Abstract
Staphylococcus aureus strains lacking agr- and sarA-dependent gene products or specific MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesins were compared for the ability to activate inflammatory responses in the lung. The mutants were evaluated for virulence in a mouse model of pneumonia and by quantifying their ability to stimulate interleukin-8 (IL-8) and granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in respiratory epithelial cells. In a neonatal mouse, only strains with intact agr and sarA loci were consistently associated with invasive, fatal pulmonary infection (P < 0.001) and sarA was specifically required to cause bacteremia (P < 0.001). The agr and/or sarA mutants were, nonetheless, fully capable of producing pneumonia and were as proficient as the wild-type strain in stimulating epithelial IL-8 expression, a polymorphonuclear leukocyte chemokine, in airway cells. In contrast, agr and especially sarA mutants induced less epithelial GM-CSF expression, and MSCRAMM mutants lacking fibronectin binding proteins or clumping factor A, a ligand for fibrinogen, were unable to stimulate epithelial GM-CSF production. The ability to induce IL-8 expression was independent of the adherence properties of intact bacteria, indicating that shed and/or secreted bacterial components activate epithelial responses. While conserved staphylococcal components such as peptidoglycan are sufficient to evoke inflammation and cause pneumonia, the agr and sarA loci of S. aureus are critical for the coordination of invasive infection of the lungs.
Staphylococcus aureus, a major cause of pulmonary infection, is associated with primary pneumonia in infants and young children and secondary pneumonia following viral infection and is one of the most common causes of hospital-acquired pneumonia (National Nosocomial Infection Surveillance Data, www.cdc.gov, 1999). S. aureus frequently contributes to airway disease in patients with cystic fibrosis, infecting 50% of patients by age 10 (Cystic Fibrosis Foundation Patient Registry Data, Annual Report 2000). Despite its association with pulmonary disease, most of the data used to explain the pathogenesis of S. aureus infection is derived from studies of infection involving skin and soft-tissue structures, endothelial surfaces, and professional immune cells. The expression of specific adhesins, fibronectin binding proteins (12), fibrinogen binding proteins, or MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (15) is important in these infections. Secreted S. aureus exoproducts, the hemolysins, coagulase, and exotoxins are associated with both local and hematogenous infections. S. aureus also evokes host inflammatory responses through the activation of chemokine and cytokine expression and the recruitment of polymorphonuclear leukocytes (PMNs) to the site of infection (24, 26, 31, 34).
The expression of many of these S. aureus virulence factors is regulated by the agr locus, a complex global regulator (7). The agr locus controls expression of RNAs II and III and is responsible for negative regulation of the synthesis of cell surface proteins, including protein A and coagulase, and positive regulation of the expression exoproducts such as the hemolysins and toxic shock syndrome toxin 1. The sarA locus, which includes three overlapping transcripts, all encoding the 14.5-kDa SarA protein (6), is important in the activation of several virulence genes, including those that encode the hemolysins and fibronectin binding proteins (33). Mutants that lack agr sarA function are less virulent in animal models of endophthalmitis (2) and endocarditis (4). However, the complex interactions of these loci make it difficult to predict the in vivo response to mutations in these global regulators.
Since S. aureus is a major respiratory pathogen, we were interested in establishing if the agr and sarA loci, which have been shown to be important in the pathogenesis of staphylococcal infection at other sites, are also critical for the establishment of infection in the respiratory tract. Using a neonatal-mouse model of respiratory tract infection, as well as a number of in vitro assays of chemokine and cytokine expression, we found that mutations affecting the global regulatory loci agr and sarA significantly impair the ability of the organisms to cause invasive infection. However, all of the staphylococcal strains examined retained the ability to induce proinflammatory responses in airway epithelial cells both in vitro and in vivo.
MATERIALS AND METHODS
Bacterial strains.
S. aureus RN6390 is a wild-type strain that, along with the mutants listed, has been previously characterized in detail (2, 4) (Table 1). RN6911 (agr) is a mutant of RN6390 with a Δagr::tetM insertion, ALC136 (sarA) is RN6390 with an sarA::Tn917LTV1 insertion, and ALC135 is RN6390 with both a Δagr::tetM insertion and an sarA::Tn917LTV1 insertion. ALC1645 (spA) is a protein A mutant of RN6390 (3). S. aureus Newman is a wild-type strain. Mutants of strain Newman include ALC60 (clfA), which lacks clumping factor, a major fibrinogen binding protein (32), and DU5886 (fnbpA fnbpB), which lacks fibronectin binding proteins A and B. CYGP medium was used for all of the S. aureus strains (20). Chemicals were obtained from Sigma Chemical Co., St. Louis, Mo., unless otherwise specified.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Reference(s) |
|---|---|---|
| RN6390 | Parental strain | 2, 33 |
| RN6911 | agr mutant of RN6390 with a Δagr::tetM insertion | 2, 33 |
| ALC136 | RN6390 with an sarA::Tn917LTV1 insertion | 2, 33 |
| ALC135 | agr sarA mutant of RN 6390 with both a Δagr::tetM and an sarA::Tn917LTV1 insertion | 2, 33 |
| ALC1645 | RN6390 spA (protein A) mutant | 3 |
| Newman | Wild type | 32 |
| ALC60 | Strain Newman clfA (clumping factor) mutant | 21 |
| DU5886 | Strain Newman fnbpA fnbpB (fibronectin binding proteins A and B) mutant | 33 |
Cell culture.
