Abstract
The sialylation of lipooligosaccharide (LOS) in Neisseria meningitidis plays a role in the resistance of the organism to killing by normal human serum. The length of the α chain extending out from the heptose I [Hep (I)] moiety of LOS influenced sialylation of N. meningitidis LOS in vitro and in vivo. The α chain required a terminal Gal and a trisaccharide or longer oligosaccharide to serve as an acceptor for sialylation. The disaccharide lactose (Galβ1-4Glc) in the α chain of immunotype L8 LOS could not function as an acceptor for the sialyltransferase, probably due to steric hindrance imposed by the neighboring Hep (II) with phosphorylethanolamine and another group attached.
Neisseria meningitidis and Neisseria gonorrhoeae are obligate human pathogens. N. meningitidis isolated from blood or cerebrospinal fluid is encapsulated (5). Besides the contribution of its capsule to the survival of N. meningitidis in the bloodstream, sialylation of lipooligosaccharide (LOS) enhances the resistance of the pathogen to the complement-mediated bactericidal activity of human serum (3, 9). Sialylation of LOS also increases the resistance of N. meningitidis to opsonophagocytosis by human neutrophils (2). Therefore, both encapsulation and LOS sialylation contribute to the survival of N. meningitidis in the human bloodstream. For N. gonorrhoeae, which does not possess a capsule, sialylation of LOS has been shown to be critical for the survival of the organism in human serum (1, 22). Fresh gonococcal isolates from urethral exudates are resistant to complement-mediated serum bactericidal activity, but the resistance is usually lost upon subculture in vitro (30). The loss of serum resistance can be restored by the sialylation of LOS on the cell surfaces of N. gonorrhoeae cells with CMP-NeuNAc (18).
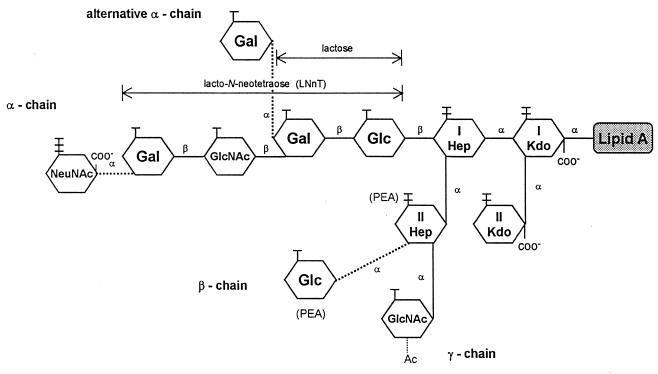
N. meningitidis can be classified into 12 immunotypes, L1 through L12, on the basis of the antigenicities of LOSs (25). Most case isolates expressed the L3,7,9 phenotype (8). Structural studies (14, 19) have shown that N. meningitidis LOS consists of a branched oligosaccharide (OS) composed of 5 to 10 monosaccharide units linked to lipid A through 3-deoxy-d-manno-octulosonic acid (Kdo) as presented in Fig. 1. Two Kdos and two heptoses, heptose I [Hep (I)] and Hep (II), are conserved in all LOS immunotypes. Lacto-N-neotetraose (LNnT) (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) and its related OSs are attached to Hep (I), and this branch is designated the α chain (6, 10, 20). Two other very short sugar chains are attached to Hep (II), a β chain with Glc at position 3 in L2 and L5 LOSs and a γ chain with GlcNAc at position 2 (14). Phosphorylethanolamine (PEA) replaces the β-chain Glc at position 3 of Hep (II) in the L1, L3, L7, and L8 OSs (6, 14). In contrast, PEA is attached to position 6 or 7 in L2, L4, and L6 LOSs (14).
FIG. 1.
Schematic presentation of N. meningitidis LOS. The α chains of L2, L3, L4, L5, L7, and L9 LOSs contain LNnT structures (14). The alternative α chain of L1 LOS is a trisaccharide, Galα1-Galβ1-Glc. The L6 LOS lacks the terminal Gal in the LNnT structure, and the L8 LOS is a lactosyl disaccharide (20). The terminal Gals of LNnT and the L1 trisaccharide are often partially sialylated. Ac, acetyl residue.
