Abstract
Although Streptococcus intermedius and Streptococcus mutans are regarded as members of the commensal microflora of the body, S. intermedius is often associated with deep-seated purulent infections, whereas S. mutans is frequently associated with dental caries. In this study, we investigated the roles of the S. mutans and S. intermedius antigen I/II proteins in adhesion and modulation of cell surface characteristics. By using isogenic mutants, we show that the antigen I/II in S. mutans, but not in S. intermedius, was involved in adhesion to a salivary film under flowing conditions, as well as in binding to rat collagen type I. Binding to human fibronectin was a common function associated with the S. mutans and S. intermedius antigen I/II. Adhesion of S. mutans or S. intermedius to human collagen types I or IV was negligible. Hydrophobicity, as measured by water contact angles, and zeta potentials were unaltered in the S. intermedius mutant. The S. mutans isogenic mutants, on the other hand, exhibited more positive zeta potentials at physiological pH values than did the wild type. The results indicate common and species-specific roles for the antigen I/II in mediating the attachment of S. mutans and S. intermedius to host components and in determining cell surface properties.
Streptococcus mutans and Streptococcus intermedius are commensal organisms found in the human oral cavity. S. mutans is a late colonizer mainly found in dental plaque (34) and is associated with dental caries (23). S. intermedius is found particularly in the gingival crevice around the margins of the teeth (30). S. intermedius also is a resident of the gastrointestinal and urogenital tracts and is associated with abscess formation and with tropism for infections of the brain and liver (51). Like other oral streptococci, S. mutans and S. intermedius are implicated as causative agents of infective endocarditis (9, 10, 12, 40, 51).
Most oral streptococci, including S. mutans and S. intermedius, express one or more members of a family of structurally and antigenically related surface proteins termed antigen I/II. These proteins have received a variety of names according to the strains or species in which they were identified, such as antigen B (42), Sr (35), I/II (14), and PAc (36) from S. mutans, Spa A (18) from Streptococcus sobrinus; PAa from Streptococcus cricetus (44); and SspA and SspB from two tandemly arranged genes in Streptococcus gordonii (8). In S. intermedius a 160-kDa protein is observed (38), whereas the antigen I/II molecular mass in other oral streptococci ranges from 170 to 215 kDa (8, 14, 18, 35, 36, 42).
Investigation of the antigen I/II adhesive properties, particularly in S. mutans and S. gordonii, has been the focus of numerous studies, since attachment to host components represents a crucial event during bacterial colonization and infection (13). Despite its importance as a commensal organism and as a causative agent of purulent lesions, functional investigations on the role of S. intermedius surface molecules are limited. Recent studies have shown, however, that the antigen I/II in S. intermedius exhibit adhesive and stimulatory properties associated with virulence mechanisms (38).
In the oral cavity, bacterial adhesion occurs on surfaces coated with salivary films. Static binding assays have demonstrated that antigen I/II is essential for S. mutans (16) but not for S. gordonii adhesion to salivary films (8). Such binding has not been investigated for S. intermedius. In the oral cavity, bacterial adhesion to salivary conditioned surfaces occurs under the influence of hydrostatic pressure and shear forces during salivary flow. These dynamic conditions may influence bacterial adhesion. For instance, in Pseudomonas aeruginosa, surface structures associated with adhesion under static conditions were not involved in adhesion to the same substrata under flowing conditions (7).
The ability of bacteria to bind extracellular matrix (ECM) components is regarded as a significant factor in the development of abscesses and infective endocarditis, since tissue damage often precedes streptococcal colonization (2, 52). Immobilized antigen I/II fragments from several oral streptococci are shown to bind soluble ECM, such as fibronectin, laminin, and collagen type I (43). Moreover, isogenic mutants of S. mutans and S. gordonii deficient in antigen I/II exhibit diminished adhesive capacity to immobilized collagen type I (24). Collagen binding, suggestively, contributes to the ability of streptococci to invade dentinal tubules (24). Streptococci may, however, interact differently with soluble and immobilized ECM (5, 25, 29).
Cell surface properties associated with adhesion, such as hydrophobicity and surface charge are influenced by bacterial surface molecules, such as those present in fimbriae and fibrils (28, 48). Although S. mutans does not exhibit such structures, a “fuzzy layer” on its surface has been associated with the antigen I/II (21). Hydrophobicity is reported to be reduced in S. mutans isogenic mutants deficient in antigen I/II (11, 27, 36).
The aim of the present study was to investigate the role of the S. mutans and S. intermedius antigen I/II in bacterial binding to and detachment from a salivary film under flowing conditions and its role in adhesion to immobilized extracellular matrix proteins. We also examined the contribution of antigen I/II to hydrophobicity and surface charge.
MATERIALS AND METHODS
Bacterial strains and culture media.
The S. intermedius strains used in this study included the type strain NCTC 11324 and its antigen I/II isogenic mutant IB08981 (38). The S. mutans strains included S. mutans LT11, a highly transformable variant of UA159 (46), and two antigen I/II isogenic mutants inactivated at different sites, IB10991 and IB03987 (described below). The strains were stored at −70°C in brain heart infusion broth (BHIB; Difco Laboratories, Detroit, Mich.) supplemented with 15% (vol/vol) glycerol. Streptococcal strains prepared for the immunoblotting, adhesion, zeta potential and contact angle assays were grown in BHIB at 37°C under microaerophilic conditions. For streptococcal transformation, Todd-Hewitt broth (THB; Difco Laboratories) containing 5% heat-inactivated horse serum (HS) was used as the growth medium, and incubation was at 37°C under microaerophilic conditions. Escherichia coli carrying the plasmid pSF151 was grown in Luria-Bertani broth (Difco Laboratories) supplemented with kanamycin (Sigma-Aldrich AS, Oslo, Norway) at a final concentration of 50 μg/ml. For the selection of isogenic mutants, kanamycin was used at a final concentration of 500 μg/ml.