1HAEo−, a simian virus 40-transformed human airway cell line obtained from D. Gruennert, University of Vermont, Burlington (8), was grown in Dulbecco’s modified essential minimal medium (DMEM)-F12, and 16HBE cells, simian virus 40-transformed human bronchial epithelial cells, were grown in minimal essential medium (MEM) with 10% fetal bovine serum as described previously (23). 9HTEo−, a human tracheal epithelial cell line, was obtained from P. Davis (Case Western Reserve University, Cleveland, Ohio) and grown in DMEM-F12 with fetal bovine serum (23). All of the cell culture media used included penicillin, streptomycin, ℓ-glutamate, and amphotericin B.
Bacterial adherence assays.
1HAEo− cells were grown in 96-well plates until confluent as previously described (8). S. aureus strains grown overnight on CYGP plates were resuspended in phosphate-buffered saline (PBS) and labeled with [35S]methionine for 1 h at 37°C, washed, and resuspended in DMEM-F12 with 0.1% fetal calf serum at a concentration of 108 CFU/ml. Following a 60-min incubation with the epithelial cells and three PBS washes, monolayers were solubilized with 0.2% NaOH and scintillations were counted. Statistical analysis was performed by analysis of variance (Microsoft Excel 5.0).
Isolation of S. aureus peptidoglycan.
Peptidoglycan was isolated from 1-liter cultures of RN6390 and RN6911 by standard methods (10). The final material isolated was lyophilized and resuspended in PBS and used at a concentration of 50 μg/ml.
S. aureus induction of IL-8, G-CSF, and GM-CSF expression.
Confluent monolayers of 16HBE− human bronchial epithelial cells grown in 10-cm dishes were incubated for 60 min with 5 × 108 CFU of S. aureus RN6390 or RN6911 per ml. The epithelial cells were washed and lysed with RLT buffer in accordance with the RNeasy Mini Kit protocol (Qiagen, Valencia, Calif.). Lysate was homogenized with a Qiashredder, and total RNA was isolated with an RNeasy Mini Kit. Reverse transcription was performed with Omniscript Reverse Transcriptase (RT) from Qiagen and oligo(dT) from Perkin-Elmer (Boston, Mass.). PCR was done with Taq from Roche Biochemicals (Indianapolis, Ind.). The PCR primers used for human granulocyte colony-stimulating factor (G-CSF) were 5′-GCTTAGAGCAAGTGAGGAAG and 5′-AGGTGGCGTAGAACGCGGTA, those used for granulocyte-macrophage colony-stimulating factor (GM-CSF) were 5′-GGAGCATGTGAATGC CATC and 5′-ATCTGGGTTGCACAGGAAG, those used for IL-8 were 5′-TACTCCAAACCTTTCCAACCC and 5′-AACTTCTCCACAACCCTCTG, and those used for β-actin were 5′-GTGGGCCGCTCTAGGCACCA and 5′-GGTTGGCCTTAGGGTTCAGGGGGG.
IL-8 and GM-CSF ELISAs.
IL-8 and GM-CSF in 1HAEo− or 16HBE− cell culture supernatants were detected by enzyme-linked immunosorbent assay (ELISA) using monoclonal anti-IL-8 immunoglobulin G (R&D, Minneapolis, Minn.) as previously described (8) and the GM-CSF Quantikine kit (R&D). 1HAEo− cells were grown in DMEM-F12 medium, and 16HBE− cells were grown in MEM. Confluent monolayers in 96-well plates were weaned from serum for 24 h and then incubated for 60 min with either PBS, standardized inocula of the S. aureus strains listed above (5 × 108 CFU/ml), S. aureus lipoteichoic acid (10 to 100 μg/ml), peptidoglycan isolated from RN6390 and RN6911 (50 to 250 μg), or IL-1β (120 ng/ml) (CalBiochemical Corp., San Diego, Calif.), which was used as a positive control. The wells were washed and sterilized by the addition of 100 μg of gentamicin per ml, supernatants were harvested 18 h later, and IL-8 or GM-CSF was measured by ELISA. Control wells were stained with trypan blue to verify cellular viability of >75%. Quintuplicate wells were inoculated, and the mean and standard deviation were calculated. The data were tested for statistically significant variance with GraphPad InStat software by a one-way analysis of variance with Bonferroni’s post test to test the null hypothesis that there was no difference in the amount of the outcome variable (IL-8 or GM-CSF production) compared with that of the control strain. Each experiment was performed on at least two separate occasions, and a representative experiment was selected.