Many N. meningitidis LOSs are heterogeneous in that they have 4.0- and 3.6-kDa predominant components and other minor components as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (24, 26). LOS heterogeneity results in the expression of multiple immunotypes in many N. meningitidis isolates (10, 20). The LNnT structure in the α chain is a common structure in the 4.0-kDa components of many immunotype LOSs of N. meningitidis (14). The terminal Gal of LNnT in 4.0-kDa components of LOSs could be sialylated with N-acetylneuraminic acid (NeuNAc) (molecular weight, 309) with only a slight increase of its apparent size to 4.1 kDa upon SDS-PAGE (16, 26). Most of the LOS in group B and C organisms is partially sialylated. The degree of sialylation differs among strains and according to growth conditions (25). We have shown that sialic acid is α2,3 linked to Gal in all LNnT-containing LOSs of the eight prototype LOSs from group B and C organisms except L1 and L6 LOSs (26). The sialic acid in L1 was reported to be α2,6 linked to the Pk epitope (28). The L6 LOS is nonsialylated due to the lack of a terminal Gal to function as the acceptor for its sialyltransferase (STase) (14).
In this study, we compared the specificities of LOS-specific STases from representative LOS strains, including 126E (L1), and also investigated the influence of the length of the LOS α chain on its sialylation in N. meningitidis.
Specificities of LOS-specific STases from N. meningitidis.
We investigated the specificities of LOS-specific STases, since sialic acid was reported to be α2,6 linked in the LOS of 126E (L1) but α2,3 linked in six other LOSs. The STases from four N. meningitidis strains, 126E (serogroup C:immunotype L1), M986 (B:L3,7), M986’s noncapsulated variant M986-NCV (L3,7), and 7889 (A:L11), were prepared, and the linkages formed between NeuNAc and Gal were characterized by using sialic acid-binding lectins. Each strain was grown in 200 ml of liquid Catlin medium for 8 h in 500-ml Wheaton bottles with shaking at 150 rpm (24). The cells were collected by centrifugation. The STase was released from the bacteria by suspending them in 10 ml of 0.5% Triton X-100 in water and then sonicating them for 15 s in an ice-water bath using an ultrasonic processor at 50 W (15). After the removal of cell debris by centrifugation, the supernatant containing the STase was concentrated to 1 ml using a 10,000 molecular-weight-cutoff membrane (Centriprep YM-10; Millipore, Bedford, Mass.). The concentrate was finally mixed with an equal volume of glycerol and stored at −20°C (15). It should be noted that the STase preparations contained a noticeable amount of endogenous LOS. Sialylation of LOS was carried out at 37°C overnight in 65 μl of a mixture containing 150 μg of LOS/ml, 7 mM CMP-NeuNAc, 2 mM MgCl2, 300 μg of bovine serum albumin (BSA)/ml, 0.02% sodium azide, and 10 μl of an STase preparation in 15 mM imidazole buffer (pH 7.0). Lectin blotting was used to characterize the sialic acid linkage in each modified LOS. The sialic acid-binding lectins used in the study were Limax flavus agglutinin (LFA), Maackia amurensis leukoagglutinin (MAL), and Sambucus nigra agglutinin (SNA). LFA binds to sialic acid regardless of its linkage (13), MAL is specific for the trisaccharide sequence NeuNAcα2-3Galβ1-4GlcNAc/Glc (12), and SNA recognizes the NeuNAcα2-6Galβ1-4GlcNAc/Glc sequence (21).
