Abstract
The BrkA protein of Bordetella pertussis inhibits killing by the antibody-dependent classical pathway of complement; however, susceptibility to complement can be highly variable. Log-phase bacteria grown in Stainer-Scholte (SS) broth plated on Bordet-Gengou (BG) agar were about 500 times more sensitive to killing by complement than stationary-phase SS-BG cultures. While always more susceptible to complement than the wild-type strain, a BrkA mutant displayed a similar growth phase variation in susceptibility to complement. Growth phase susceptibility to complement was also observed for a mutant constitutive for Bvg activation of BrkA, suggesting that modulation of virulence factor expression was not responsible for sensitivity to complement. Susceptibility was not due to differential antigenic expression, since serum adsorbed with complement-resistant, stationary-phase SS-BG cultures lacked bactericidal activity against B. pertussis harvested at all times during the growth cycle. These results suggest that log-phase susceptibility to complement is not due to variable expression of BrkA or antigenic differences and may be an inherent property of rapidly growing cultures. Implications for vaccine development are discussed.
Bordetella pertussis is the causative agent of whooping cough in humans. The BrkA protein of B. pertussis confers resistance to killing by the classical pathway of complement (3, 10, 11). Virulence factors of B. pertussis, including BrkA, are regulated by the two-component global regulatory system, BvgA and BvgS, reviewed by Akerley and Miller (1). Under permissive conditions B. pertussis expresses Bvg-regulated virulence factors, while in the presence of high concentrations of sulfate ions or nicotinic acid, transcription of the Bvg-regulated genes is shut down, a condition termed modulation (13). While the presence of BrkA confers added resistance to killing by complement, we have observed that susceptibility to complement can be highly variable depending on the growth conditions. In this study we set out to determine what factors, in addition to BrkA, influence susceptibility to complement.
B. pertussis is very fastidious and can be difficult to culture, particularly in broth. In previous studies we used bacteria harvested directly from Bordet-Gengou (BG) agar (3, 10, 16, 18). However, plate-grown bacteria cannot be assigned to a specific growth stage. We have recently adopted a technique in which the bacteria are grown in Stainer-Scholte (SS) broth plated on agar (SS-BG) to overcome these issues (7, 8).
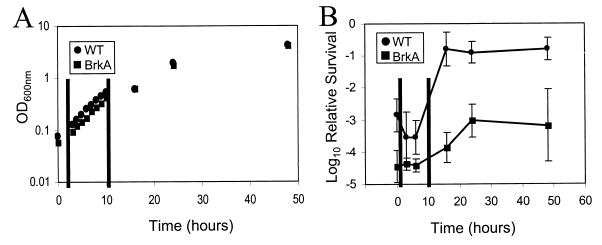
A wild-type complement-resistant strain, BP338 (17), and a BrkA complement-sensitive derivative, RFBP2152 (11), were harvested after 2 days’ growth on BG agar plates to an optical density (OD) at 600 nm of 0.1 in warm (37°C) SS broth, and 7 ml was plated onto BG agar as previously described (7). Nalidixic acid (30 μg/ml) was added to media for both strains, and gentamicin (30 μg/ml) was added to medium for RFBP2152. The broth cultures were harvested at the indicated times. The bacteria were pelleted by centrifugation and suspended in an equivalent volume of broth, and bacterial growth was determined by OD (Fig. 1A). Growth of the wild type and growth of the BrkA mutant were similar during the 48 h of these experiments. Logarithmic growth occurred during the first 10 h and tapered off.
FIG. 1.
Susceptibility of B. pertussis to complement as a function of growth phase. B. pertussis was grown for 2 days on BG agar, harvested at an OD at 600 nm (OD600nm) of 0.1 in SS broth, and grown in 7 ml of SS on BG agar for the indicated length of time. Abbreviations: WT, wild type (BP338); BrkA, BrkA mutant (RFBP2152). (A) The graph represents the average OD at 600 nm of at least three experiments. Vertical lines represent the approximate boundaries of the log-phase growth. (B) Serum killing assays were performed with 10% human serum for 1 h. Results are presented as mean log10 of survival relative to control experiments with heat-inactivated serum ± standard error (error bars) of at least six separate experiments.
Growth phase and susceptibility to complement.
Complement killing assays were performed as previously described (3). Briefly, bacteria were harvested at the indicated times and diluted to an OD at 600 nm of 0.005 (about 107 bacteria per ml) in warm SS broth. Ten microliters of bacteria and two microliters of serum were brought to 20 μl with SS broth in a 96-well U-bottom plate. Samples were incubated at 37°C with shaking at 150 rpm for 1 h. Phosphate-buffered saline with 10 mM EDTA was added to inhibit complement killing, and serial dilutions were plated onto BG agar. Heat-inactivated serum, which does not kill B. pertussis (10), was used to determine total bacterial numbers, and relative survival was calculated as follows: (CFU of bacteria treated with test serum)/(CFU of bacteria treated with heat-inactivated serum). Statistical analysis of at least three separate experiments was performed using Student’s t test.
