the possible involvement of LPS could be ruled out. These results indicated that IFN-γ-inducing ability of recombinant PLY was not associated with cytolytic, pore-forming activity and not due to the contaminating LPS.
FIG. 2.
IFN-γ-inducing ability and cytotoxicity of truncated PLYs with C-terminal deletions. Normal spleen cells were stimulated with PLY437 (A) and PLY426 (B). The levels of IFN-γ (column) and LDH release (points connected by a line) in the culture supernatant were measured after 24 and 6 h, respectively. Data are shown as the mean of triplicate determinations ± the SD. The result shown here is the representative of three similar experiments.
FIG. 3.
Effect of polymyxin B (PMB) on IFN-γ production induced by recombinant PLYs. Normal spleen cells were stimulated with recombinant PLYs or LPS for 24 h in the presence (open column) or absence (closed column) of 5 μ g of polymyxin B/ml. The culture supernatant was collected, and the IFN-γ was measured by EIA. Data are shown as the mean of triplicate determinations ± the SD. The same experiment was repeated twice, and a similar result was obtained.
Expression of iNOS in spleen cells stimulated with recombinant PLYs.
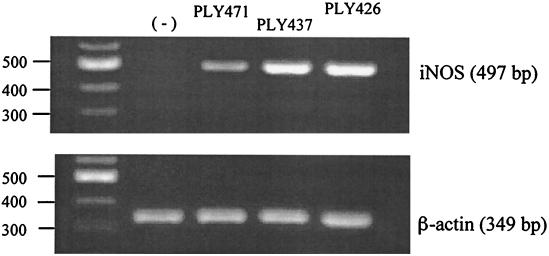
IFN-γ is highly capable of inducing iNOS expression and NO production in macrophages (22) and spleen cells (46). There is a report showing that PLY induced iNOS expression and NO production in murine macrophages through a pathway dependent on IFN-γ signaling (12). Based on these reports, we examined the induction of iNOS by the recombinant PLYs that showed IFN-γ-inducing ability but no pore-forming activity. Normal spleen cells were stimulated with cholesterol-treated PLY471 (25 nM), PLY437 (400 nM), and PLY426 (800 nM) at the concentrations effective for inducing IFN-γ production. After 24 h of stimulation, total RNA was extracted from the cells and subjected to RT-PCR for amplification of iNOS mRNA. A significant level of iNOS expression was observed in the spleen cells after stimulation with all three recombinant preparations (Fig. 4).
FIG. 4.
Expression of iNOS mRNA in the spleen cells stimulated with recombinant PLYs. Normal spleen cells were stimulated for 24 h with PLY471 (25 nM), PLY437 (400 nM), or PLY426 (800 nM), at the concentration optimal for IFN-γ induction for each recombinant protein. Total RNA was extracted and subjected to RT-PCR. PCR products obtained by using specific primer sets for iNOS and β-actin were electrophoresed. The predicted sizes of PCR products for iNOS and β-actin are indicated. Bands in the left lane indicate the positions of DNA size markers. The same experiment was repeated twice, and a similar result was obtained.
NO production by spleen cells stimulated with recombinant PLYs.
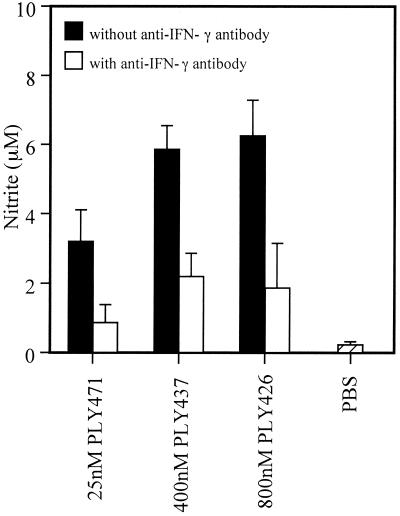
We next measured the actual production of NO in the culture supernatant of spleen cells. Spleen cells were stimulated with the same preparations used for RT-PCR for 48 h and then the level of nitrite, the end product of NO, was assayed by Griess reaction. As shown in Fig. 5, nitrite production was observed in the culture after stimulation with recombinant PLY preparations that could induce IFN-γ production. NO production was not observed at all by stimulation with 0.01 to 50 nM of PLY471 when not pretreated with cholesterol (data not shown). The amount of nitrite production appeared to depend on the level of IFN-γ induced. To determine the involvement of PLY-induced IFN-γ in NO production, the effect of neutralization of IFN-γ was examined. PLY-induced nitrite production was inhibited significantly by the addition of anti-mouse IFN-γ antibody to the cell culture (Fig. 5). These results showed that PLY was capable of inducing NO production by means of the induced IFN-γ.