Mouse model of infection.
BALBc/ByJ mice (10 to 14 days of age; mean weight, 5.96 ± 2.1 g) were intranasally inoculated with an inoculum of 2 × 108 CFU of S. aureus in 10 μl as previously described (28) and returned to their mothers. Mice were sacrificed 24 h later by using Avertin (2,2,2-tribromoethanol; Aldrich, Milwaukee, Wis.). The left lung and the spleen were homogenized in 100 μl of PBS and cultured on CYGP plates. Colonies were tested for the expected antibiotic resistance markers. The right lung was fixed in formalin for routine histopathological studies. Pneumonia was defined as the recovery of >102 CFU of S. aureus per 100 μl of minced lung tissue, as well as histopathology indicative of acute bacterial pneumonia (PMN infiltration, hemorrhage, and edema). Bacteremia was defined as recovery of any staphylococci from the spleen. Animals found dead were treated similarly. All procedures were done in accordance with institutional animal care guidelines. Differences in the rates of pneumonia, bacteremia, and mortality at 24 h between the control group infected with RN6390 and the mutant strains were analyzed for statistical significance by chi-square analysis.
Histopathology and immunocytochemistry.
Snap-frozen sections of formalin-fixed mouse lungs were cryosectioned onto poly-ℓlysine-coated slides and dried at room temperature. Sections were incubated with 10 μg of goat anti-mouse GM-CSF antibody per ml and subsequently with a secondary antibody. Sections were then treated with Vectastain (Vector Labs, Burlingame, Calif.) ABS reagent, peroxidase substrate, and hematoxylin counterstain and mounted with Vectamount.
RESULTS
The agr locus is associated with virulence.
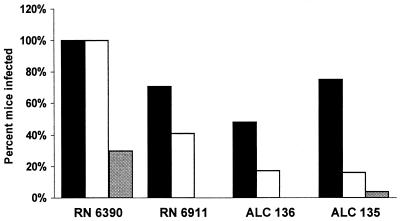
The virulence of S. aureus strains was tested in neonatal BALBc/ByJ mice following intranasal inoculation with either wild-type S. aureus RN6390 or a mutant strain. Since expression of agr- and sarA-dependent virulence factors is contingent upon cell density and growth phase (7, 22), the mutants were first compared to ascertain that, at least under laboratory conditions, their growth rates did not differ significantly (data not shown). At 18 h following inoculation, wild-type strain RN6390 was the most virulent, causing pneumonia and bacteremia in 100% of the mice inoculated and 30% mortality (Fig. 1). agr mutant strain RN6911 was less virulent than the parental strain; only 41% of the mice were bacteremic (P < 0.01 compared with RN6390), and RN6911 caused no deaths (P < 0.001). The mutants lacking sarA expression, with either a single mutation (ALC136) or the agr sarA double mutation (ALC135), respectively, caused bacteremia in 16 and 17% of the mice inoculated (P < 0.001 for each compared with RN6390). Only a single death was associated with ALC135 infection, and none was associated with ALC136, indicating the importance of the sarA locus in overall virulence.
FIG. 1.
Virulence of S. aureus strains in a neonatal-mouse model of infection. Numbers of mice used: RN6390, 30; RN6911, 31; ALC136, 32; ALC135, 46. The percentage of the total number of mice inoculated with each strain that developed pneumonia or bacteremia or died at 18 h following infection is indicated on the y axis. Black bars, % pneumonia; open bars, % bacteremia; shaded bars, % mortality.