Susceptibility of B. pertussis to complement was examined at 0, 3, 6, 16, 24, and 48 h (Fig. 1B). The zero hour time point represents bacteria harvested directly from BG agar plates under conditions similar to those used in previous studies that showed that BrkA is essential for complement resistance (3, 10, 11). Susceptibility of the wild-type strain was highly variable at different time points; however, the wild-type strain was more complement resistant than the BrkA mutant at all time points. At time zero, a large number of wild-type bacteria were killed; however, killing of the BrkA mutant was 20 times greater (P < 0.0002; n = 7). At 6 h, during log-phase growth, about five times more wild-type BP338 was killed than at 0 h (Fig. 1B [WT, 0 and 6 h]) (P < 0.04; n = 7). By 16 h, as BP338 was exiting log phase, resistance increased and about 500 times more B. pertussis survived relative to that at 6 h, and 100 times more survived relative to that at time zero (Fig. 1B [WT, 0 and 16 h]) (P < 0.00003; n = 7). Between 0 and 24 h, RFBP2152 also displayed increased serum resistance, with about 30-fold-greater survival at 24 h (Fig. 1B [BrkA, 0 and 24 h]) (P < 0.0003; n = 7).
Role of modulation in susceptibility to complement.
Virulence factors of B. pertussis, including BrkA, are regulated by the two-component BvgA/S system (1). Certain environmental signals can down regulate expression of the virulence factors, a process called modulation (13). To determine whether modulation of BrkA expression could influence susceptibility to complement, BrkA expression was examined by Western blotting, as previously described (3). BrkA was detected in the wild type grown under permissive conditions at all time points, although a high degree of variability of expression of BrkA was observed, with less expression observed during the first hours in culture. When the bacteria were preincubated under modulating conditions and then transferred to SS-BG, BrkA expression was not detected until 7 h in culture. Since the levels of BrkA expression were variable, these studies did not rule out a role for modulation in growth phase susceptibility to complement.
To further investigate the contribution of modulation to the changes in complement resistance, a mutation in bvgS (mutation of nucleic acid G to A at position 2557) that confers a BvgS constitutive (virulent) phenotype (14) was introduced into the wild-type strain, using plasmid pJM503 to create strain BPCV. Plasmid pJM503 (14) was introduced into BP338 by conjugation, and gentamicin-resistant, nonhemolytic, single-crossover mutants were selected. The resulting bvgS knockout was passaged on BG agar without gentamicin three times to allow growth of gentamicin-sensitive double-crossover mutants lacking the plasmid. The bacteria were incubated with 5% serum for 5 min at 37°C to enrich for isolates that regained expression of virulence factors, specifically BrkA. Constitutive mutants were identified based on regaining hemolytic ability on modulating BG agar and loss of resistance to gentamicin.
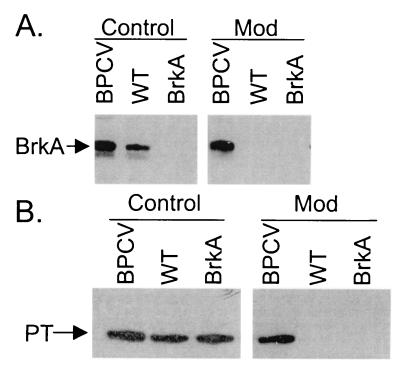
To confirm the constitutive virulence phenotype, expression of BrkA and pertussis toxin subunit S1, another Bvg-regulated virulence factor, under modulating conditions was examined by Western blotting. The wild type (BP338) and BPCV each express BrkA when grown on BG agar (Fig. 2A, Control). However, only BPCV expressed BrkA when grown in the presence of modulating levels of sulfate ion (Fig. 2A, Mod). RFBP2152, the brkA mutant, did not express BrkA under any condition (Fig. 2A, BrkA). All three strains expressed S1 when grown under control conditions, but only BPCV, the constitutive mutant, expressed S1 under modulating conditions (Fig. 2B).
FIG. 2.
Virulence factor expression of BPCV. Bacteria were grown on BG agar or modulating BG agar for 48 h, harvested, and analyzed for expression of BrkA (A) or pertussis toxin (PT) subunit S1 (B) by Western blotting. Abbreviations: BPCV, constitutively virulent mutant; WT, BP338; BrkA, RFBP2152; Control, bacteria grown under permissive conditions; Mod, bacteria grown under modulating conditions.