DNA isolation.
The streptococcal integration vector pSF151 was isolated from E. coli with the Qiagen Plasmid Maxi kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s recommendations for high-copy-number plasmids. pSF151 replicates in E. coli but not in streptococci and expresses kanamycin resistance in both organisms (45).
Streptococcal chromosomal DNA was isolated by the modified CTAB (cetyltrimethylammonium bromide) method, as previously described (39).
PCR amplification of target insert.
The forward primer (5′-GTCAGTGGCAACGATTTATCCAA) and the reverse primer (5′-AATAATTCGTTGAACCGGCAAGA) were used to amplify a homologous region in S. mutans LT11 and S. intermedius NCTC 11324. The amplified fragments corresponded to a conserved region in the antigen I/II protein downstream of the S. mutans and S. intermedius antigen I/II proline-rich regions (26, 44). The final PCR volume of 100 μl contained 245 ng of bacterial DNA template, 3 U of Dynazyme (Finnzymes Oy, Espoo, Finland), 0.8 mM deoxynucleoside triphosphate mixture, and 1× Dynazyme buffer with 1.5 mM MgCl2. The following parameters for PCR amplification were used: 94°C for 3 min (initial denaturing period); 25 cycles of 94°C for 30 s (denaturing period), 55°C for 1 min (annealing period), 72°C for 1 min (extension period); and 72°C for 5 min (final extension period).
Insertional inactivation.
The antigen I/II targeting insert for the inactivation of the antigen I/II at homologous sites in S. mutans LT11 and S. intermedius NCTC 11324 was derived from a conserved antigen I/II gene sequence described above. The amplified fragments were extracted from 0.7% agarose gel by high-speed centrifugation (53), purified with glass milk according to the recommended protocol (Geneclean; Bio 101 Inc., Carlsbad, Calif.), and digested with the restriction enzyme Tsp509I. The plasmid pSF151 was digested with EcoRI and ligated to the Tsp509I-restricted fragments. The ligation mixtures (10 μg of pSF151 and 0.75 μg of the targeting insert) were used to transform S. mutans LT11 and S. intermedius NCTC 11324. Transformation of S. intermedius by the addition of synthetic competence factor resulted in the isogenic mutant IB08981, previously described (38). S. mutans transformation was performed as described by Perry and Kuramitsu (37) with modifications (39). Briefly, S. mutans overnight culture was transferred to a fresh THB-HS medium in a 1:40 dilution and incubated at 37°C for 3 h. At this point, the pSF151-targeting insert ligation mixture was added to 1 ml of the bacterial culture and incubated for an additional 2 h. The transformants were selected by plating the cells on THB-HS agar plates containing kanamycin. An isogenic mutant was randomly selected for further characterization and named IB10991. For the inactivation of the S. mutans antigen I/II gene at the 5′-terminal end, a similar strategy was used, except that the target insert was derived from Sau3AI-digested pSAD7B ligated to BamHI-restricted pSF151. pSAD7B carries the N-terminal encoding sequence of the S. mutans OMZ 175 antigen I/II (43). An isogenic mutant that did not react with the antibody for the antigen I/II was randomly selected for further characterization and named IB03987.
Immunoblotting.
Bacterial pellets and supernatants were obtained from streptococcal cultures in the exponential phase. The pellets were washed twice with phosphate-buffered saline (pH 7.4; PBS), resuspended in extraction buffer (0.05 M Tris pH 6.8; 2% sodium dodecyl sulfate [SDS]; 10% glycerol; 0.1 M dithiothreitol; 0.004% pyronin) and were boiled for 3 min. The culture supernatants were lyophilized and resuspended in loading buffer. Polypeptides derived from equivalent amounts of bacterial pellets and supernatants were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on 7.5% gels with the discontinuous buffer system of Laemmli (17) and then electroblotted onto nitrocellulose membranes. The blots were incubated with rabbit anti-I/II immunoglobulin G raised against purified Sr (anti-SR I/II) from S. mutans OMZ 175. Antibody binding was revealed with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G and enzyme substrate (BCIP [5-bromo-4-chloro-3-indolylphosphate] and nitroblue tetrazolium [both from Sigma]).
Southern blotting and sequencing.
Chromosomal DNA was digested with EcoRI, electrophoresed on 0.7% agarose gel, and transferred to a positively charged nylon membrane (Boehringer Mannheim GmbH, Mannhein, Germany). The random primed method was used for digoxigenin-11-dUTP (Boehringer Mannheim) labeling of the pSF151 probe, as recommended by the manufacturer. Prehybridization and hybridization were performed with DIG Easy Hyb (Boehringer Mannheim) at 45°C. Chemiluminescent substrate was used for the detection of hybridization signals on X-ray films.
Insertion inactivation at homologous sites in the selected isogenic mutants S. mutans IB10991 and S. intermedius IB08981 was confirmed by sequencing (Fig. 1). The forward primer described above for gene inactivation, 5′-GTCAGTGGCAACGATTTATCCAA, and the reverse primer annealing to pSF151, 5′-AGCGGATAACAATTTCACACAGGA, were used. The parameters for PCR amplification were the same as described above for amplification of the target insert. The PCR products were prepared for sequencing by purification with shrimp alkaline phosphatase and exonuclease I (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer’s instructions. Sequencing was undertaken by using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems, Stockholm, Sweden), and the final products were run on the ABI Prism 377 (PE Applied Biosystems) by using 5% Long Ranger Gels (FMC Bioproducts, Rockland, Maine). The DNA sequences were determined on both strands.
FIG. 1.
DNA and amino acid sequence of the pSF151 insertion site at homologous antigen I/II regions within the chromosome of the S. intermedius IB08981 and S. mutans IB10991 isogenic mutants. This region corresponds to the 1,099 to 1,154 amino acid residues in the full-length (1,527-amino-acid) S. mutans MT8148 antigen I/II (accession no. P11657). The amplified fragment used for insertional inactivation was flanked by the Tsp509I restriction sites, as indicated. The forward primer used for sequencing and amplification of the target site is underlined. ✽, Identical bases.