FIG. 5.
NO production by spleen cells after stimulation with recombinant PLYs. Normal spleen cells were stimulated with recombinant PLYs for 48 h in the presence or absence of anti-mouse IFN-γ antibody. The culture supernatant was collected and nitrite was quantified by Griess reaction. Data are shown as the mean of triplicate determinations ± the SD. The result shown here is representative of three similar experiments.
DISCUSSION
PLY has been proved to be an important virulence factor of S. pneumoniae from in vivo experiments with isogenic mutant strains of the ply gene (1, 6–10, 34, 35). The cytolytic activity and complement-activating ability of PLY are believed to contribute to virulence especially in the early stage of infection (1, 6–8, 34). However, several studies suggested the involvement of some properties of PLY other than cytolytic and complement-activating activities in virulence (1, 6, 8).
In the present study, we showed that PLY471, a full-length form of recombinant PLY, was capable of inducing IFN-γ production in spleen cells when it was treated with cholesterol for the abolishment of pore-forming activity (Fig. 1). However, at the concentration of >25 nM, at which cytotoxicity was not completely blocked by cholesterol due to the limit in the solubility of cholesterol, the level of IFN-γ production became lower and the IFN-γ-inducing ability was not observed at all in the cytotoxic PLY471 without cholesterol treatment. These results implied that the IFN-γ-inducing mechanism was different from that in the pore formation and that the cytotoxicity of PLY471 seemed to interfere with the IFN-γ-inducing ability.
A much higher level of IFN-γ production was induced by PLY437 and PLY426, truncated forms of PLY that were confirmed to have neither cholesterol-binding nor pore-forming activity against not only erythrocytes (4) but also spleen cells (Fig. 2), although a higher concentration was needed than for PLY471 (Fig. 2). From these results, it can be concluded that IFN-γ-inducing ability was independent of cholesterol-binding and pore-forming activities.
The reason is not clear why the higher concentration was required to induce IFN-γ in truncated PLYs. In a study of recombinant PFO, it was reported that C-terminal truncation caused a disruptive change of overall molecular structure (39). Therefore, it is plausible that truncation of PLY affected the molecular structure effective for IFN-γ induction in addition to abolishment of cytolytic activity. In comparing two truncated recombinants, a higher dose of PLY426 was required than of PLY437 for optimal IFN-γ induction. If we take into consideration that PLY426 is shorter than PLY437 by 11 amino acids, it is likely that the molecular structure is critically involved in the IFN-γ induction. In any case, the structural requirement for cytokine induction should be determined in detail.
We sought to rule out the possible involvement of LPS contamination in the E. coli-derived samples. In the working concentrations of PLY, the amount of contaminating LPS determined by Limulus assay never exceeded 10 pg/ml, and IFN-γ production was not induced in the spleen cells by addition of 10 pg of LPS derived from E. coli/ml (Fig. 3). In addition, treatment of our preparations with 0.5 μ g of polymyxin B/ml never affected the activity, whereas the response to LPS was completely inhibited by the same treatment (Fig. 3). From these observations, the results presented here can be regarded as the activity of recombinant protein itself and not an artifact by contaminating LPS.
