Abstract
Historically, resistance to phagocytosis has been determined by incubating group A streptococci in human blood and comparing the numbers of CFU before and after incubation. Utilizing a flow cytometry-based technique, we have investigated the phagocytosis of M+ group A streptococci by polymorphonuclear leukocytes (PMNs) in heparinized human peripheral whole blood. Intracellular labeling of streptococci with a nontoxic fluorescent dye allowed us to quantify the association and phagocytosis of M+ streptococci by PMNs in whole blood in the presence or absence of C5a, a physiologically important chemotactic activator of PMNs. We found that wild-type strains of group A streptococci that are resistant to phagocytosis (determined by the classical Lancefield method) readily associate with C5a-activated whole-blood PMNs. In the absence of opsonizing M-type-specific antibodies, the M+ streptococci associated with PMNs are phagocytized and killed. In addition, blockade of the β2 integrin, CD11b/CD18, with anti-human CD11b monoclonal antibody inhibited association between M+ streptococci and C5a-activated PMNs. These findings establish a new relationship between M+ streptococci and PMNs, in which C5a-activated PMNs have the capacity to kill M+ streptococci in whole blood through a receptor-mediated phagocytic mechanism.
Resistance to phagocytosis is a hallmark of virulent group A streptococci. Activation of the alternative complement pathway in response to M+ streptococci is limited. This characteristic has traditionally been attributed to M protein, which limits the deposition of C3b on the streptococcal surface (10, 17). The precise mechanism by which M protein limits the deposition of C3b and activation of the alternative complement pathway is unresolved. In contrast, M− streptococci become circumferentially covered with C3b and efficiently activate the alternative complement pathway.
In addition to M protein, virulent strains of group A streptococci possess a highly specific surface endopeptidase that cleaves C5a at its polymorphonuclear leukocyte (PMN) binding site (3). Streptococcal C5a peptidase (SCPA) was shown to retard the infiltration of granulocytes to subdermal sites of group A streptococcus infection (12). SCPA destroys the chemotactic signal generated by the alternative complement pathway, thereby inhibiting recruitment of phagocytes to the site of infection. Mutations in the C5a peptidase gene (scpA) support this proposed mechanism. Elimination of SCPA by mutation enhances granulocyte infiltration and clearance of M49+ streptococci from subdermal and intranasal sites of infection, even though M49+ streptococci are resistant to killing in the Lancefield bactericidal assay (12–14). These data led us to question whether C5a-activated PMNs have the capacity to phagocytize M+ group A streptococci.
Schnitzler et al. developed a flow cytometry-based assay to quantitate the phagocytosis of group A streptococci in whole blood (21). These investigators demonstrated that M+ streptococci, resistant to phagocytosis in the traditional Lancefield assay, are not resistant to phagocytosis in whole blood pretreated with phorbol-12-myristate-14-acetate (PMA). PMA is an activator of PMNs that is known to phosphorylate the β2-subunit (CD18) of β2-integrins LFA-1 and CR3 (22). Utilizing this flow cytometry-based assay, we sought to determine whether PMNs activated with the more physiologically relevant C5a could phagocytize and kill M+ streptococci that are resistant to killing in the Lancefield bactericidal assay. In addition, we investigated the roles of CD11b/CD18 (CR3) receptors in the phagocytosis of M+ streptococci by C5a- and PMA-activated PMNs.
MATERIALS AND METHODS
Bacterial strains.
Group A streptococcus strain CS101 is a spontaneous streptomycin-resistant derivative of a serum opacity-positive (OF+) serotype M49-positive strain. Strain MJY1-3 is an isogenic CS101 mutant with a defined internal in-frame deletion of the mrp, emm, and enn genes. The phenotype of this strain has been fully characterized to ensure that mga and scpA49 are normally expressed and that products from the deleted genes are not produced (14).
Group A streptococcus strain 90-226, an invasive serotype M1+ strain, cultured from the blood of a patient with sepsis (18), was obtained from the World Health Organization Center for Reference and Research on Streptococci at the University of Minnesota (4,5,6). Strain 90-226emm1::Km was constructed by insertional inactivation of emm1 with the aph-A3 (kanamycin resistance) gene (4, 6).
Group A streptococcus strain JRS4 is a spontaneous streptomycin-resistant derivative of an invasive serotype M6-positive, rheumatic fever-associated isolate (6). Strain JRS145 is an M6− JRS4 derivative in which the chloramphenicol acetyltransferase gene from Bacillus pumilus has been fused to the emm6 promoter in place of the emm6.1 gene (2). Strain JRS251 is a JRS4 derivative containing an emm6 allele in which the two C repeats and the spacer between the C repeats have been deleted (20). All JRS strains were provided by J. R. Scott (20).
Collection of human whole-blood samples.
Whole-blood samples were drawn from healthy human volunteers via a 21-gauge Vacutainer blood collection set into a silicone-coated collection tube (Becton Dickinson, Franklin Lakes, N.J.). Heparin (Upjohn, Kalamazoo, Mich.) was immediately added to the collected blood at a concentration of 5 USP units/ml of whole blood.
Preparation of streptococci for flow cytometry.
Streptococci were prepared for flow cytometry as previously described (14, 22). Streptococcal strains were precultured in 10 ml of Todd-Hewitt broth supplemented with 5 g of yeast extract (Difco Laboratories, Detroit, Mich.) per liter at 37°C and 5% CO2 for 18 h.
For strain CS101 or MJY1-3, streptococci were harvested when the optical density at 600 nm (OD600) reached 0.38 to 0.42. For strain 90-226 or JRS4-derived strains, streptococci were harvested when the OD560 reached 0.5. In addition, strain 90-226 and JRS4-derived cultures were diluted 1:4 in phosphate-buffered saline (PBS) (pH 7.4) (Gibco BRL, Grand Island, N.Y.) that was sterile filtered with a 0.22-μm-pore-size bottle top filter (Millipore, Bedford, Mass.) before centrifugation.