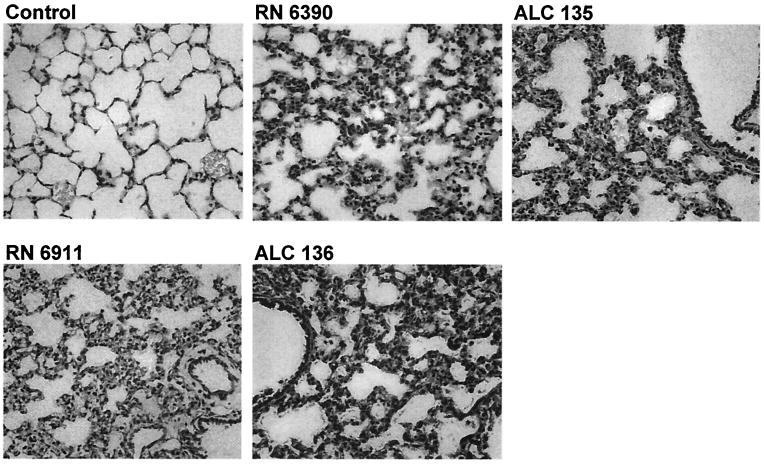
While agr and sarA were important in overall virulence, neither locus was essential for the induction of airway inflammation. Infection with an agr, sarA, or agr sarA mutant was associated with pneumonia in 71, 48, and 75%, of the mice, respectively, as defined by recovery of the organisms from the lungs (Fig. 1) and confirmed by histopathology (Fig. 2). Compared with the normal, uninfected lungs, a significant cellular inflammatory response was evident in those of all infected mice, with infiltration of PMNs. Edema of the tissues and cellular infiltration and consolidation were prominent. The S. aureus mutants caused significant inflammation, even though many animals did not appear ill, nor were they necessarily bacteremic.
FIG. 2.
Histology of S. aureus infection in the murine lung. Control, BALBc/ByJ mouse inoculated with PBS. RN6390, wild type (note edema, PMN infiltration, and loss of air spaces). ALC135, agr sarA mutant. RN6911, agr mutant. ALC136, sarA mutant (PMN infiltration and edema are similar to those associated with infection due to the wild-type strain). Magnification, ×250.
Staphylococcal stimulation of epithelial cytokine and chemokine expression.
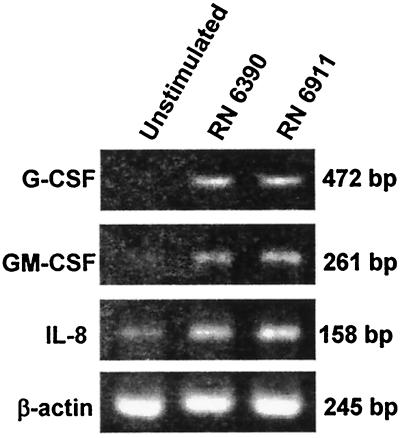
Bacterial interactions with airway epithelial cells induce the expression of proinflammatory chemokines such as IL-8 and the cytokines GM-CSF and G-CSF. These cytokines serve to recruit, activate, and inhibit PMN apoptosis (1, 17), as demonstrated in vitro and in animal studies of airway infection (18). Having found that wild-type and agr mutant strains elicit a PMN response in murine airways, we compared the abilities of the mutants to activate expression of these proinflammatory chemokines and cytokines in human airway cell lines (Fig. 3). Expression of G-CSF, GM-CSF, and IL-8 was assessed by RT-PCR. Following a 60-min incubation with equivalent inocula of RN6390 and RN6911, there was apparent induction of both cytokines and IL-8.
FIG. 3.
Induction of chemokine and cytokine expression by S. aureus. G-CSF, GM-CSF, and IL-8 expression in airway epithelial cells was assessed by RT-PCR in unstimulated control cells or following 60 min of incubation with 5 × 108 CFU of S. aureus RN6390 or RN6911 per ml.
Stimulation of IL-8 expression by staphylococci and staphylococcal components.
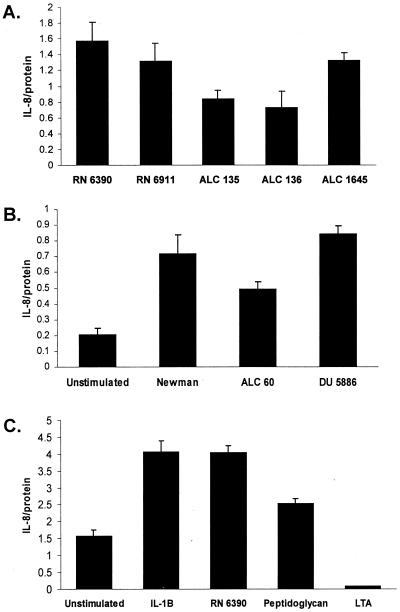
IL-8 is a major PMN chemokine expressed by human airway epithelial cells in response to bacterial infection. The amount of IL-8 produced by 1HAEo− epithelial monolayers following stimulation with S. aureus mutants was quantified by ELISA (Fig. 4A). For each of the strains tested, there was a linear IL-8 dose response for the 106 to 108 CFU/ml applied (data not shown). At the single concentration shown, there were no statistically significant differences in the amount of IL-8 production elicited by the agr mutant and parental strain RN6390. Both of the strains with sarA mutations elicited less IL-8 than did the wild type (P < 0.001 for both ALC135 and ALC136), although the amount of IL-8 expression induced by these sarA mutants was, nonetheless, within a range that is biologically significant. The protein A mutant (ALC1645) was fully capable of inducing IL-8 expression. S. aureus strain Newman and the isogenic mutant lacking fibronectin binding proteins A and B were equivalent in the ability to stimulate IL-8 production. ALC60, a clfA mutant lacking clumping factor, the major fibrinogen binding protein, stimulated 30% less IL-8 than did the parental strain (P < 0.001) (Fig. 4B). Staphylococcal lipoteichoic acid at concentrations ranging from 10 to 100 μg/ml did not elicit an IL-8 response (only one concentration shown), whereas S. aureus peptidoglycan from RN6390 (or RN6911 [data not shown]) stimulated equivalent amounts of IL-8 (Fig. 4C).