Adhesion to a salivary film in a parallel plate flow chamber.
Bacterial adhesion to the bottom glass plate of a parallel plate flow chamber was determined with a phase-contrast microscope coupled to a CCD-MXR camera and an image analyzer. Bacterial overnight cultures collected by centrifugation were washed twice and resuspended in adhesion buffer to a final concentration of 3 × 108 cells/ml. Pooled freeze-dried human whole saliva, prepared as previously described (49), was reconstituted in adhesion buffer (2 mM potassium phosphate, 50 mM potassium chloride, 1 mM calcium chloride; pH 6.8) to a final concentration of 1.5 mg/ml. The bottom glass plate was coated overnight at 4°C with the reconstituted saliva.
The bacterial flow rate was adjusted to 1.4 ml/min under the influence of a hydrostatic pressure at a shear rate of 15 s−1, which corresponds to physiological conditions likely to be found on the tooth surface (6). Twenty-five live images were taken every 1 to 2 min for the first 30 min and thereafter at 10 to 30 min intervals for up to 4 h, when the flow was stopped. The images obtained were subtracted from the out-of-focus image, after which the number of attached cells was determined. The initial deposition rate was calculated as the number of microorganisms that adhered during the first 30 min per unit of time and area. An air bubble was then passed through the chamber, and the percentage of detached cells was calculated.
Adhesion to matrix proteins.
S. mutans LT11 and the isogenic mutant IB03987 were assayed for adhesion to immobilized matrix proteins according to the protocol previously described for S. intermedius NCTC 11324 and its isogenic mutant IB08981 (38). Bacterial cells were radioactively labeled by overnight growth in the presence of [methyl-3H]thymidine (6 μCi/ml, 85 Ci/mmol; Amersham Corp.), giving between 1 × 10−3 to 4 × 10−3 dpm per cell. The cells were collected by centrifugation, washed twice with PBS, and resuspended to an optical density at 600 nm (OD600) of 2.4. Cell suspensions were sonicated for 10 s at a power output of 20%. Microscopic examination confirmed the disruption of the majority of the chains.
Detachable microtiter plates (MaxiSorp; Nunc A/S, Roskilde, Denmark) were coated with human fibronectin (2 μg/well; Sigma), human collagen I (2.5 μg/well; Sigma), human collagen IV (2.5 μg/well; Sigma), or rat collagen I (5 μg/well; Collaborative Biomedical Products, Bedford, Mass.) in a volume of 100 μl/well. At least two experiments, with five parallels each, were conducted. Fibronectin was diluted in 0.05 M Tris-buffered saline (pH 7.5; TBS) and collagen in 0.01 M acetic acid. Wells coated with TBS or acetic acid were used as the respective controls. The plates were incubated at room temperature for 2 h. After incubation the wells were washed four times with PBS. Nonspecific protein-binding sites were blocked with 200 μl of 1% bovine serum albumin (Sigma) in PBS by incubation at room temperature for 30 min. The content of the wells was then aspirated, and 50 μl of 0.2% PBS-bovine serum albumin and 50 μl of the bacterial suspension were added to each well. The plates were incubated with gentle shaking at 37°C for 2 h. Unbound cells were removed by washing the plates four times with PBS containing 0.05% Tween 20 (Sigma). The wells were then detached and transferred to separate vials containing 5 ml of scintillation liquid. Radioactivity was measured as disintegrations per minute in a liquid scintillation analyzer (Packard 1900 TR; Packard Instrument Company, Meriden, Conn.).
Cell surface analysis.
For contact angle measurements, a layer of overnight-grown bacterial cells washed and resuspended in distilled water was deposited on membrane filters (0.45 μm [pore size]; Millipore Corp., Bedford, Mass.) under negative-pressure filtration. After 30 min of air drying at 25°C, sessile droplets of water were placed on the bacterial lawn for the assessment of contact angles.
Bacterial zeta potentials of overnight-grown cells washed with distilled water and resuspended in 10 mM potassium phosphate with adjusted pH values of 2, 3, 4, 5, 7, and 9 were measured with a Lazer Zee meter equipped with an image analysis program.
Microbial adhesion to the hydrocarbon hexadecane was measured as described by Westergren and Olsson (50). Cells at early, mid-, late, and stationary phases were washed twice with PBS and resuspended to an OD450 of 1.0. Volumes of 1.2-ml bacterial suspensions were mixed with 75, 150, or 200 μl of hexadecane. The OD in the aqueous phase was then measured (at 450 nm), and the reduction in binding to hexadecane was calculated.
Statistical analysis.
Student’s t test or the paired t test was used for two group comparisons. One-way analysis of variance followed by the Student-Newman-Keuls test was used for multiple comparisons.
RESULTS
Construction of antigen I/II isogenic mutants.
Disruption of the antigen I/II gene at homologous sites in the isogenic mutants S. intermedius IB08981 and S. mutans IB10991 was verified by sequencing (Fig. 1). These isogenic mutants expressed a truncated antigen I/II gene product of ca. 125 kDa in S. intermedius IB08981 and of 150 kDa in S. mutans IB10991, which was found predominantly in the supernatants (Fig. 2). This was in contrast to the wild types S. intermedius NCTC 11324 and S. mutans LT11, in which antigen I/II was found primarily in the whole-cell extracts. The antigen I/II molecular masses in S. intermedius NCTC 11324 and S. mutans LT11 were ca. 160 and 185 kDa, respectively (Fig. 2). Neither the cell extract nor the supernatant of the isogenic mutant S. mutans IB03987 reacted with anti-SR (Fig. 2). The insertion of pSF151 in the chromosome of the isogenic mutants was confirmed by Southern hybridization.