The streptococci were recovered by centrifugation, washed twice in PBS, and resuspended in 1 ml of PBS. Bis-carboxyethyl-carboxyfluorescein-pentaacetoxy-methylester (BCECF-AM; Molecular Probes, Eugene, Oreg.), a nonvital intracellular dye, was added to the streptococcal suspension to a final concentration of 1 mM. After a 30-min incubation at 37°C, the now green fluorescent streptococci were sonicated to disrupt chains. The fluorescent streptococci were washed three times in PBS and then used immediately in the fluorescence-activated cell sorting (FACS) phagocytosis assay.
Flow cytometry-based phagocytosis assay.
Phagocytosis of BCECF-AM-labeled streptococci by C5a-activated whole-blood PMNs was quantified via flow cytometry as previously described (14, 22). A 1-ml volume of heparinized whole blood (5 USP units/ml) from healthy human donors containing approximately 2 × 106 PMNs was incubated in a sterile 1.5-ml Eppendorf microcentrifuge tube with 50 μl of 10 μM recombinant human C5a (rhC5a) (Sigma, St. Louis, Mo.) or without C5a for 30 min at 37°C on a Labquake rotator at 8 rpm (Lab Industries, Berkeley, Calif.). In some experiments, PMA (Sigma) or mouse anti-human CD11b monoclonal antibody (MAb) (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) was added to heparinized whole blood 5 min prior to the addition of the bacteria. The final concentrations were 20 ng/ml for PMA and 15% (vol/vol) for the CD11b MAb.
After the incubation period, 100 μl of 107 BCECF-AM-labeled streptococci in PBS was added and the tubes were incubated at 37°C on a Vortex-Genie 2 equipped with a turbo-mix microcentrifuge attachment (Scientific Industries, Inc., Bohemia, N.Y.) at 1,000 rpm. Samples of 100 μl were removed at various incubation time points and immediately mixed with 2 ml of ice-cold FACS Lysing Solution (Becton Dickinson Immunocytometry Systems).
The collected samples were incubated for 20 min at room temperature in the dark. The granulocytes were then isolated from the lysis solution by centrifugation (5 min, 4°C, 300 × g) (GS-6R centrifuge; Beckman Instruments Inc., Palo Alto, Calif.) and washed three times in 1 ml of ice-cold PBS. The PMNs were resuspended in a final volume of 500 μl of ice-cold PBS, and flow cytometric analysis was performed with a FACScan flow cytometer using CellQuest software (Becton Dickinson Immunocytometry Systems). PMNs were selectively analyzed by gating the sample by relative size and granularity. Association of BCECF-AM-labeled streptococci with PMNs is expressed as the increase in green fluorescence of the PMNs.
Lancefield bactericidal assay.
Lancefield bactericidal assays were performed as previously described (17). Strains CS101 and MJY1-3 were grown, washed, and resuspended in 1 ml of PBS as previously described. Diluted culture (0.1 ml) and fresh human blood (0.9 ml) were mixed and rotated at 37°C. Viable counts after 0, 30, 60, 90, and 120 min were determined by plating diluted samples onto blood agar.
FACS of group A streptococcus-associated PMNs from whole blood.
Strains CS101 and MJY1-3 were prepared for flow cytometry as previously described (14). One-milliliter samples of heparinized whole blood were preincubated with or without C5a or PMA as described previously. Following the addition of streptococci, a 400-μl sample was withdrawn and added to 8 ml of ice-cold ACK lysing buffer (Biofluids Inc., Rockville, Md.). After 30 min of coincubation, a 400-μl sample was withdrawn and added to ice-cold ACK lysing buffer.
The samples were incubated at room temperature for 20 min, and the granulocytes were isolated and washed as described previously (19) with one notable change: the wash buffer consisted of heat-inactivated fetal calf serum diluted to a final concentration of 2% in PBS. The lysed blood samples were sorted under sterile conditions at 4°C into 1 ml of ice-cold PBS containing 2% FCS via a FACS Vantage flow cytometer (Becton Dickinson Immunocytometry Systems).
Approximately 100,000 streptococcus-associated PMNs were sorted from each sample. FACS parameters were identical to those established in the previous experiments utilizing FACScan analysis. Therefore, only PMNs expressing green fluorescence were sorted from the granulocytes. Group A streptococci without blood were sorted from lysis buffer to distinguish between killing caused by the lysis buffer from that caused by FACS. The sorted PMNs were quantified using a hemocytometer and diluted to 50,000 group A streptococcus-associated PMNs/ml. Dilutions of 10−1, 10−2, and 10−3 were lysed in distilled H2O and plated on blood agar. After overnight incubation at 37°C, the CFU/PMN ratio was calculated.
Double-immunofluorescence microscopy.
Strain CS101 (108 CFU) was inoculated into 1 ml of human blood with PMA-activated PMNs. This mixture was rotated for 10 min as described above. After red blood cells were lysed, PMNs were spread on a glass slide and fixed with ACK lysing buffer. Slides were blocked with 10% goat serum and exposed first to rabbit anti-carbohydrate group A serum for 1 h and then to cyanin (Cy3)-conjugated mouse anti-rabbit antibody for 1 h at room temperature. Cells were permeabilized by 0.2% saponin before a second exposure to rabbit anti-carbohydrate A and fluorescein isothiocyanate (FITC)-conjugated mouse anti-rabbit antibody, each for 1 h. Slides were viewed with a confocal microscope (Bio-Rad MRC 1024).
RESULTS
Whole-blood PMNs preactivated with C5a associate with M49+ SCPA+ group A streptococci.