In the presence of the substrate CMP-NeuNAc and with the nonsialylated LNnT-containing M986-NCV LOS (L3,7) as an acceptor, all STases sialylated the LOS. The band for sialylated LOS shifted slightly upward upon SDS-PAGE analysis on 16% polyacrylamide gel (see Fig. 2, lanes 3 and 3A). For lectin blotting, the LOS components resolved by SDS-PAGE were electrophoretically transferred onto a nylon membrane (Magna, 0.2-μm pore size; Micron Separations Inc., Westborough, Mass.) at 100 mA for 2 h at 4°C. The membrane was rinsed with phosphate-buffered saline, blocked with 1% BSA in phospate-buffered saline for an hour, and probed with alkaline phosphatase-labeled lectins (1 to 2 μg/ml; E-Y Laboratories, San Mateo, Calif.) overnight in 25 mM Tris-buffered saline (pH 7.4) containing 0.5 mg of BSA/ml and 1 mM MgCl2. The LOSs on the membrane were then visualized with BCIP (5-bromo-4-chloro-3-indolylphosphate)-Nitro Blue Tetrazolium sodium substrate (Boehringer Mannheim Corp., Indianapolis, Ind.) after three washes with Tris-buffered saline containing 0.05% Tween 20. Digoxigenin-labeled MAL (Boehringer Mannheim Corp.) was also used as a probe (26) in this study with the same results. All sialylated LOSs produced by the STases, including the 126E (L1) enzyme with the LNnT-containing LOS (L3,7) as the acceptor, bound LFA and MAL (see Fig. 2, lanes 3 and 3A). In the SNA blot, SNA bound to control glycoproteins, transferrin and fetuin, but did not bind to any of the sialylated LOSs (not shown). These results show that these STases had very similar specificities and that NeuNAc was α2,3 linked to the terminal Gal of LNnT of the LOS, since the sialylated LOS bound the lectin MAL but not SNA. Although it was reported that the L1 LOS had a NeuNAc α2,6 linked to its Galα1-4Galβ1-4Glc terminus (28), no differences in the specificities of the STases isolated from strains 126E (C:L1), M986 (B:L3,7), M986-NCV (L3,7), and 7889 (A:L11) were observed when an LNnT-containing LOS was used as an acceptor. Thus, the STases, including that of 126E, formed α2,3-linked NeuNAc.
FIG. 2.
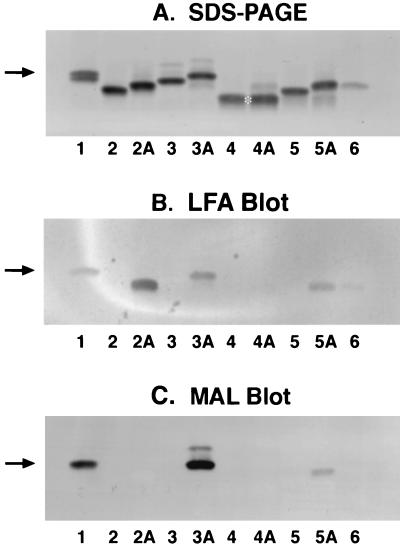
Sialylation of purified LOSs from N. meningitidis strains grown in Catlin medium. (A) Silver-stained LOSs after SDS-PAGE; (B) LFA blot for detecting sialylated LOSs; (C) MAL blot for detecting α2,3-linked sialic acid. Lanes 1, M986 LOS markers with a pair of doublets (the upper 4.1-kDa component is sialylated and the lower 4-kDa component is nonsialylated); lanes 2 and 2A, nonsialylated 126E (L1) LOS without and with addition of CMP-NeuNAc, respectively; lanes 3 and 3A, M986NCV (L3,7); lanes 4 and 4A, A1 (L8); lanes 5 and 5A, 7880 (L10); lanes 6, the control with 126E STase and CMP-NeuNAc only. Sample loads were 100 ng of LOS for silver staining (23) and double that amount for lectin blots. The arrows indicate the location of the sialylated 4.1-kDa LOS; the asterisk indicates the location of the nonsialylated 3.6-kDa L8 LOS.
Purified N. meningitidis LOS and outer-membrane-bound LOS as acceptors for STase in the sialylation of LOS.