FIG. 2.
Immunoblot analysis of antigen I/II expression in the cell extract (lanes 1 to 5) and supernatant (lanes 6 to 10) of S. intermedius NCTC 11324 (lanes 1 and 6) and the isogenic mutant IB08981 (lanes 2 and 7) and of S. mutans LT11 (lanes 3 and 8) and the isogenic mutants IB10991 (lanes 4 and 9) and IB03987 (lanes 5 and 10). The positions of the molecular mass markers are indicated.
Binding to salivary film.
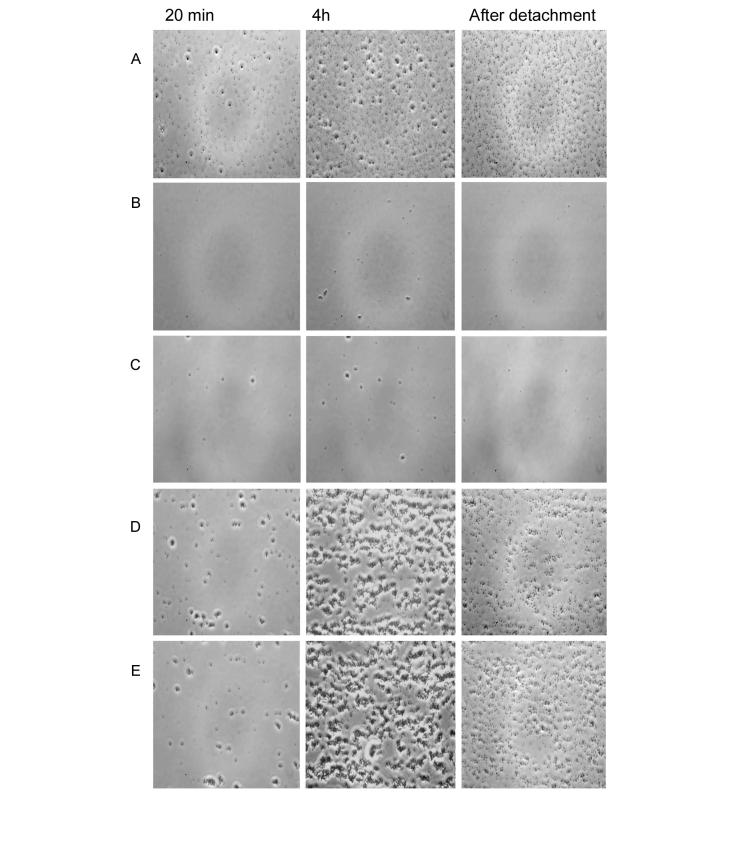
The role of the S. mutans and S. intermedius antigen I/II in adhesion to a salivary film under flowing conditions was investigated by comparing isogenic mutants defective in production of antigen I/II with the respective wild types (Table 1). Phase-contrast microscope images of adherent cells are shown in Fig. 3. Compared to S. mutans LT11, the S. mutans IB10991 initial adhesion rate and final number of attached cells (4 h) to the salivary coated glass was reduced by 90%. No significant difference was found between the S. mutans isogenic mutants IB10991 and IB03987. S. intermedius NCTC 11324 and the isogenic mutant IB08981 adhered similarly and in significant numbers to the salivary film.
TABLE 1.
Bacterial adhesion to saliva-coated glass
| Strain | Mean no. of attached cells/cm2 (SD)a
|
% DetachmentB(SD) after air bubble passagec | |
|---|---|---|---|
| Initial bacterial adhesion rate (s−1) | After 4 h of flowing (106) | ||
| S. mutans | |||
| LT11 | 962 (314) | 8.4 (1.5) | 46 (14) |
| IB10991 | 103 (119)b | 0.4 (0.2)b | - |
| IB03987 | 26 (12)b | 0.3 (0.2)b | - |
| S. intermedius | |||
| NCTC 11324 | 1,558 (536) | 9.9 (1.0) | 49 (11) |
| IB08981 | 1,370 (840) | 11.0 (0.9) | 40 (8) |
Mean and standard deviation values were derived from triplicate experiments.
Significantly different from the wild type (P < 0.05) as calculated by one-way analysis of variance, followed by the Student-Newman-Keuls test.
-, The number of adhering cells was too low for accurate analysis (<0.15 × 106/cm2).
FIG. 3.
Phase-contrast microscope images of bacterial adhesion to salivary film in the parallel plate flow chamber. (A) S. mutans LT11. (B) S. mutans IB10991. (C) S. mutans IB03987. (D) S. intermedius NCTC 11324. (E) S. intermedius IB08981. The images represent 25 consecutive shots taken at 20 min or 4 h of flowing, and after air-bubble passage.
Bacterial detachment after 4 h of flowing was calculated as the percentage of detached cells by air bubble passage (Table 1). Detachment of S. mutans LT11 was ca. 45%, while for the isogenic mutants the remaining adherent bacteria were in too low numbers to allow accurate analysis. No significant difference in detachment was observed between S. intermedius NCTC 11324 and its isogenic mutant IB08981.
Binding to immobilized matrix proteins.
The isogenic mutant IB03987 was compared to the wild-type S. mutans LT11 for its ability to adhere to fibronectin and collagen immobilized onto microtiter plate wells (Table 2). Adhesion of the isogenic mutant to fibronectin and rat collagen I was reduced by 19 and 65%, respectively. Neither the wild type nor the mutant exhibited significant binding to human collagen types I and IV. In S. intermedius IB08981, adhesion to fibronectin was reduced by 75%, whereas neither the wild type nor the mutant adhered significantly to rat collagen I or human collagen types I and IV.
TABLE 2.