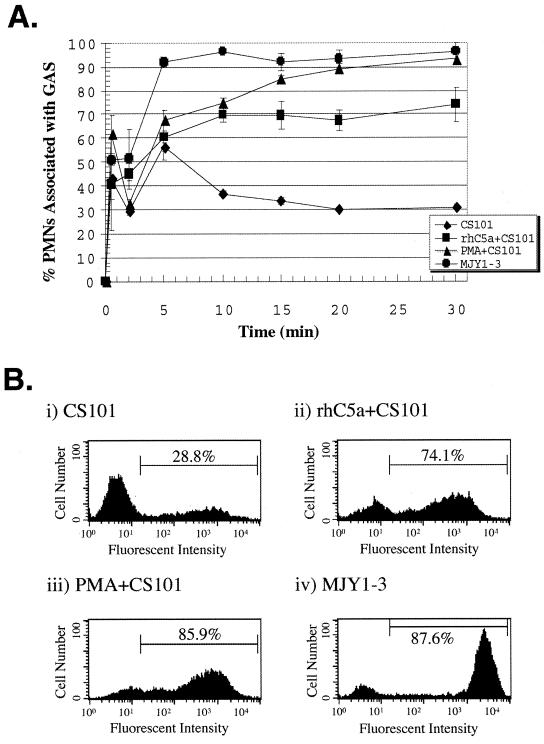
Experiments were performed to determine whether PMNs pretreated with C5a, a physiologically relevant activator of PMNs, develop the capacity to associate with M+ streptococci. Heparinized whole blood was preincubated with 0.5 μM C5a, and the kinetics of BCECF-AM-labeled streptococcus association with C5a-activated PMNs in whole blood was examined (Fig. 1A). In nontreated blood, there was an initial rapid association between strain CS101 and PMNs, which abruptly decreased to reach a steady-state level after 10 to 15 min. Approximately 30% of the PMNs remained associated with streptococci throughout the assay (Fig. 1A).
FIG. 1.
C5a-activated PMNs associate with M49+ streptococci. (A) The data for strains CS101, C5a-activated CS101 (rhC5a+CS101), PMA-activated CS101 (PMA+CS101), and strain MJY1-3 are the mean percentages of gated granulocytes associated with group A streptococci (GAS) from three independent experiments. The differences in the values for PMNs associated with strain CS101, comparing C5a-activated to nonactivated whole-blood PMNs, are statistically significant at the 2-min (P = 0.005), 10-min (P = 0.0007), 15-min (P = 0.004), 20-min (P = 0.001), and 30-min (P = 0.004) time points determined by using an unpaired two-tailed Student’s t test. The error bars depict the standard errors of the means. (B) Representative histograms of granulocytes gated 15 min after whole blood was inoculated with streptococci. For each histogram, 10,000 granulocytes were counted.
Kinetic analysis indicated that attachment of M49+ streptococci to PMNs in whole blood depended upon PMN pretreatment with C5a or PMA. In blood with C5a- or PMA-activated PMNs, PMN association with M49+ streptococci was more intense and rapid than that of nontreated blood (Fig. 1A). Differences in the percentages of PMNs associated with fluorescent M49+ streptococci were greatest after 15 min of incubation, as shown in Fig. 1B. Seventy-four percent of C5a-activated PMNs and 86% of PMA-activated PMNs associated with strain CS101, whereas only 29% of PMNs in nontreated blood associated with strain CS101 (Fig. 1B). Strain MJY1-3, the Δmrp Δemm Δenn deletion mutant, was associated without prior C5a or PMA activation of PMNs.
C5a-activated PMNs associate with M1+ and M6 + streptococci but do not associate with M6+ streptococcus in whole blood.
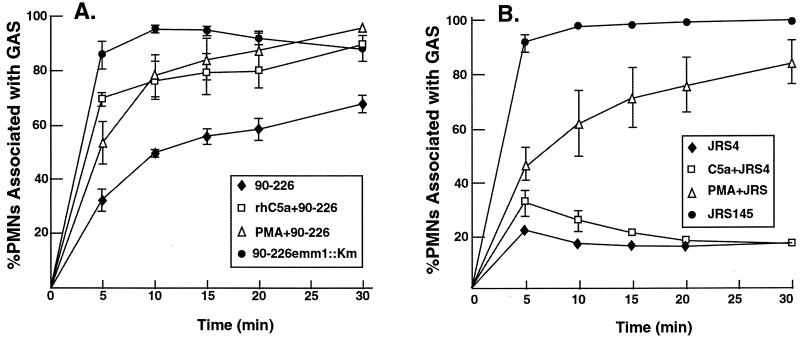
To test the generality of the previous experiment, FACS assays were conducted with M1+ and M6+ streptococcal strains. Similar to the previously described FACS experiments utilizing strain CS101, strain 90-226 rapidly associated with PMA- or C5a-activated PMNs (Fig. 2A). Differences were greatest in the first 5 min of incubation. After 15 min of incubation, approximately 85 to 90% of C5a- and PMA-activated PMNs associated with strain 90-226, whereas only 50% of PMNs preincubated without a chemical activator associated with M1+ streptococci. The M1− strain 90-226emm1::Km associated with PMNs without prior C5a or PMA activation.
FIG. 2.
C5a-activated PMNs in whole blood associate with strain 90-226 streptococci. (A) The data for strain 90-226, C5a-activated 90-226 (rhC5a+90-226), PMA-activated 90-226 (PMA+90-226), and strain 90-226emm1:: Km are the mean percentages of gated granulocytes associated with group A streptococci (GAS) from four independent experiments. The differences in the values for PMNs associated with strain 90-226, comparing C5a-activated to nonactivated whole-blood PMNs, are statistically significant at the 5-min (P = 0.005), 10-min (P = 0.01), 15-min (P = 0.03), 20-min (P = 0.04), and 30-min (P = 0.0008) time points determined by using an unpaired two-tailed Student’s t test. (B) Strain JRS4 is a wild-type M6+ streptococcus. Strain JRS145 is an M6− mutant that was derived from JRS4 (20). Data collected from nonactivated whole-blood PMNs incubated with wild-type strain JRS4 and the M6− strain JRS145 are the mean percentages of PMNs associated with streptococci from three independent experiments. The error bars depict the standard errors of the means.
Preactivation of PMNs with C5a in whole blood led to a minor increase in PMN association with strain JRS4 (Fig. 2B). The association of strain JRS4 with C5a-activated PMNs was transient and diminished during the assay. By 5 min, only 32% of the C5a-activated PMNs and 21% of the nonactivated PMNs associated with strain JRS4 (Fig. 2B). The small differences at 5- and 10-min points were statistically significant, however. As expected, PMNs without prior C5a or PMA activation associated efficiently with the isogenic M6− mutant strain JRS145. Preactivation of PMNs in whole blood with PMA led to a sharp and significant increase in PMN association with strain JRS4. This suggests that PMNs activated with PMA express a receptor that interacts with M6+ streptococci that is not up-regulated by C5a activation. It is possible that other characteristics, such as differences in the size of hyaluronic acid capsules, could account for differences in ingestion rates by activated PMNs.