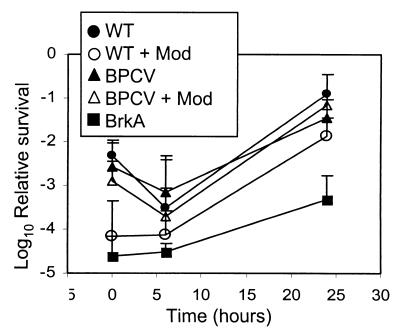
Resistance to complement was determined for the wild type and BPCV following growth under control and modulating conditions and for the BrkA mutant under control conditions (Fig. 3). Susceptibility to complement for the wild-type strain grown under control conditions was similar to that for the constitutive mutant (BPCV) grown under control or modulating conditions. Each strain displayed an initial increase in susceptibility at 6 h but became extremely resistant by 24 h. Susceptibility of the wild type grown under modulating conditions was similar to that of the BrkA mutant at 0 and 6 h, times when it lacks BrkA expression, as determined by Western blotting (data not shown). However, resistance to killing was fully recovered by 24 h. Susceptibility to complement varied with growth phase independent of BrkA or Bvg regulation, suggesting that factors in addition to BrkA play a role in conferring resistance to complement.
FIG. 3.
Susceptibility to complement following modulation. B. pertussis was grown under control or modulating conditions and then was transferred to SS-BG and grown under conditions that permit expression of the Bvg-regulated virulence factors. Serum killing assays were performed with 10% human serum using cells harvested at the indicated times. Results are presented as mean log10 survival relative to control experiments with heat-inactivated serum and standard errors (error bars) of at least three separate experiments. Abbreviations: WT, B338; BPCV, constitutively virulent mutant; BrkA, RFBP2152; Mod, bacteria grown on modulating BG agar.
Antigenic variability and susceptibility to complement.
Since the antibody-dependent classical pathway of complement kills B. pertussis, changes in susceptibility to complement over time could be due to differential expression of bacterial antigens. To address this hypothesis, serum was adsorbed with wild-type bacteria grown for 24 h in BG-SS. This should remove all antibodies directed against epitopes present at that time but leave antibodies directed against antigens unique to other time points, such as 0 and 6 h.
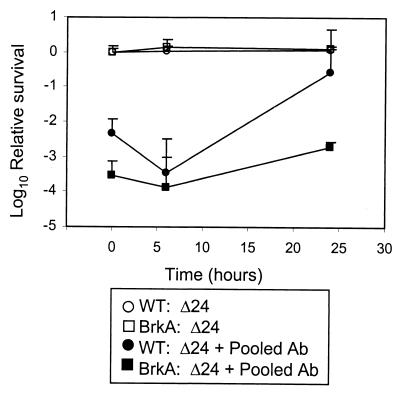
As a source of complement for these studies, human serum (25 ml) was depleted of immunoglobulin by sequential incubation with agarose-conjugated immunoglobulin binding proteins for 30 min on ice, as follows: two times with 5 ml of protein A (lot 60K7018; Sigma, St. Louis, Mo.), once with 5 ml of protein G (lot 40K7025; Sigma), once with 2 ml of protein L (lot BE43650; Pierce, Rockford, Ill.), and twice with 5 ml of protein LA (lot 119H1653; Sigma). The serum was filter sterilized, and aliquots were stored frozen at −80°C until use. The antibody-depleted serum lacked bactericidal activity against the wild type or the BrkA mutant (data not shown) but mediated killing of both strains when heat-inactivated immune serum was added as a source of antibody (Fig. 4).
FIG. 4.
Antigenic variability and susceptibility to complement. B. pertussis was grown under control conditions prior to being transferred to SS-BG and grown under conditions that permit expression of the Bvg-regulated virulence factors. Serum killing assays were performed at the indicated times with 20% antibody-depleted complement in the presence of antibody adsorbed with 24-h-grown bacteria (open symbols) or with addition of immune antibody (closed symbols). Results are presented as the mean log10 survival relative to control experiments with heat-inactivated serum of at least three separate experiments (error bars show standard errors) Circles, BP338; squares, RFBP2152.
A bactericidal assay performed with adsorbed sera in the presence of antibody-depleted complement showed that neither wild-type nor BrkA mutant bacteria were killed at 0, 6, or 24 h (Fig. 4). These data show that removal of antibodies to antigens present at 24 h removes all bactericidal antibodies from serum and suggest that changes in complement sensitivity during the growth cycle were not due to variable antigenic expression during growth phase.
Gram-positive bacteria are not killed by complement. It is thought that the thick peptidoglycan layer prevents access of the membrane attack complex to the bacterial membrane. Gram-negative bacteria can be killed by complement, but the mechanism by which complement kills gram-negative bacteria is not well understood (15). It is widely accepted that complement deposited on the outer membrane does not kill the bacteria, and damage to the inner membrane is thought to be necessary to kill gram-negative bacteria, but how complement gains access to the inner membrane is not known. One hypothesis suggests that complement must first lyse the outer membrane to gain access to the inner membrane (9). Another hypothesis suggests that only complement deposited at specific locations on the outer membrane, such as the Bayer’s junctions (4), can gain access to the inner membrane and mediate bacterial killing (19).