We could also observe iNOS gene expression (Fig. 4) and NO production (Fig. 5) in the spleen cells stimulated with the PLY preparations that showed IFN-γ-inducing ability. NO production was not seen in the condition where no IFN-γ was induced. In addition, the NO production induced by IFN-γ-inducing PLY preparations was inhibited by the addition of anti-mouse IFN-γ antibody (Fig. 5). These results indicated that PLY was capable of inducing NO production through the induction of IFN-γ production.
IFN-γ has been reported to induce iNOS expression and NO production in macrophages (22) and spleen cells (46). Recently, Braun et al. reported that PLY could induce NO production from murine macrophages at a range of 5 to 750 nM and suggested that the NO production depended on the IFN-γ signaling pathway by using mice lacking IFN-γ receptor or IFN regulatory factor 1 (12). Their findings are consistent with our present results showing that a high level of IFN-γ production was actually induced by PLY, resulting in NO production. In their report, however, nothing was mentioned about the dissociation between cytolytic activity and NO-inducing ability.
The most important finding in our study is that even truncated PLY without cytolytic activity was capable of inducing NO production. NO seems now to contribute to septic shock caused by gram-positive bacteria resulting in hypotension, vascular hyporeactivity to vasoconstrictors, and multiple organ failure (24, 42). There are reports on the virulence of an S. pneumoniae mutant in which various point mutations were introduced in the ply gene (1, 6–8, 34). In these reports, it was suggested that PLY must have additional properties for virulence expression that were not abolished by the point mutations abrogating cytolytic activity (1, 6, 8). Our finding of the IFN-γ-inducing, NO-inducing activity of noncytolytic truncated PLY may explain their findings regarding the dissociation between cytolytic activity and virulence expression in vivo.
Although NO was not measured, Benton et al. reported that IFN-γ was detected in the plasma from mice infected with S. pneumoniae at a septic state just prior to death but not in mice infected with the ply-negative mutant strain (5). Therefore, there is a possibility that the IFN-γ- and NO-inducing abilities of PLY presented here may contribute to the virulence of S. pneumoniae in the late stage near death in which a large number of this bacteria exist in circulating blood.
In conclusion, we demonstrated clearly that PLY is capable of inducing IFN-γ production in spleen cells by a mechanism different from pore formation and that the induced IFN-γ leads to NO production that might contribute to the virulence of S. pneumoniae in a septic state.
Acknowledgments
This study was supported by the “Research for the Future” Program from The Japan Society for the Promotion of Science; a Grant-in-Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science, and Technology; and a grant from The Ministry of Health, Labor, and Welfare of Japan.
Editor: E. I. Tuomanen
REFERENCES
- 1.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24: 167–174 [DOI] [PubMed] [Google Scholar]
- 2.AlonsoDeVelasco, E., A. F. M. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59: 591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alouf, J. E. 1999. Introduction to the family of the structurally related cholesterol-binding cytolysins (‘sulfhydryl-activated’ toxins), p. 443–456. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, England
- 4.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2001. Essential role of domain 4 of pneumolysin from Streptococcus pneumoniae in cytolytic activity as determined by truncated proteins. Biochem. Biophys. Res. Commun. 281: 37–44 [DOI] [PubMed] [Google Scholar]
- 5.Benton, K. A., M. P. Everson, and D. E. Briles. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23: 201–209 [DOI] [PubMed] [Google Scholar]
- 7.Berry, A. M., J. E. Alexander, T. J. Mitchell, P. W. Andrew, D. Hansman, and J. C. Paton. 1995. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect. Immun. 63: 1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67: 981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57: 2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182: 197–205 [DOI] [PubMed] [Google Scholar]
- 12.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67: 3750–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46(RR-8): 1–24 [Google Scholar]
- 14.Cockeran, R., H. C. Steel, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Pneumolysin potentiates production of prostaglandin E2 and leukotriene B4 by human neutrophils. Infect. Immun. 69: 3494–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183: 604–611 [DOI] [PubMed] [Google Scholar]