Adherence to ECM proteins
| Strain | Mean no. of cells bound (106) (SD)a
|
Source or reference | |||
|---|---|---|---|---|---|
| Human fibronectin (n = 3) | Rat collagen I (n = 3) | Human collagen I (n = 2) | Human collagen IV (n = 2) | ||
| S. mutans | |||||
| LT11 | 37 (9.0) | 21 (12.0) | NS | NS | This study |
| IB03987 | 30 (10.8)* | 7 (14.4)* | NS | NS | This study |
| S. intermedius | |||||
| NCTC 11324 | 1.6 (0.5) | NS | NS | NS | 39 |
| IB08981 | 0.4 (0.2)* | NS | NS | NS | 39 |
| Control (S. mutans OMZ 175) | - | 26 (3.0)b | 22 (4.3) | 16 (1.2) | 39 |
*, Significantly different from the wild type (P < 0.05) as calculated by paired t test. NS, counts not significantly different from background values. -, Not tested.
n = 2.
Cell surface properties associated with antigen I/II.
Inactivation of the S. mutans and S. intermedius antigen I/II gene had no significant effect on the contact angles with water (Table 3).
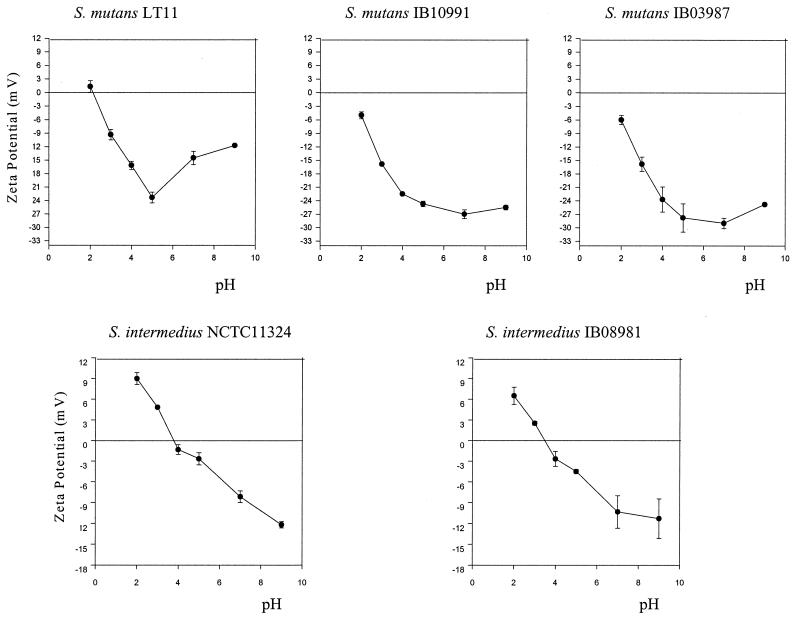
The effect of antigen I/II inactivation on bacterial zeta potentials differed between S. mutans and S. intermedius (Fig. 4). At physiological pH values (pH 5 and 7), S. mutans LT11 exhibited a more positive zeta potential than its isogenic mutants IB10991 and IB03987, whereas no difference was found between S. intermedius NCTC 11324 and IB08981. In S. mutans, disruption of the antigen I/II gene in the mutants resulted in the shift of the isoelectric point toward more acidic values than in the wild type.
FIG. 4.
Effect of antigen I/II inactivation on bacterial zeta potentials measured at various pH values. The means and standard errors (bars) of triplicate assays are shown.
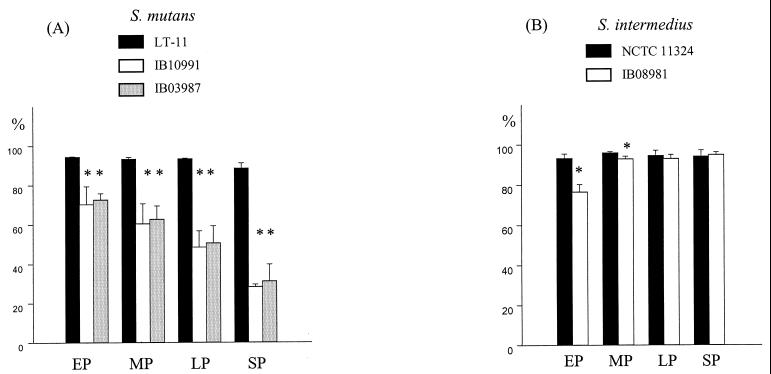
The adhesion to hexadecane was calculated as the percent loss in OD relative to that of the initial bacterial suspension (Fig. 5). Maximum S. mutans adhesion occurred with 150 μl of hexadecane. Reduction in adherence of the S. mutans isogenic mutants to hexadecane varied from 25% at early growth phase to 68% at the stationary phase. In contrast, binding of the S. intermedius mutant to hexadecane was reduced at the early and mid-phases of exponential growth by 18 and 4%, respectively.
FIG. 5.
Hydrophobicity of S. mutans LT11 and the isogenic mutants IB10991 and IB03987 (A) and of S. intermedius NCTC 11324 and the isogenic mutant IB08981 (B) during early (EP), mid- (MP), late (LP), and stationary (SP) phases. Hydrophobicity was calculated as the percent adhesion to hexadecane. Error bars indicate the standard errors of triplicate samples. ✽, Significantly different from the wild type (P < 0.05), as calculated by one-way analysis of variance followed by the Student-Newman-Keuls test (A) or Student’s t test (B).