FIG. 4.
IL-8 expression in airway epithelial cells. Equivalent inocula of the S. aureus strains listed (5 × 108 CFU/ml), IL-1β (120 ng/ml), peptidoglycan isolated from strain RN6390 (50 μg/ml), and lipoteichoic acid (50 μg/ml) were incubated with 1HAEo− (A and B) or 16HBE− (C) cells for 60 min. The monolayers were sterilized with gentamicin and refed. Cell culture supernatants were harvested 18 h later, and IL-8 content was determined by ELISA and expressed as nanograms per milligram of protein. Each experimental point was determined in quintuplicate, and a mean and standard error were generated and subjected to analysis of variance. Each experiment was repeated at least twice, and representative results are shown.
GM-CSF expression is differentially induced by S. aureus mutants.
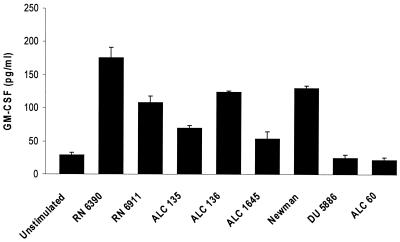
The proinflammatory cytokine GM-CSF is also expressed by epithelial cells in response to bacterial infection and has a major effect in preventing the constitutive apoptosis of activated PMNs (29). Staphylococcal mutants were tested for the ability to activate epithelial GM-CSF expression and to establish if the same staphylococcal ligands activate expression of IL-8 and GM-CSF, which are both dependent upon NF-κB for expression (Fig. 5). In contrast to the results obtained with IL-8, agr-dependent gene products were required to induce GM-CSF production. Both the agr and agr sarA mutants stimulated approximately 30% less GM-CSF than did the parental strain (P < 0.001 for each), and the agr sarA mutant stimulated 61% less (P < 0.001). A protein A mutant was also less effective in its activation of GM-CSF expression, stimulating a response equal to 31% of that of the parental strain (P < 0.001). S. aureus strain Newman stimulated slightly less GM-CSF production than did RN6390, and mutants lacking expression of the MSCRAMM ligands responsible for fibronectin binding and fibrinogen binding were unable to activate GM-CSF production (P < 0.001). There were no significant differences in the induction of G-CSF expression by the wild-type and mutant strains (data not shown).
FIG. 5.
GM-CSF expression in airway epithelial cells following exposure to S. aureus. 1HAEo− cell culture supernatants harvested 18 h following a 60-min incubation with a 5 × 108-CFU/ml inoculum of each strain listed were screened for GM-CSF content by ELISA. The cells were weaned to 0.1% fetal calf serum for 24 h prior to inoculation, and duplicate wells were stained with trypan blue to assess viability. Each experimental point was determined in quintuplicate, and a mean and standard error were generated and subjected to analysis of variance.
GM-CSF expression in the murine lung.
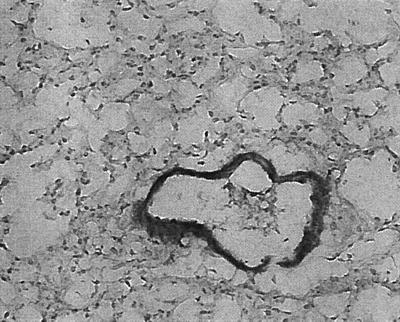
To document that GM-CSF is expressed in vivo in response to pulmonary S. aureus infection, sections of mouse lung were screened for GM-CSF 24 h following inoculation with the S. aureus strains (Fig. 6). There was prominent expression of GM-CSF in the cells lining the large airways, stimulated with either the wild-type or the agr mutant S. aureus strain, and much less GM-CSF production was associated with the cells lining the smaller airways.
FIG. 6.