The above experiments showed significant differences in the capacity of nonactivated PMNs to associate with different strains of streptococci. Background association differences between strains may reflect phenotypic differences, which depend on different histories in the laboratory. For example, the M6 and M49 strains are more stable than the M1 strain with regard to M-protein expression. The M6 strain, an original Lancefield culture, has been passaged multiple times in mice, which selects for a more stable M+ state. Moreover, expression of different M proteins in an isogenic background demonstrated that different M proteins recognize different repertoires of receptors on the surfaces of eukaryotic cells and vary with regard to their capacity to impart resistance to phagocytosis (1). The increased association of strain 90-226 with PMNs in untreated blood could be explained by interactions of M1 protein with cellular receptors or macromolecules that are not recognized by M49 protein.
Preactivation of whole-blood PMNs with C5a restricts the growth of M49+ streptococci.
Historically, resistance to phagocytosis was determined by incubating streptococci in fresh human blood and comparing the numbers of CFU before and after blood exposure. Interpretation of this assay is complicated by the fact that both growth of streptococci and their capacity to resist ingestion by phagocytes impinge on the number of residual viable streptococci. Resistance to phagocytosis by strain CS101 and its corresponding M− isogenic mutant, MJY1-3, was tested in a classical Lancefield bactericidal assay using blood from three different donors (Fig. 3).
FIG. 3.
Time course of M49+ streptococci killed by PMNs in blood pretreated with C5a. Approximately 1,000 CFU of strain CS101 or MJY1-3 was added to 1 ml of heparinized human whole blood and rotated at 37°C. Diluted samples of blood were plated on agar plates at the indicated time points to determine the number of CFU. The growth ratio (y axis) equals the number of CFU at a given time point divided by the initial CFU at time zero. Data are the mean growth ratios from three independent experiments. The error bars depict the standard errors of the means.
Group A streptococci were added to fresh human blood, which had been preincubated with or without C5a or PMA. The blood used in these assays was devoid of opsonizing M-type-specific antibodies. After 30 min of blood exposure, approximately 100% of the M49− streptococci were killed. Compared to growth in untreated blood, growth of strain CS101 in blood preincubated with C5a decreased 1.2-fold after 90 min (P = 0.05) and 1.3-fold after 120 min (P = 0.05) (Fig. 3). Growth of M49+ streptococci was restricted even more in blood pretreated with PMA. Compared to untreated blood, growth of strain CS101 in PMA-treated blood decreased twofold at 90 min (P = 0.004) and 3.4-fold at 120 min (P = 0.0002). Neither C5a nor PMA influenced growth of streptococci in serum (data not shown)
FACS was employed to confirm that M+ streptococci which were associated with PMNs in whole blood are actually killed (Table 1). The Lancefield bactericidal assay indicated that growth of strain CS101 was moderately inhibited in blood pretreated with C5a or PMA. The viability of streptococci that associated with PMNs was compared to those associated with PMNs after 30 min of incubation. If PMNs kill associated streptococci, the ratio of CFU per sorted fluorescent PMN should decrease with time.
TABLE 1.
Group A streptococci associated with PMNs in whole blood are killeda
| Treatment | % PMNs associated with GAS
|
CFU per GAS-associated PMNb
|
Fold decrease in CFU per GAS-associated PMNs | Mean fluorescence intensity of GAS-associated PMNs
|
|||
|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 0 min | 30 min | 0 min | 30 min | ||
| Blood + CS101 | 11.5 | 40.9 | 1.2 ± 0.05 | 0.18 ± 0.004 | 6.7 | 59.2 | 70.8 |
| Blood + rhC5a + CS101 | 7.2 | 61.0 | 1.7 ± 0.09 | 0.13 ± 0.009 | 13.1 | 58.9 | 122.2 |
| Blood + PMA + CS101 | 10.6 | 60.6 | 1.1 ± 0.02 | 0.19 ± 0.018 | 5.8 | 68.9 | 84.5 |
| Blood + MJY1-3 | 22.1 | 97.8 | 3.5 ± 0.37 | 0.18 ± 0.003 | 19 | 141.8 | 618.0 |
Group A streptococcus (GAS)-associated PMNs were sorted from whole blood of blood donor 1 by FACS.
Values are means ± standard deviations.
Similar to the previous FACS and Lancefield assays, blood was preincubated with or without C5a or PMA. At the 0-min time point, 107 BCECF-AM labeled streptococci (multiplicity of infection of ∼100) were added to the blood. The 0-min time point represents a sample withdrawn from the blood and placed in ice-cold lysis buffer immediately following the brief manual mixing of the blood to evenly distribute the bacteria (Table 1). It is assumed that this short exposure of streptococci to PMNs in whole blood allows them to associate with PMNs but does not afford PMNs enough time to ingest and kill associated streptococci. For each time point, 100,000 streptococcus-associated PMNs were sorted from the lysed blood samples. The sorted streptococcus-associated PMNs were then plated on blood agar and the number of CFU per PMN was calculated (Table 1).
Activation of PMNs with rhC5a or PMA increased the fraction of PMNs associated with group A streptococcus after 30 min of incubation. The ratio of CFU per sorted PMN decreased significantly from 5.8- to 19-fold over the 30-min incubation period. Preincubation of blood with rhC5a produced a greater decrease in viability than blood that received only buffer. The mean fluorescence of these PMNs was also significantly greater than that of the buffer controls, suggesting that C5a up-regulated receptors required for association with PMNs. Although PMA-activated PMNs were significantly more fluorescent than those in the buffer control, the viability of PMN-associated streptococci did not decrease significantly relative to that of the buffer control or C5a-activated PMNs. This suggests that PMA may up-regulate receptors that promote association with group A streptococcus but that association does not always result in intracellular killing. The M− strain MJY1-3 associated to a greater extent with PMNs at both 0- and 30-min time points and was more readily killed than strain CS101 independent of preincubation conditions. Microscopic inspection of PMNs from the 30-min time point revealed that they were associated with an extraordinarily large number of streptococci. In fact, there were so many M49− streptococci associated with the sorted PMNs that it was not possible to accurately quantitate this association.