DISCUSSION
The region C terminal to the antigen I/II proline-rich segment (26) was used as a target to disrupt the gene in S. mutans and S. intermedius at homologous sites. Disruption at this site resulted in truncated proteins, observed as faint bands on the immunoblotting of whole-cell preparations. The transformants revealed high levels of truncated proteins in the supernatants, in contrast to the wild types, which showed low levels of the native protein. This is in accordance with previous observations that the S. mutans antigen I/II surface anchoring region is localized at the C terminus (20, 32, 33). The known antigen I/II sequences show the conserved LPXTG C-terminal motif commonly found in other gram-positive C-terminal anchored proteins (8, 14, 35, 36, 44, 47). The respective secreted truncated proteins were ca. 35 kDa smaller than the antigen I/II expressed by S. intermedius and S. mutans wild types. This suggests that the difference in size of the S. intermedius and S. mutans native antigen I/II, which corresponds to ca. 160 and 185 kDa, respectively, is most likely due to differences upstream of the insertion site. As the present study was being finalized, the entire sequence of the S. intermedius antigen I/II became available (gi 14571813, deposited by H. Tamura). It reveals that the antigen I/II amino acid sequence in S. intermedius exhibits ca. 250 fewer residues than in S. mutans, confirming the size difference observed by immunoblotting. The overall degree of sequence identity is ca. 70%, with the S. intermedius sequence varying mostly due to fewer residues in the alanine-rich repetitive region and in the conserved proline-rich sequence.
In this study we showed that adhesion to salivary film under conditions of flow was reduced in the S. mutans isogenic mutant, whereas both S. intermedius wild-type and mutant strains adhered in significant numbers to the salivary film. The results indicate that while antigen I/II is essential for adhesion of S. mutans to the salivary film, adhesion of S. intermedius was unaffected by the inactivation of the antigen I/II gene. Antigen I/II segments C terminal of the proline-rich region and N-terminal sequences, including the alanine-rich region, have been identified as being involved in S. mutans binding to salivary components (14, 15, 31). Protein sequence homology search with BLAST (1) reveals that the S. mutans segment C terminal of the proline-rich region (amino acid residues 1025 to 1044) (14) shows homology to one of the sequenced segments in S. intermedius (44). Four- to five-amino-acid substitutions are, however, observed. One of the substitutions is in the E1037 residue, being K in S. intermedius. Site-directed mutagenesis studies have identified E1037 as one of the two residues important for S. mutans binding to salivary components. Since binding of antigen I/II requires sialic acid residues in S. gordonii, a different receptor to salivary components has been suggested. In S. mutans binding seems to occur in a sialic-independent manner (15). In S. gordonii antigen I/II, a K residue substitutes the E1037 residue, as in S. intermedius. It has been suggested that such a substitution could affect the recognition of salivary receptors by this domain (15). It is also possible that the difference in size of the alanine-rich sequence in S. intermedius, compared to S. mutans, may have an effect on the capacity of the S. intermedius antigen I/II to recognize salivary receptors. A definitive conclusion as to the difference in the role of the antigen I/II observed between S. mutans and S. intermedius in binding to salivary components requires, however, further investigation.
Bacterial surface structures associated with adhesion under static conditions may not play a similar role in adhesion under flowing conditions (7, 41). Factors such as shear forces and physical collisions between suspended and adhering microorganisms are part of the dynamic conditions present in a flowing system. In the oral cavity, bacterial adhesion occurs under salivary flowing conditions. The S. mutans antigen I/II binding to the salivary film under flowing conditions was in accordance with static binding assays showing adhesion of S. mutans to salivary components by antigen I/II (16, 19). The two S. mutans isogenic mutants exhibited more than 90% reduction in the initial adhesion rate to the salivary film. This difference was still observed after 4 h of flow. There was no difference between the S. mutans isogenic mutants IB10991 and IB03987. Accordingly, inactivation of the gene at any site will result in loss of the anchoring motif and release of the truncated protein.
In the oral cavity, the microorganisms are exposed to high detachment forces as, for instance, during eating and swallowing. During such events, air-liquid interfaces frequently pass over the salivary conditioned enamel surface. In our study, the percentage of detached cells after the passage of an air bubble was similar for both the S. intermedius wild type and the mutant strain, suggesting that the wild type and the isogenic mutant present similar binding affinities to the salivary film. The low number of adhering S. mutans isogenic mutants after air bubble passage restrained comparison of detachment values in the isogenic mutants and wild type.
Tissue damage may expose ECM macromolecules and allow bacterial adhesion. Streptococcal adhesion to fibronectin is purported, for instance, as a pivotal step in the pathogenesis of endocarditis (2, 52). ECM proteins may expose different microbial binding domains when they are in soluble or immobilized forms (22). Binding of antigen I/II derived polypeptides to soluble fibronectin has previously been reported (43). In this study we show that the S. mutans, as well as the S. intermedius antigen I/II were associated with binding also to immobilized human fibronectin.
Streptococcal adhesion to collagen type I may be a critical factor for invasion of dentinal tubules during dental caries. The antigen I/II produced by S. mutans and S. gordonii are related to both tubule invasion and binding to collagen type I (24). We found that the antigen I/II in S. mutans LT-11 was associated with binding to rat collagen type I, but not to human collagen I. The results stress the importance of the matrix protein source used, since the structure of human ECM proteins may differ from those of other animals (22). Adhesion of S. mutans and S. intermedius to human collagen types I and IV was feeble for both the wild type and the mutants. In contrast to S. mutans, the S. intermedius antigen I/II is not associated with binding to rat collagen type I (38).
Bacterial hydrophobicity and surface charge are postulated as driving forces for the initial adhesion of microorganisms. These properties may be influenced by the presence of specific cell surface receptor sites (3). Adhesion to hydrocarbons and water contact angles are currently used as measurements of bacterial hydrophobicity. The role of the antigen I/II in binding to the hydrocarbon hexadecane varied among S. mutans and S. intermedius. Disruption of the antigen I/II in S. mutans resulted in mutants with progressively reduced adhesion to hexadecane with growth. In S. intermedius, the mutant bound less to the hexadecane at initial growth phases, compared to the wild type, whereas no significant difference was observed between the wild type and its mutant at late log or stationary phase. Our results confirmed previous reports that the S. mutans antigen I/II is related to binding to hexadecane at the stationary phase (11, 27, 36). Measurement of hydrophobicity based on adhesion to hydrocarbons may, however, be influenced by the negatively charged surface of hydrocarbons in the commonly used buffer systems (4). Measurement of water contact angles is regarded, therefore, as a more accurate method to estimate bacterial hydrophobicity. Accordingly, the S. mutans LT11 exhibited a more positive zeta potential at physiological pH values than the isogenic mutant. Our results indicate therefore that disruption of the antigen I/II affected the S. mutans surface charge rather than the bacterial hydrophobicity.