A recent study showed that galactosides, lactose, and LNnT may serve as acceptors for STase to form an α2,3-linked NeuNAc (4). However, these acceptors lack lipid A and an inner core, which are integral parts of LOS. Therefore, in this study we used LOS and LOS-containing outer-membrane vesicles (OMV) as acceptors for STase. LOSs and OMV were isolated from organisms grown in Catlin medium as previously described (7). We selected 126E (L1), M986-NCV (L3,7), A1 (L8), and 7880 (L10) LOSs as acceptors, since these LOSs all possess a terminal Gal in the α chain. The M986-NCV LOS serves to represent seven of the LNnT-containing LOSs among the 12 prototypes (L2 through L9, except for L6), since it is nonsialylated and contains a terminal LNnT in the α chain. Both 126E and M986-NCV STases were used in the initial studies on sialylation of the four LOS acceptors. However, we observed no apparent difference in results with the two STases after SDS-PAGE and lectin blot analyses. Since the 126E STase preparation had much higher enzymatic activity, it was used in most of the experiments.
Figure 2 shows the results of SDS-PAGE analysis and LFA and MAL blots of the sialylation of the four LOSs as acceptors. In Fig. 2A, except for the A1 LOS (lanes 4 and 4A), all LOSs shifted slightly upward in the presence of STase and CMP-NeuNAc, indicating sialylation of three LOSs. Although the A1 (L8) LOS possesses a terminal lactosyl structure, it did not change its mobility. The additional faint band above the A1 LOS (lanes 4A) was from the endogenous LOS present in the preparation of 126E STase, i.e., the sialylated 126E LOS, as is evident from the enzyme control depicted in lane 6. Sialylation of the three LOSs, but not A1 LOS, was confirmed by blotting with the sialic acid-binding lectin LFA, as shown in Fig. 2B. The 126E LOS appeared to have higher reactivity with LFA due in part to a higher level of loading, as shown in Fig. 2A. The lectin blot of MAL, which binds the trisaccharide NeuNAcα2-3Galβ1-4GlcNAc/Glc sequence, shows that MAL bound to the M986 (L3,7) and 7880 (L10) LOSs (Fig. 2C, lanes 3A and 5A). Thus, NeuNAc is α2,3 linked to Gal in these two LOSs.
Although both the M986-NCV (L3,7) and 7880 (L10) LOSs bound the MAL lectin, the binding of the latter was much weaker. Preliminary structural analysis and monoclonal antibody characterization indicate that the acceptor in 7880 (L10) LOS is Galβ1-4Glcβ1-4Glc (11, 27). Thus, the binding epitope in M986-NCV LOS, NeuNAcα2-3Galβ1-4GlcNAc, has a higher affinity than that in 7880 LOS, NeuNAcα2-3Galβ1-4Glc (12). The epitope of the former also extends out further from the Hep (I) moiety, thereby rendering it more accessible to the lectin. For M986-NCV LOS, there was a second minor higher-molecular-weight MAL-binding band (Fig. 2C, lane 3A). This very minor LOS component bound well with MAL, indicating the presence of the NeuNAcα2-3Galβ1-4GlcNAc structure at its terminus. Its size was about two monosaccharide units larger than that of the major sialylated LOS component, as judged from its relative mobility on SDS-PAGE (Fig. 2A). Therefore, this minor component is probably derived from the addition of N-acetyllactosamine to the LNnT in the α chain and is readily sialylated. The SNA lectin, which recognizes the NeuNAcα2-6Galβ1-4GlcNAc/Glc sequence, did not bind to any of the sialylated LOS components.
N. meningitidis sheds or releases OMV during cell growth and propagation (7). The conformation of LOS in OMV probably remains the same as that of LOS existing on the organism. Hence, we also used LOS in OMV as an acceptor to investigate sialylation of membrane-associated LOS. Besides LOS, the OMV also contains outer membrane proteins, phospholipids, and some polysaccharide if it is from a capsulated strain. Sialylation of the LOS in the OMV was carried out as for purified LOS. Overall, the results were the same as those obtained using purified LOSs as acceptors (not shown). Thus, the levels of accessibility of the α chain of LOS to STase were not different whether the LOS acceptor was in a purified LOS or whether it was present in an OMV. Our results also showed that in the α chain of LOS, either in a purified LOS or in an OMV, not all terminal Gal was accessible to STase, since A1 (L8) LOS with a terminal lactose (Galβ1-4Glc) was not sialylated. This result is different from that of a previous study indicating that free lactose is a good acceptor for STase (4).