Immunohistological detection of GM-CSF expression by the airway epithelial cells lining the large murine bronchi. Frozen sections of murine lung were examined for GM-CSF expression 18 h following intranasal administration of RN6390. A representative section is shown demonstrating immunoreactive material in the cells lining the large airways. Original magnification, ×250.
S. aureus adherence does not correlate with virulence.
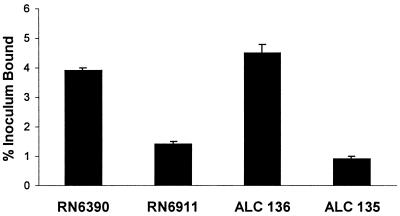
For many pathogens, virulence can be correlated directly with tissue binding, as has been demonstrated for S. aureus soft-tissue infections. The abilities of agr, sarA, and agr sarA mutant and wild-type strains of S. aureus to bind to respiratory epithelial cells were quantified (Fig. 7). Approximately 3.9% of the inoculum of parental strain RN6390 was adherent, almost threefold more than that of agr mutant RN6911 (P < 0.001). The sarA mutant ALC136 bound slightly more than the wild-type strain (consistent with the negative effect of sarA on surface protein expression). The agr sarA double mutant ALC135 bound much less than did the wild-type strain (P < 0.001) and slightly less than the agr mutant, with less than 1% of the inoculum adherent. Binding was dependent upon the presence of a small amount of serum (0.1%) and did not correlate with the ability to cause pneumonia in the murine lung.
FIG. 7.
Adherence properties of S. aureus strains. The relative adherence of 35S-labeled organisms to 1HAEo− airway epithelial cell monolayers was measured. The percentage of the added inoculum (5 × 108 CFU/ml) that bound is indicated.
DISCUSSION
In the studies detailed in this report, the interactions of S. aureus and airway epithelial cells were examined to determine the contributions of the agr and sarA loci to the pathogenesis of pulmonary infection. As expected, these global regulatory loci were important for coordination of the expression of the many gene products required for invasive infection. In contrast, the ability to elicit PMN-dominated airway inflammation was a fundamental property of all of the S. aureus strains studied and was not dependent upon the agr locus. Much of the initial inflammatory response to inhaled bacteria is signaled by the mucosal cells lining the respiratory tract. S. aureus has the potential to activate host cytokine and chemokine responses by the direct effects of cellular components such as peptidoglycan, lipoteichoic acid, or other adhesins on the mucosal epithelium (27). agr+ staphylococci activate IL-8 expression by airway cells through the ligation of asialo-GM1 receptors, stimulation of Ca2+ fluxes, mitogen-activated protein kinase phosphorylation, and activation of NF-κB (24). As shown in this report, agr mutant organisms can also activate IL-8, as well as GM-CSF, expression, indicating that several S. aureus gene products are likely to be involved in this host-bacterium interaction.
S. aureus has the potential to interact with mucosal cells in several different ways; through superficial interactions of shed bacterial products such as protein A or peptidoglycan and epithelial receptors, by adherence of intact bacteria to host epithelial cells, or by invasion and internalization of the bacteria (9, 25). Several reports have documented S. aureus stimulation of endothelial cytokine responses (26, 31, 35) and have identified genes that are activated by the internalization of these bacteria by different cell types (9). The in vitro studies detailed, with brief exposure of the airway cells to the bacteria, are likely to reflect superficial interactions between the organisms and the epithelium. The ability of S. aureus to adhere to airway epithelial cells did not directly correlate with induction of IL-8 expression or with the ability to induce pneumonia in mice. The agr mutants had less than 25% of the binding capability of either the wild-type or the sarA mutant strain but virtually the same ability to induce IL-8 expression. This is consistent with the hypothesis that loosely bound or shed components such as peptidoglycan or protein A are immunostimulatory and neither attachment of intact organisms nor frank invasion of the epithelial cell is required to activate airway cytokine and chemokine responses. This differs from other models of S. aureus infection in which the expression of a specific adhesin, such as fibronectin binding protein (25), for example, is required to initiate disease. Instead, induction of airway inflammation can be mediated by several different staphylococcal adhesins and corresponding receptors and is not necessarily dependent upon the expression of single virulence factors that are critical for the pathogenesis of S. aureus infection at other sites.