The mean fluorescence intensity of a streptococcus-associated PMN is a useful qualitative measurement of the number of streptococci associated with a PMN (Table 1). A PMN with a fluorescence intensity greater than 10 is associated with at least one fluorescent streptococcus. A sorted low-fluorescence PMN is associated with fewer streptococci than a PMN with a very high fluorescence intensity. Because of the large inoculum of streptococci and the constant mixing, an individual PMN undergoes multiple contacts with streptococci during the assay. Therefore, a time-dependent increase in the fluorescence intensity of a C5a- or PMA-activated PMN reflects the accumulation of bacteria during the assay (Table 1). Increased fluorescence intensity also indicated that the decrease in PMN-associated CFU after 30 min of incubation was not due to dissociation or elution of streptococci from PMNs over time. Coupled with the decrease in CFU per fluorescent PMN with time, these data indicate that M49+ streptococci are killed by C5a- or PMA-activated PMNs.
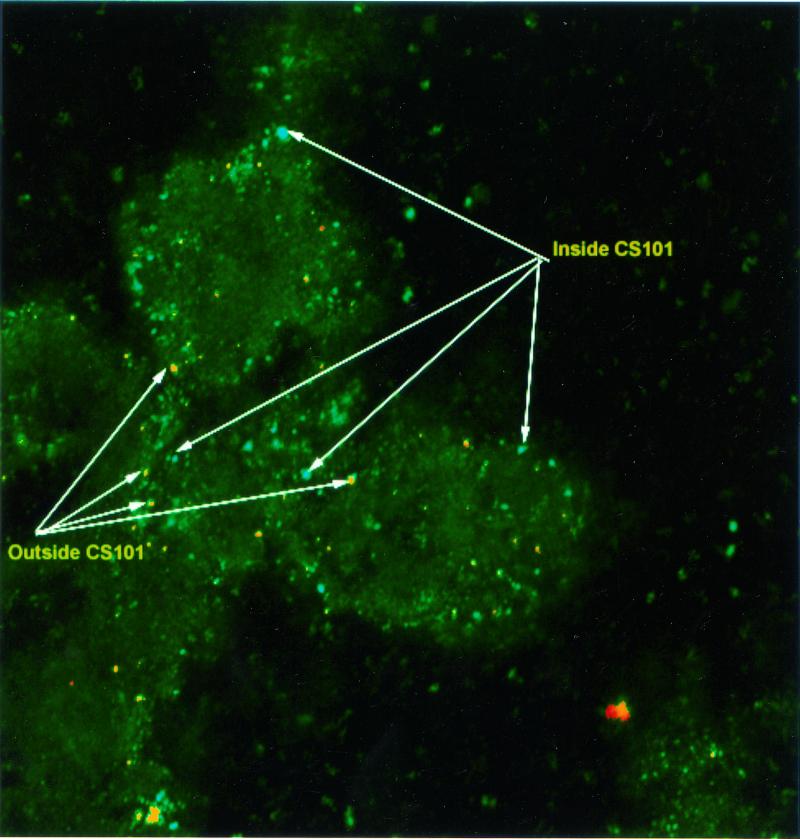
Results from Figure 3 and Table 1 suggested that rhC5a- or PMA-activated PMNs are able to kill M+ streptococci but did not establish that PMNs actually ingest these streptococci. Two-color immunofluorescence confocal microscopy (Fig. 4) demonstrated that at least 50% of PMN-associated streptococci were internalized. Strain CS101 was incubated with blood containing PMA-activated PMNs for 30 min and then processed for staining. Extracellular streptococci were stained with rabbit anti-group A carbohydrate antibody and FITC-conjugated mouse anti-rabbit immunoglobulin (IgG). Both intracellular and extracellular bacteria were stained with rabbit anti-group A carbohydrate antibody and goat anti-rabbit IgG conjugated to Cy3 after permeabilization with saponin (6). In Fig. 4, extracellular bacteria appear red and green (yellow when micrographs are overlaid), whereas intracellular streptococci are green. Both red-yellow and green streptococci were found to be associated with PMNs. The ratio of intracellular to extracellular streptococci was determined to be approximately 1:1 by counting 110 streptococci or chains that were associated with PMNs.
FIG. 4.
Distinction between intracellular and extracellular streptococci associated with PMNs by double-immunofluorescence microscopy. Streptococci in whole-blood smears were identified with rabbit anti-carbohydrate group A serum and Cy3-conjugated mouse anti-rabbit antibody (red) and with FITC-conjugated mouse anti-rabbit antibody. Red and green fluorescent images were overlaid on identified streptococci that were labeled with both Cy3 and FITC. Intracellular streptococci are green, and extracellular streptococci are red or red-yellow. Magnification, ×400.
PMN association with M49+ streptococci in whole blood is inhibited by anti-CD11b MAb.
Schnitzler et al. has shown that blockade of CR3 receptors with CD11b MAb reduces the association of M49+ streptococci with PMA-activated PMNs in whole blood (22). C5a also up-regulates the function and expression of CR3 receptors on PMNs (19, 25). Therefore, we performed experiments to determine whether the association between M49+ streptococci and C5a-activated PMNs in whole blood is dependent on CR3.
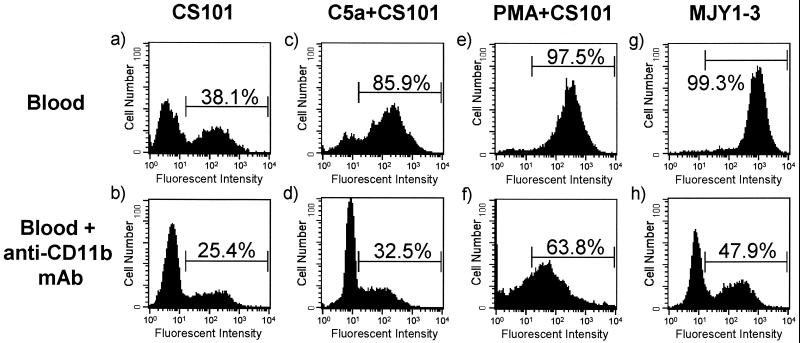
Blockade of CR3 receptors with anti-CD11b MAb in C5a-activated whole-blood PMNs resulted in a striking reduction in the number of PMNs associated with M49+ streptococci. Addition of anti-CD11b MAb to heparinized whole blood 5 min prior to the addition of M49+ streptococci reduced the association of C5a- or PMA-activated PMNs with strain CS101 (Fig. 5). After a 30-min exposure to strain CS101 in blood preincubated with anti-CD11b MAb, the number of C5a-activated PMNs associated with strain CS101 decreased 54%. In untreated blood, the number of PMNs associated with strain CS101 decreased 13% in the presence of anti-CD11b MAb. This may mean that 20 to 30% of PMN association with M49+ streptococci is unrelated to the availability of CR3 receptors in untreated and C5a-activated blood. In the presence of anti-CD11b MAb, the number of PMA-activated PMNs associated with strain CS101 decreased 34%.