The inactivation of the antigen I/II-encoding gene provided evidence for the species-specific role of the antigen I/II protein in the ability of S. mutans to bind to a salivary film under flowing conditions, and to influence S. mutans surface charge, compared to S. intermedius. On the other hand, the antigen I/II was associated with binding of both S. mutans and S. intermedius to fibronectin. There has been considerable interest in developing strategies to interfere with S. mutans adhesion by targeting the antigen I/II protein. Since most oral streptococci express proteins belonging to the antigen I/II family, identification of common, as well as species-specific, antigen I/II functions might have future clinical implications.
TABLE 3.
Contact angles with water (θw)a
| Strain | Mean θw (SD) |
|---|---|
| S. mutans | |
| LT11 | 29 (2.7) |
| IB10991 | 31 (1.0) |
| IB03987 | 33 (1.0) |
| S. intermedius | |
| NCTC 11324 | 51 (3.0) |
| IB08981 | 44 (10.0) |
The mean and standard deviation values derived from triplicate experiments are shown.
Acknowledgments
We thank J. Ogier for the kind gift of anti-SR I/II and pSAD7B and U. R. Dahle for his generous assistance with the sequencing. We are grateful to J. Smit, M. Axelsson, S. Stig, and M. Weidemann for excellent technical assistance.
Editor: E. I. Tuomanen
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M. 1994. Virulence factors among gram-positive bacteria in experimental endocarditis. Infect. Immun. 62: 2143–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busscher, H. J., M. M. Cowan, and H. C. van der Mei. 1992. On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol. Rev. 8: 199–209. [DOI] [PubMed] [Google Scholar]
- 4.Busscher, H. J., B. van de Belt-Gritter, and H. C. van der Mei. 1995. Implications of microbial adhesion to hydrocarbons for evaluating cell-surface hydrophobicity. 1. Zeta-potentials of hydrocarbon droplets. Colloids Surfaces B. Biointerfaces 5: 111–116. [Google Scholar]
- 5.Chia, J. S., C. Y. Yeh, and J. Y. Chen. 2000. Identification of a fibronectin binding protein from Streptococcus mutans. Infect. Immun. 68: 1864–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawes, C., S. Watanabe, P. Biglow-Lecomte, and G. H. Dibdin. 1989. Estimation of the velocity of the salivary film at some different locations in the mouth. J. Dent. Res. 68: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 7.De Kievit, T. R., R. Gillis, S. Marx, C. Brown, and B. H. Iglewski. 2001. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 67: 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demuth, D. R., Y. Duan, W. Brooks, A. R. Holmes, R. McNab, and H. F. Jenkinson. 1996. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol. Microbiol. 20: 403–413. [DOI] [PubMed] [Google Scholar]
- 9.Gossling, J. 1988. Occurrence and pathogenicity of the Streptococcus milleri group. Rev. Infect. Dis. 10: 257–285. [DOI] [PubMed] [Google Scholar]
- 10.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44: 331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington, D. J., and R. R. B. Russell. 1993. Multiple changes in cell wall antigens of isogenic mutants of Streptococcus mutans. J. Bacteriol. 175: 5925–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, J. A. 1997. The “Streptococcus milleri” group: Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius. Rev. Med. Microbiol. 8: 73–80. [Google Scholar]
- 13.Jenkinson, H. F., and D. R. Demuth. 1997. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23: 183–190. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, C., P. Evans, L. Bergmeier, S. F. Lee, A. Progulske-Fox, A. C. Harris, A. Aitken, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 258: 127–132. [DOI] [PubMed] [Google Scholar]
- 15.Kelly, C. G., J. S. Younson, B. Y. Hikmat, S. M. Todryk, M. Czisch, P. I. Haris, I. R. Flindall, C. Newby, A. I. Mallet, J. K. Ma, and T. Lehner. 1999. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat. Biotechnol. 17: 42–47. [DOI] [PubMed] [Google Scholar]
- 16.Koga, T., N. Okahashi, I. Takahashi, T. Kanamoto, H. Asakawa, and M. Iwaki. 1990. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect. Immun. 58: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 18.LaPolla, R. J., J. A. Haron, C. G. Kelly, W. R. Taylor, C. Bohart, M. Hendricks, J. P. Pyati, R. T. Graff, J. K. Ma, and T. Lehner. 1991. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect. Immun. 59: 2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, M. S., C. Seok, and D. A. Morrison. 1998. Insertion-duplication mutagenesis in Streptococcus pneumoniae: targeting fragment length is a critical parameter in use as a random insertion tool. Appl. Environ. Microbiol. 64: 4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. F., and L. Gao. 2000. Mutational analysis of the C-terminal anchoring domains of Streptococcus mutans P1 antigen: role of the LPXTGX motif in P1 association with the cell wall. Can. J. Microbiol. 46: 584–592. [PubMed] [Google Scholar]
- 21.Lee, S. F., A. Progulske-Fox, G. W. Erdos, D. A. Piacentini, G. Y. Ayakawa, P. J. Crowley, and A. S. Bleiweis. 1989. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect. Immun. 57: 3306–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ljungh, A., and T. Wadstrom. 1995. Binding of extracellular matrix proteins by microbes. Methods Enzymol. 253: 501–514. [DOI] [PubMed] [Google Scholar]