Previous studies have shown that gram-negative organisms like Escherichia coli are more sensitive to killing by complement when they are undergoing logarithmic growth (2). In one study (5), simultaneous disruption of outer membrane integrity and inner membrane integrity occurred upon addition of complement to E. coli in the logarithmic phase. However, when complement was added to bacteria in the log phase, immediate disruption of outer membrane integrity was observed, but inner membrane integrity was maintained until division was initiated.
In this study we have shown that complement susceptibility of B. pertussis is also growth stage specific, and stationary-phase bacteria are much more resistant than bacteria undergoing logarithmic growth. Growth stage susceptibility was not due to differential expression of BrkA or antigenic differences.
While increased susceptibility to complement during active bacterial growth might seem to put B. pertussis at risk for killing by complement, animal experiments suggest that this may not be the case. In one study, more than a million bacteria were recovered 5 days after inoculating mice with 500 bacteria (12). Bacterial growth plateaued after about 10 days, but colonization persisted for several weeks. B. pertussis is only killed by complement in the presence of antibody (10, 11, 16, 18); however, it can take up to a week to generate antibody in a primary infection. Thus, rapid bacterial replication, when the bacteria are susceptible to complement, can be completed before antibody production begins. A role for complement killing following prior exposure or vaccination seems likely. Disease in adolescents and adults can be less severe, and often the only symptom is a persistent cough (6). A secondary antibody response could be generated in a few days, and it is possible that complement killing plays a role in limiting bacterial replication during a secondary infection. Previous studies have suggested that the current acellular pertussis vaccines are not very effective at generating antibodies that promote killing by complement (16, 18). Designing vaccines that activate complement killing early in infection could improve efficacy.
Acknowledgments
This study was supported by grant RO1 AI45715 to A.A.W. M.G.B. was the recipient of a Graduate Student Summer Research Fellowship from the University of Cincinnati.
Editor: D. L. Burns
REFERENCES
- 1.Akerley, B. J., and J. F. Miller. 1996. Understanding signal transduction during bacterial infection. Trends Microbiol. 4: 141–146. [DOI] [PubMed] [Google Scholar]
- 2.Allen, R. J., and G. K. Scott. 1980. The effect of purified lipopolysaccharide on the bactericidal reaction of human serum complement. J. Gen. Microbiol. 117: 65–72. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69: 3067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, M. E. 1968. Areas of adhesion between wall and membrane of Escherichia coli. J. Gen. Microbiol. 53: 395–404. [DOI] [PubMed] [Google Scholar]
- 5.Bhakdi, S., G. Kuller, M. Muhly, S. Fromm, G. Seibert, and J. Parrisius. 1987. Formation of transmural complement pores in serum-sensitive Escherichia coli. Infect. Immun. 55: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2): S112–S117. [DOI] [PubMed] [Google Scholar]
- 7.Craig-Mylius, K. A., T. H. Stenson, and A. A. Weiss. 2000. Mutations in the S1 subunit of pertussis toxin that affect secretion. Infect. Immun. 68: 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig-Mylius, K. A., and A. A. Weiss. 2000. Antibacterial agents and release of periplasmic pertussis toxin from Bordetella pertussis. Antimicrob. Agents Chemother. 44: 1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feingold, D. S., J. N. Goldman, and H. M. Kuritz. 1968. Locus of the lethal event in the serum bactericidal reaction. J. Bacteriol. 96: 2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez, R. C., and A. A. Weiss. 1994. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect. Immun. 62: 4727–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez, R. C., and A. A. Weiss. 1998. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol. Lett. 163: 57–63. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58: 3445–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton, A. R., and A. A. Weiss. 1989. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol. 171: 6206–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, J. F., S. A. Johnson, W. J. Black, D. T. Beattie, J. J. Mekalanos, and S. Falkow. 1992. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J. Bacteriol. 174: 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor, P. W. 1992. Complement-mediated killing of susceptible gram-negative bacteria: an elusive mechanism. Exp. Clin. Immunogenet. 9: 48–56. [PubMed] [Google Scholar]
- 16.Weingart, C. L., W. A. Keitel, K. M. Edwards, and A. A. Weiss. 2000. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect. Immun. 68: 7175–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss, A. A., P. S. Mobberley, R. C. Fernandez, and C. M. Mink. 1999. Characterization of human bactericidal antibodies to Bordetella pertussis. Infect. Immun. 67: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright, S. D., and R. P. Levine. 1981. How complement kills E. coli. I. Location of the lethal lesion. J. Immunol. 127: 1146–1151. [PubMed] [Google Scholar]