FIG. 5.
Blockade of CR3 receptors with MAb reduces the number of PMNs associated with M49+ streptococci. Representative histograms of the granulocyte gated 30 min after whole blood was inoculated with streptococci. The whole-blood PMNs in the second row of histograms were pretreated with anti-CD11b MAb. Histograms a and b depict strain CS101 in blood with nonactivated PMNs, the whole-blood PMNs in histograms c and d were pretreated with rhC5a, and the whole-blood PMNs in histograms e and f were pretreated with PMA. Histograms g and h depict strain MJY1-3 in blood with nonactivated PMNs. The PMNs in the bracketed region are associated with BCECF-AM-labeled streptococci. For each histogram, 10,000 granulocytes were counted.
Sixty-four percent of PMN association with M49+ streptococci in blood containing PMA-activated PMNs may not depend on the availability of CR3 receptors (Fig. 5). The difference in PMA- and C5a-activated PMNs may reflect activation of different intracellular signaling pathways. PMN activation by C5a or PMA up-regulates many of the same receptors; however, the function of a given receptor may be dependent upon the signaling pathway activated. Association of the M49− strain MJY1-3 with PMNs from blood without prior C5a or PMA activation of PMNs decreased 51% in the presence of anti-CD11b MAb (Fig. 5). Thus, uptake of M− streptococci may partially involve preexisting CR3 receptors.
M6+ streptococci that are unable to bind complement factor H associate with from nonactivated PMNs in whole blood.
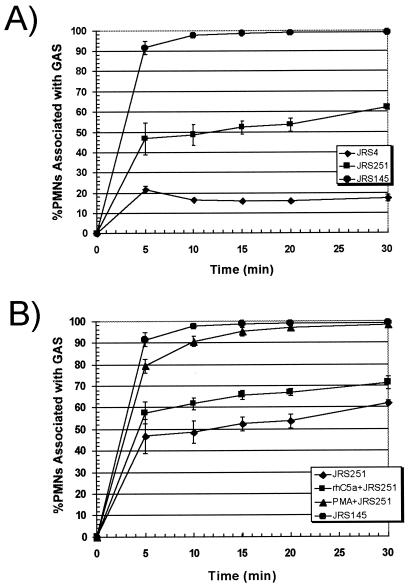
The above experiments demonstrated that M+ streptococci are phagocytized and killed by C5a-activated PMNs in whole blood via a CR3 receptor-dependent mechanism. Although considerably less C3b is deposited on M+ streptococci (10, 11), there must be sufficient iC3b on their surface to mediate ingestion by activated PMNs. Factor H is a serum protein that serves as a cofactor for the conversion of C3b to iC3b (9). Factor H binds to the C-repeat domain of M protein and promotes degradation of C3b in vitro (7, 9). The degradation of C3b to iC3b inhibits the formation of C3 convertase and the deposition of C3b, which is required for M+ streptococci to bind CR1 that is expressed on the surfaces of quiescent neutrophils. Failure to be opsonized through this mechanism was proposed to account for resistance to phagocytosis that is mediated by M protein (7, 9). Therefore, streptococci with a mutant M protein that is unable to bind factor H should be less resistant to phagocytosis. Perez-Casal et al. constructed a mutant, strain JRS251, in which the entire C-repeat domain of M protein was deleted (20). Strain JRS251 bound significantly less factor H than its parent, strain JRS4. This M6 protein mutant, however, survived and multiplied in human blood in tests with the Lancefield bactericidal assay (20). This contradicted the suggestion that factor H binding is responsible for resistance to phagocytosis. Kotarsky confirmed that resistance to phagocytosis was not dependent on bound factor H (16). These reports led us to question whether C5a- or PMA-activated whole-blood PMNs had the capacity to phagocytize strain JRS251 streptococci.
FACS analysis revealed that strain JRS251 streptococci readily associate with nonactivated PMNs in whole blood (Fig. 6A). After 30 min of coincubation, 62% of nonactivated whole-blood PMNs were associated with strain JRS251 while only 18% associated with strain JRS4. Preactivation of PMNs with C5a increased the level of PMNs associated with strain JRS251 by only 10% (Fig. 6B). PMA-activated PMNs rapidly associated with strain JRS251, but PMA did not dramatically increase uptake of the wild-type streptococcal strain, JRS4 (Fig. 6B). The isogenic M6− strain JRS145 was phagocytized without prior C5a or PMA activation (Fig. 6A). These results are consistent with the suggestion of Horstmann et al. that resistance to phagocytosis depends in part on factor H binding to the C repeats of M protein (9). Although other explanations are possible, our data are also consistent with the possibility that more C3 convertase and C3b are deposited on the surface of the mutant strain JRS251 than on the surface of wild-type streptococcal strain. Moreover, the finding that activation of CR3 on PMNs by C5a has little impact on ingestion of strain JRS251 is consistent with the expectation that little iC3b is formed on the streptococcal surface in the absence of bound factor H.
FIG. 6.
Deletion of the factor H binding site from M6 protein increases susceptibility to phagocytosis. Strain JRS4 is the M6+ wild-type strain. Strain JRS251 expresses an M6 protein that is unable to bind factor H due to a deletion of the C repeats, and strain JRS145 is an M6− mutant (20). Data for strains JRS4, JRS145, and JRS251 are from experiments performed simultaneously with peripheral human whole-blood samples from the same individual. (A) Streptococci were incubated in blood with PMNs that had not been preactivated with PMA or rhC5a. The mean percentages of PMNs associated with streptococci from three independent experiments are shown. The difference in PMNs associated with strain JRS4 or JRS251 from nonactivated whole-blood PMNs is statistically significant at the 5-min (P = 0.005), 10-min (P = 0.0007), 15-min (P = 0.004), 20-min (P = 0.001), and 30-min (P = 0.004) time points. (B) The differences in the values for PMNs associated with JRS251 comparing C5a-activated to nonactivated whole-blood PMNs are statistically significant at the 15-min (P = 0.03), 20-min (P = 0.02), and 30-min (P = 0.04) time points. Significance was determined by using an unpaired two-tailed Student’s t test. The error bars depict the standard errors of the means.