- 23.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50: 353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love, R. M., M. D. McMillan, and H. F. Jenkinson. 1997. Invasion of dentinal tubules by oral streptococci is associated with collagen recognition mediated by the antigen I/II family of polypeptides. Infect. Immun. 65: 5157–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowrance, J. H., D. L. Hasty, and W. A. Simpson. 1988. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect. Immun. 56: 2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, J. K., C. G. Kelly, G. Munro, R. A. Whiley, and T. Lehner. 1991. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect. Immun. 59: 2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride, B. C., M. Song, B. Krasse, and J. Olsson. 1984. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect. Immun. 44: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNab, R., H. Forbes, P. S. Handley, D. M. Loach, G. W. Tannock, and H. F. Jenkinson. 1999. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J. Bacteriol. 181: 3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNab, R., A. R. Holmes, J. M. Clarke, G. W. Tannock, and H. F. Jenkinson. 1996. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect. Immun. 64: 4204–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mejare, B., and S. Edwardsson. 1975. Streptococcus milleri (Guthof), an indigenous organism of the human oral cavity. Arch. Oral Biol. 20: 757–762. [DOI] [PubMed] [Google Scholar]
- 31.Moisset, A., N. Schatz, Y. Lepoivre, S. Amadio, D. Wachsmann, M. Scholler, and J. P. Klein. 1994. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect. Immun. 62: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami, Y., Y. Nakano, Y. Yamashita, and T. Koga. 1997. Identification of a frameshift mutation resulting in premature termination and loss of cell wall anchoring of the PAc antigen of Streptococcus mutans GS-5. Infect. Immun. 65: 794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami, Y., Y. Yamashita, Y. Nakano, H. O. Ito, H. Yu, and T. Koga. 1997. Role of the charged tail in localization of a surface protein antigen of Streptococcus mutans. Infect. Immun. 65: 1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyvad, B., and M. Kilian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24: 267–272. [DOI] [PubMed] [Google Scholar]
- 35.Ogier, J. A., M. Scholler, Y. Leproivre, A. Pini, P. Sommer, and J. P. Klein. 1990. Complete nucleotide sequence of the sr gene from Streptococcus mutans OMZ 175. FEMS Microbiol. Lett. 56: 223–227. [DOI] [PubMed] [Google Scholar]
- 36.Okahashi, N., C. Sasakawa, M. Yoshikawa, S. Hamada, and T. Koga. 1989. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol. Microbiol. 3: 673–678. [DOI] [PubMed] [Google Scholar]
- 37.Perry, D., and H. K. Kuramitsu. 1981. Genetic transformation of Streptococcus mutans. Infect. Immun. 32: 1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen, F. C., S. Pasco, J. Ogier, J. P. Klein, S. Assev, and A. A. Scheie. 2001. Expression and functional properties of the Streptococcus intermedius surface protein antigen I/II. Infect. Immun. 69: 4647–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen, F. C., and A. A. Scheie. 2000. Genetic transformation in Streptococcus mutans requires a peptide secretion-like apparatus. Oral Microbiol. Immunol. 15: 329–334. [DOI] [PubMed] [Google Scholar]
- 40.Piscitelli, S. C., J. Shwed, P. Schreckenberger, and L. H. Danziger. 1992. Streptococcus milleri group: renewed interest in an elusive pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 11: 491–498. [DOI] [PubMed] [Google Scholar]
- 41.Reddy, K., and J. M. Ross. 2001. Shear stress prevents fibronectin binding protein-mediated Staphylococcus aureus adhesion to resting endothelial cells. Infect. Immun. 69: 3472–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell, R. R. 1979. Wall-associated protein antigens of Streptococcus mutans. J. Gen. Microbiol. 114: 109–115. [DOI] [PubMed] [Google Scholar]
- 43.Sciotti, M. A., I. Yamodo, J. P. Klein, and J. A. Ogier. 1997. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol. Lett. 153: 439–445. [DOI] [PubMed] [Google Scholar]
- 44.Tamura, H., T. Kikuchi, R. Shirato, and H. Kato. 2001. Cloning and DNA sequencing of the surface protein antigen I/II (PAa) of Streptococcus cricetus. FEMS Microbiol. Lett. 196: 251–256. [DOI] [PubMed] [Google Scholar]
- 45.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120: 105–110. [DOI] [PubMed] [Google Scholar]
- 46.Tao, L., T. J. MacAlister, and J. M. Tanzer. 1993. Transformation efficiency of EMS-induced mutants of Streptococcus mutans of altered cell shape. J. Dent. Res. 72: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 47.Tokuda, M., N. Okahashi, I. Takahashi, M. Nakai, S. Nagaoka, M. Kawagoe, and T. Koga. 1991. Complete nucleotide sequence of the gene for a surface protein antigen of Streptococcus sobrinus. Infect. Immun. 59: 3309–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Mei, H. C., P. S. Handley, R. Bos, and H. J. Busscher. 1998. Oral biofilms and plaque control. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 49.van Hoogmoed, C. G., M. van Der Kuijl-Booij, H. C. van Der Mei, and H. J. Busscher. 2000. Inhibition of Streptococcus mutans NS adhesion to glass with and without a salivary conditioning film by biosurfactant-releasing Streptococcus mitis strains. Appl. Environ. Microbiol. 66: 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westergren, G., and J. Olsson. 1983. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect. Immun. 40: 432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiley, R. A., D. Beighton, T. G. Winstanley, H. Y. Fraser, and J. M. Hardie. 1992. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J. Clin. Microbiol. 30: 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willcox, M. D., C. Y. Loo, D. W. Harty, and K. W. Knox. 1995. Fibronectin binding by Streptococcus milleri group strains and partial characterisation of the fibronectin receptor of Streptococcus anginosus F4. Microb. Pathog. 19: 129–137. [DOI] [PubMed] [Google Scholar]
- 53.Wu, W., M. J. Welsh, P. B. Kaufman, and H. H. Zhang. 1997. Methods in gene biotechnology. CRC Press, Inc., Boca Raton, Fla.