- 16.Doern, G. V., A. B. Brueggemann, H. Huynh, E. Wingert, and P. Rhomberg. 1999. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98. Emerg. Infect. Dis. 5: 757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrante, A., B. Rowan-Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46: 585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French, N., J. Nakiyingi, L. M. Carpenter, E. Lugada, C. Watera, K. Moi, M. Moore, D. Antvelink, D. Mulder, E. N. Janoff, J. Whitworth, and C. F. Gilks. 2000. 23-Valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355: 2106–2111 [DOI] [PubMed] [Google Scholar]
- 19.Hackett, S. P., and D. L. Stevens. 1992. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J. Infect. Dis. 165: 879–885 [DOI] [PubMed] [Google Scholar]
- 20.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62: 1501–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65: 187–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, T. W. Mak, T. Taniguchi, and J. Vilcek. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263: 1612–1615 [DOI] [PubMed] [Google Scholar]
- 23.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31: 1709–1722 [DOI] [PubMed] [Google Scholar]
- 24.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188: 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell, T. J. 1999. Pneumolysin: structure, function and role in disease, p. 476–495. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, England
- 26.Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Boulnois. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol. 5: 1883–1888 [DOI] [PubMed] [Google Scholar]
- 27.Nandoskar, M., A. Ferrante, E. J. Bates, N. Hurst, and J. C. Paton. 1986. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology 59: 515–520 [PMC free article] [PubMed] [Google Scholar]
- 28.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect. Immun. 64: 3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399: 590–593 [DOI] [PubMed] [Google Scholar]
- 30.Örtqvist, Å., J. Hedlund, L. Å. Burman, E. Elbel, M. Höfer, M. Leinonen, I. Lindblad, B. Sundelöf, and M. Kalin. 1998. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 351: 399–403 [DOI] [PubMed] [Google Scholar]
- 31.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4: 103–106 [DOI] [PubMed] [Google Scholar]
- 32.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41: 1212–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43: 1085–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubins, J. B., D. Charboneau, C. Fasching, A. M. Berry, J. C. Paton, J. E. Alexander, P. W. Andrew, T. J. Mitchell, and E. N. Janoff. 1996. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 153: 1339–1346 [DOI] [PubMed] [Google Scholar]
- 35.Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubins, J. B., T. J. Mitchell, P. W. Andrew, and D. E. Niewoehner. 1994. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect. Immun. 62: 3829–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27: 337–346 [DOI] [PubMed] [Google Scholar]
- 38.Schrag, S. J., B. Beall, and S. F. Dowell. 2000. Limiting the spread of resistant pneumococci: biological and epidemiologic evidence for the effectiveness of alternative interventions. Clin. Microbiol. Rev. 13: 588–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada, Y., M. Nakamura, Y. Naito, K. Nomura, and Y. Ohno-Iwashita. 1999. C-terminal amino acid residues are required for the folding and cholesterol binding property of perfringolysin O, a pore-forming cytolysin. J. Biol. Chem. 274: 18536–18542 [DOI] [PubMed] [Google Scholar]
- 40.Sibelius, U., F. Rose, T. Chakraborty, A. Darji, J. Wehland, S. Weiss, W. Seeger, and F. Grimminger. 1996. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect. Immun. 64: 674–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanabe, Y., H. Xiong, T. Nomura, M. Arakawa, and M. Mitsuyama. 1999. Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O. Infect. Immun. 67: 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiemermann, C. 1997. Nitric oxide and septic shock. Gen. Pharmacol. 29: 159–166 [DOI] [PubMed] [Google Scholar]
- 43.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55: 1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 45.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Requirement of initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect. Immun. 65: 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, J., I. Kawamura, H. Zhu, and M. Mitsuyama. 1995. Involvement of natural killer cells in nitric oxide production by spleen cells after stimulation with Mycobacterium bovis BCG. Study of the mechanism of the different abilities of viable and killed BCG. J. Immunol. 155: 5728–5735 [PubMed] [Google Scholar]