DISCUSSION
Bacterial phagocytosis is a multistep process consisting of three primary stages: (i) recognition, (ii) attachment and engulfment, and (iii) lysosomal fusion and killing (24). In general, phagocytosis of bacteria requires the interaction of specific receptors on the phagocyte surface with bacterial bound opsonin. Streptococcal virulence is influenced by the ability of streptococci to resist phagocytosis, which is attributed to M protein (17). Fixation of C3b on the surface of M+ streptococci is significantly reduced compared to that of M− streptococci (10, 11).
Schnitzler et al. developed a flow cytometry-based assay to quantify phagocytosis of streptococci in whole blood and conducted preliminary experiments, which demonstrated that PMNs phagocytize M49+ streptococci in whole-blood PMNs activated with PMA (21). In addition to confirming their findings, we have demonstrated that whole-blood PMNs activated with the more physiologically relevant C5a readily associate with and kill M49+ SCPA+ streptococci. Stimulation of PMNs with C5a or PMA causes a dramatic increase in the number and functionality of CR3 receptors (19, 25, 23). Schnitzler et al. demonstrated that the association of PMNs with M49+ streptococci in whole blood pretreated with PMA is CR3 receptor dependent (21). In addition to confirming their findings, we have demonstrated that the association of C5a-activated whole-blood PMNs with M49+ SCPA+ streptococci is also CR3 receptor dependent. Blockade of CR3 receptors with anti-CD11b MAb inhibited C5a-activated PMN association with strain CS101 streptococci by 62%.
Previously, Jacks-Weis et al. employed [3H]thymidine-labeled streptococci and group C phage lysin to demonstrate that M49+ streptococci associated with PMNs were intracellular (10, 11). Schnitzler et al. used phase-contrast microscopy coupled with fluorescence microscopy to demonstrate that M49+ streptococci associated with PMNs were internalized (21). We confirmed both earlier studies by double-immunofluorescence microscopy. Equal numbers of adherent and internalized streptococci were observed to be associated with PMNs. Sorting PMNs associated with fluorescent M49+ streptococci in whole blood pretreated with or without C5a or PMA also showed a decrease in viability over time. In blood pretreated with C5a or PMA, the number of CFU per sorted PMN associated with M49+ streptococci decreased while the mean fluorescent intensity of PMNs associated with M49+ streptococci increased during the assay. The decrease in viable streptococci and increase in fluorescent intensity of the PMNs indicate that dead streptococci were accumulating within the PMNs.
Jacks-Weis et al. demonstrated that the alternative complement pathway is rapidly activated when streptococci are introduced into whole serum (10, 11). However, M+ streptococci bind significantly smaller amounts of factor B and properdin than M− streptococci (8). Factor B and properdin promote the formation of stable C3 convertase. Reduced binding of these ligands suggested a lack of intact C3b on the surface of M+ streptococci. Relative to M49− streptococci, the amount of C3 fixed to the surface of M49+ streptococci is significantly reduced (10). Therefore, the limited formation of stable C3 convertase would diminish amplification of alternative complement activation, effectively limiting the production of membrane attack complex and the amount of C5a produced to recruit PMNs to the site of infection.
In the FACS association assay, approximately 55% of the nonactivated PMNs from blood were associated with M49+ streptococci after 5 min of incubation (Fig. 1). However, after 30 min, only 30% of the PMNs were associated with M49+ streptococci. It appears that the early association of strain CS101 with nonactivated PMNs in blood does not result in PMN activation. This suggests that the basal expression and functional state of surface CR3 receptors on quiescent PMNs are not sufficient to mediate phagocytosis. Early PMN association with M49+ streptococci may represent a small, basal population of activated PMNs. The subsequent decrease in association may represent the removal of this PMN subset by lysis or apoptosis. Alternatively, CR3 receptors on the surface of an unstimulated PMN are in an inactive or nonfunctional state. Incubation of M49+ streptococci in heparinized whole blood does not alter the basal density of PMN CR3 receptors (23). Therefore, the inability of PMNs to remain associated with M49+ streptococci in blood with nonactivated PMNs may reflect the degradation of C3b on the surface of strain CS101 streptococci and their release from CR1 receptors. Degradation of C3b to iC3b causes a 100-fold decrease in CR1 affinity, effectively inhibiting the use of this receptor in the phagocytic uptake of the bacteria (15, 25).
Although the amount of C3 deposited on the surface of M+ bacteria is small relative to that for M− bacteria (10), the amount of C3 deposited on M49+ streptococci is sufficient for phagocytosis by activated PMNs. Experiments involving the blockade of CR3 receptors with anti-CD11b MAb demonstrated that M49+ must have enough iC3b opsonin present on their surface for phagocytosis to occur. Association and killing of M49+ streptococci were dependent upon C5a- or PMA-induced activation and up-regulation of functional CR3 receptors. The amount of CR1 receptors expressed on the cell surface of a quiescent PMN is presumed to be sufficient to arm the cell to ingest C3b opsonized M− streptococci. However, our data are consistent with the possibility that surface-bound C3b has been degraded to iC3b on M+ streptococci, possibly initiated by M-protein-bound factor H. The binding of factor H by strain CS101 has been detected by immunofluorescence microscopy (11). M+ streptococci do not have a sufficient amount of surface-bound C3b to promote their uptake via CR1 receptors by a quiescent PMN but instead may require interaction of CR3 receptors with iC3b. Therefore, activated PMNs, which have up-regulated functional CR3 receptors, are able to associate with iC3b bound to M+ streptococci by the PMNs.