Bacterial induction of epithelial IL-8 expression, while critical in the recruitment of a PMN response to bacterial infection in the airway (14), is not the only proinflammatory response element expressed. To determine if there is a global proinflammatory response to S. aureus, we also monitored the expression of G-CSF and GM-CSF, which are NF-κB-dependent cytokines expressed by pulmonary epithelial cells in response to bacteria (18). Whereas the ability to induce IL-8 expression was relatively conserved among the S. aureus strains, the ability to stimulate GM-CSF production was significantly diminished by loss of agr function. S. aureus mutants lacking fibronectin binding proteins or lacking fibrinogen binding were unable to stimulate GM-CSF production, suggesting a more specific ligand-receptor interaction in the GM-CSF response. The fibrinogen binding domain of this adhesin has been mapped (13), and it is possible that a similar locus is available on respiratory cells. Alternatively, staphylococcal “clumping,” a property conferred by clfA, may be necessary to provide a sufficient bacterial stimulus to activate epithelial signaling. Although expression of both IL-8 and GM-CSF is regulated by NF-κB, there is some specificity to the activation of the epithelial response to staphylococcal gene products.
Staphylococcal peptidoglycan and lipoteichoic acid activate NF-κB-dependent gene expression through recognition of the Toll-like receptors, specifically, TLR2 (35). However, the distribution and function of Toll-like receptors and associated adapter proteins and signaling kinases have not been established for airway epithelial cells. We were surprised to find that lipoteichoic acid does not activate IL-8 expression, as we have unpublished data that indicate that TLR-2 is available on the cell lines used for these assays. Peptidoglycan, as expected, was a potent stimulus for IL-8 expression and did not appear to be affected by agr function. A more complete analysis of epithelial Toll-like receptor function in mucosal cells is necessary for identification of the additional components of the respiratory epithelial signaling system.
Whereas the ability to elicit epithelial inflammatory responses was common to all of the S. aureus strains studied, only organisms with intact agr and sarA loci caused severe invasive infection in mice, with 100% rates of bacteremia and pneumonia. Both loci are involved in the coordinate expression of surface proteins and secreted exoproducts (5), their regulation by bacterial growth phase (7), and expression of autoinducers (16). The agr mutant caused bacteremia in fewer than half of the animals inoculated and caused no mortality. The sarA mutants, with minimal fibronectin binding protein A expression, as well as decreased hemolysin and toxin expression, produced bacteremia in fewer than 20% of the mice. These results are consistent with the known function of SarA in activating the expression of S. aureus hemolysins that facilitate local tissue destruction and entry into the bloodstream (5). The expression of S. aureus fibronectin binding proteins is sufficient for bacterial invasion in vitro (25). Fibronectin binding protein A, which is increased in the agr mutant (33) and required for internalization (9), may contribute to the 40% rate of bacteremia associated with infection due to agr mutant strain RN6911. While it is difficult to ascribe the virulence phenotype of a complex mutant to any single gene product, the phenotypes observed can be explained by known effects of the agr and sarA loci on major virulence factors. Interference with the receptor histidine kinase of the highly conserved agr regulon has been recently targeted as a potential inhibitor of S. aureus virulence (19) and might be useful in this setting for the prevention of invasive disease.
This distinction between the staphylococcal gene products that initiate local inflammation in the airways and those associated with invasive infection may be clinically relevant. An analysis of S. aureus isolated from cystic fibrosis patients demonstrated that many strains lack expression of agr-dependent gene products in vivo (11). These cystic fibrosis patients have significant airway inflammation but not invasive S. aureus infection. The histology of these infections indicates that most organisms are enmeshed in mucus and are neither adherent to the epithelium nor intracellular (30). The experiments described in this report may help to explain these clinical observations. Shed staphylococcal cell wall components, as well as whole organisms, whether lysed or intact, contribute to the activation of epithelial inflammatory responses. From our studies, direct attachment of intact bacteria to epithelial receptors is not necessary for induction of epithelial cytokine responses associated with PMN-dominated inflammation, although it may be essential for initiation of invasive infection. The pathogenesis of S. aureus infection of the airways, as introduced by inhalation, has several important differences from S. aureus infection of other sites. Although invasive infection may require agr-mediated coordination of virulence gene expression, the ability to stimulate PMN recruitment appears to be a widely conserved property of S. aureus. Strategies for the inhibition or prevention of pulmonary S. aureus infection may require targets different from those expected to interfere with the pathogenesis of other types of staphylococcal infection.
Acknowledgments
This work was supported by National Institutes of Health (HL56194 and HL60293) and Cystic Fibrosis Foundation grants to A.P. G.H. was supported by a stipend from the Cystic Fibrosis Foundation.