Although early studies suggested that factor H binding to the C repeats of M protein was responsible for the ability of streptococci to resist phagocytosis (7, 9), subsequent work has questioned this conclusion. Deletion of the C repeats did not alter the capacity of streptococci to resist phagocytosis in whole blood by using the Lancefield phagocytosis system (20). Most recently, Kotarsky et al. (16) demonstrated the existence of a higher-affinity factor H binding site near the N-terminal end of the M5 protein. Again, deletion of this binding site did not increase sensitivity to phagocytosis. Our experiments contradict these more recent findings. We observed that a C-repeat mutant (20) associated more efficiently with PMNs than wild-type streptococci. Therefore, our data agree with those of Horstmann et al. (9) and suggest that factor H binding to M protein contributes to resistance to phagocytosis. Differences between our results and those of Kotarsky et al. could reflect strain differences of the means by which resistance to phagocytosis was measured.
Resistance to phagocytosis by M+ streptococci is a complex process that is slowly being unraveled. The work presented in this paper clearly demonstrates that activated PMNs in whole blood have the capacities to phagocytize and kill M+ streptococci. Other experiments suggested that SCPA inhibits the recruitment and activation of PMNs by destroying the chemotactic gradient established by alternative complement activation (12). Animal studies demonstrated that M49+ SCPA− streptococci are effectively cleared from intranasal and subdermal sites of infection (12–14). Thus, resistance to phagocytosis in vivo may require both M protein and the C5a peptidase. The above experiments suggest that coexpression of SCPA with M protein could prevent activation of CR3 receptors on PMNs, receptors that are required for ingestion of iC3b-coated M+ streptococci.
Acknowledgments
This work was funded in part by NIAID grant AI20016.
Editor: T. R. Kozel
REFERENCES
- 1.Berkower, C., M. Ravins, A. E. Moses, and E. Hanski. 1999. Expression of different group A streptococcal M proteins in an isogenic background demonstrates diversity in adherence to and invasion of eukaryotic cells. Mol. Microbiol. 31: 1463–1475. [DOI] [PubMed] [Google Scholar]
- 2.Caparon, M. G., R. T. Geist, J. Perez-Casal, and J. R. Scott. 1992. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J. Bacteriol. 174: 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleary, P., U. Prabu, J. Dale, D. Wexler, and J. Handley. 1992. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 60: 5219–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66: 4593–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cue, D. R., and P. P. Cleary. 1997. High-frequency invasion of epithelial cells by Streptococcus pyogenes can be activated by fibrinogen and peptides containing the sequence RGD. Infect. Immun. 65: 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31: 859–870. [DOI] [PubMed] [Google Scholar]
- 7.Fischetti, V. A. 2000. Surface protein on gram-positive bacteria, p. 11–24. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 8.Hong, K., T. Kinoshita, J. Takeda, H. Kozono, P. Pramoonjago, Y. U. Kim, and K. Inoue. 1990. Inhibition of the alternative C3 convertase and classical C5 convertase of complement by group A streptococcal M protein. Infect. Immun. 58: 2535–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control factor H. Proc. Natl. Acad. Sci. USA 85: 1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacks-Weis, J., Y. Kim, and P. P. Cleary. 1982. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J. Immunol. 128: 1897–1902. [PubMed] [Google Scholar]
- 11.Jacks-Weis, J. 1982. Opsonization and phagocytosis of group A streptococci. Ph.D. thesis, University of Minnesota, Minneapolis.
- 12.Ji, Y., L. McLandsborough, A. Kondagunta, and P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, Y., B. Carlson, A. Kondagunta, and P. P. Cleary. 1997. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect. Immun. 65: 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji, Y., N. Schnitzler, E. DeMaster, and P. P. Cleary. 1998. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect. Immun. 66: 5399–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalli, K. R., J. M. Ahearn, and D. T. Fearon. 1991. Interaction of iC3b with recombinant isotypic and chimeric forms of CR2. J. Immunol. 147: 590–594. [PubMed] [Google Scholar]
- 16.Kotarsky, H., M. Gustafsson, H. G. Svensson, P. F. Zipfel, L. Truedsson, and U. Sjobring. 2001. Group A streptococcal phagocytosis resistance is independent of complement factor H and factor H-like protein 1 binding. Mol. Microbiol. 41: 817–826. [DOI] [PubMed] [Google Scholar]
- 17.Lancefield, R. C. 1962. Current knowledge of the type specific M antigens of group A streptococci. J. Immunol. 89: 307–313. [PubMed] [Google Scholar]
- 18.Lapenta, D., C. Rubens, E. Chi, and P. P. Cleary. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 91: 12115–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo, S. K., P. A. Detmers, S. M. Levin, and S. D. Wright. 1989. Transient adhesion of neutrophils to endothelium. J. Exp. Med. 167: 1779–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Casal, J., N. Okada, M. G. Caparon, and J. R. Scott. 1995. Role of the conserved C-repeat region of Streptococcus pyogenes. Mol. Microbiol. 15: 907–916. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzler, N., G. Haase, A. Bussing, A. Kaufhold, P. Beyhs, and A. Podbielski. 1995. Measuring resistance to phagocytosis of group A and G streptococci: comparison of direct bactericidal assay and flow cytometry. Med. Microbiol. Immunol. 18: 417–422. [DOI] [PubMed] [Google Scholar]
- 22.Schnitzler, N., K. Schweizer, A. Podbielski, G. Haase, B. Spellerberg, R. Holland, and R. Lutticken. 1997. Activation of granulocytes by phorbol-12-myristate-14-acetate (PMA) enhances phagocytosis of Streptococcus pyogenes. Adv. Exp. Med. Biol. 418: 892–902. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzler, N., G. Haase, A. Podbielski, R. Lutticken, and K. G. Schweizer. 1999. A co-stimulatory signal through ICAM-β2 integrin-binding potentiates neutrophil phagocytosis. Nat. Med. 5: 231–235. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein, S. C., R. M. Steinman, and Z. A. Cohn. 1977. Endocytosis. Annu. Rev. Biochem. 46: 699–722. [DOI] [PubMed] [Google Scholar]
- 25.Sutterwala, F. S., L. A. Rosenthal, and D. M. Mosser. 1996. Cooperation between CR1 (CD35) and CR3 (CD11b/CD18) in the binding of complement-opsonized particles. J. Leukoc. Biol. 59: 883–890. [DOI] [PubMed] [Google Scholar]