Editor: E. I. Tuomanen
REFERENCES
- 1.Bergmann, M., P. J. Barnes, and R. Newton. 2000. Molecular regulation of granulocyte macrophage colony-stimulating factor in human lung epithelial cells by interleukin (IL)-1beta, IL-4, and IL-13 involves both transcriptional and post-transcriptional mechanisms. Am. J. Respir. Cell Mol. Biol. 22: 582–589. [DOI] [PubMed] [Google Scholar]
- 2.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65: 1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung, A. L., K. Eberhardt, and J. H. Heinrichs. 1997. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect. Immun. 65: 2243–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94: 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien, Y., and A. L. Cheung. 1998. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 273: 2645–2652. [DOI] [PubMed] [Google Scholar]
- 6.Chien, Y., A. C. Manna, S. J. Projan, and A. L. Cheung. 1999. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 274: 37169–37176. [DOI] [PubMed] [Google Scholar]
- 7.Coulter, S. N., W. R. Schwan, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. R. Folger, A. S. Bayer, and C. K. Stover. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30: 393–404. [DOI] [PubMed] [Google Scholar]
- 8.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101: 2598–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziewanowska, K., A. R. Carson, J. M. Patti, C. F. Deobald, K. W. Bayles, and G. A. Bohach. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68: 6321–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.). 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 11.Goerke, C., S. Campana, M. G. Bayer, G. Döring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 13.Hartford, O. M., E. R. Wann, M. Hook, and T. J. Foster. 2001. Identification of residues in the Staphylococcus aureus fibrinogen-binding MSCRAMM clumping factor A (ClfA) that are important for ligand binding. J. Biol. Chem. 276: 2466–2473. [DOI] [PubMed] [Google Scholar]
- 14.Heeckeren, A., R. Walenga, M. W. Konstan, T. Bonfield, P. B. Davis, and T. Ferkol. 1997. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Investig. 100: 2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hienz, S. A., T. Schennings, A. Heimdahl, and J. I. Flock. 1996. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J. Infect. Dis. 174: 83–88. [DOI] [PubMed] [Google Scholar]
- 16.Ji, G., R. C. Beavis, and R. P. Novick. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92: 12055–12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, J. B., M. J. Rane, J. A. Scherzer, P. Y. Coxon, R. Kettritz, J. M. Mathiesen, A. Buridi, and K. R. McLeish. 2000. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 164: 4286–4291. [DOI] [PubMed] [Google Scholar]
- 18.Kube, D., U. Sontich, D. Fletcher, and P. B. Davis. 2001. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 280: L493–L502. [DOI] [PubMed] [Google Scholar]
- 19.Lyon, G. J., P. Mayville, T. W. Muir, and R. P. Novick. 2000. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. USA 97: 13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novick, R. P. 1990. The staphylococcus as a molecular genetic system. VCH Publishers, New York, N.Y.
- 21.Palma, M., D. Wade, M. Flock, and J. I. Flock. 1998. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J. Biol. Chem. 273: 13177–13181. [DOI] [PubMed] [Google Scholar]
- 22.Pohlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Doring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68: 4865–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan, S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23: 304–312. [DOI] [PubMed] [Google Scholar]
- 24.Ratner, A. J., R. Bryan, A. Weber, S. Nguyen, D. Barnes, A. Pitt, S. E. Gelber, A. Cheung, and A. Prince. 2001. Cystic fibrosis pathogens activate Ca++-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 8: 8. [DOI] [PubMed] [Google Scholar]
- 25.Sinha, B., P. Francois, Y. A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. H. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68: 6871–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soell, M., M. Diab, G. Haan-Archipoff, A. Beretz, C. Herbelin, B. Poutrel, and J. P. Klein. 1995. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect. Immun. 63: 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuyt, R. J., M. G. Netea, S. H. Kim, D. Novick, M. Rubinstein, B. J. Kullberg, J. W. Van Der Meer, and C. A. Dinarello. 2001. Differential roles of interleukin-18 (IL-18) and IL-12 for induction of gamma interferon by staphylococcal cell wall components and superantigens. Infect. Immun. 69: 5025–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang, H. B., E. DiMango, R. Bryan, M. Gambello, B. H. Iglewski, J. B. Goldberg, and A. Prince. 1996. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 64: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarr, P. E. 1996. Granulocyte-macrophage colony-stimulating factor and the immune system. Med. Oncol. 13: 133–140. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich, M., S. Herbert, J. Berger, G. Bellon, D. Louis, G. Munker, and G. Doring. 1998. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am. J. Respir. Cell Mol. Biol. 19: 83–91. [DOI] [PubMed] [Google Scholar]
- 31.Wang, J. E., P. F. Jorgensen, M. Almlöf, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 68: 3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolz, C., D. McDevitt, T. J. Foster, and A. L. Cheung. 1996. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect. Immun. 64: 3142–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolz, C., P. Pohlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36: 230–243. [DOI] [PubMed] [Google Scholar]
- 34.Yao, L., F. D. Lowy, and J. W. Berman. 1996. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect. Immun. 64: 3407–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163: 1–5. [PubMed] [Google Scholar]