Sialylation of LOS in N. meningitidis with CMP-NeuNAc in culture.
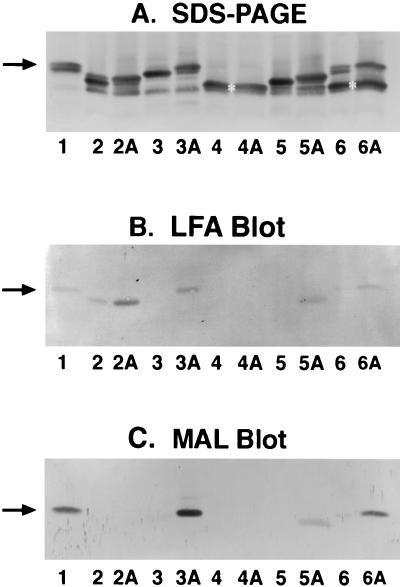
We grew strains 126E (C:L1), M986-NCV (L3,7), A1(A:L8), and 7880 (A:L10) in the presence of 200 μg of CMP-NeuNAc/ml to investigate LOS sialylation in the organism. These strains were grown in 4 ml of tryptic soy broth (TSB) with or without CMP-NeuNAc for 8 h in 20-ml glass vials with shaking at 150 rpm. The cells were collected and suspended in 0.2 ml of water, and the LOS was extracted from the cells by mixing with an equal volume of 90% hot phenol (7). After phase separation, the crude LOS preparations in the water phase were used for SDS-PAGE and lectin blot analyses. In addition to the LOS components seen in cells grown in Catlin medium (Fig. 2), these LOSs also had 3.6-kDa and other minor components when these strains were grown in TSB (Fig. 3A). When CMP-NeuNAc was added to the growth medium, the major components of the 126E (L1), M986-NCV (L3,7), and 7880 (L10) LOSs but not the A1(L8) LOS shifted slightly upward on the SDS-PAGE gel, results which are similar to the results obtained when purified LOSs were used as acceptors for STase in vitro. Thus, the major LOS components in the L1, L3,7, and L10 strains but not the L8 strain became almost completely sialylated when CMP-NeuNAc was added to the growth medium. Sialylation of the three LOSs and the presence of α2,3-linked sialic acid in the M986-NCV (L3,7) and 7880 (L10) LOSs were confirmed by blotting with LFA (Fig. 3B) and with MAL (Fig. 3C). The LFA blot also showed a low degree of LOS sialylation in strain 126E (C:L1) even without CMP-NeuNAc added, since this strain is capable of producing that substrate. No sialylation of L8 LOS was demonstrated in strain M978 (B:L8), the L8 prototype strain (Fig. 3, lanes 6 and 6A). Although M978 LOS had a major 3.6-kDa L8 component and a minor 4.0-kDa component that possesses an LNnT epitope (25), only the minor 4.0-kDa LOS component was sialylated. When strain M978 was grown in Catlin medium containing 50, 200, and 500 μg of CMP-NeuNAc/ml, the minor 4.0-kDa LOS component became completely sialylated at the substrate concentration of 50 μg/ml. However, there was no sialylation of the major L8 3.6-kDa component, which has a terminal lactosyl structure, even at the 10-times-higher substrate concentration.
FIG. 3.
Sialylation of LOS in N. meningitidis grown in TSB with and without CMP-NeuNAc. (A) Silver-stained LOSs on SDS-PAGE; (B) LFA blot for detecting sialylated LOSs; (C) MAL blot for detecting α2,3-linked sialic acid. Lanes 1, M986 LOS markers as in Fig. 2; lanes 2 and 2A, 126E (C:L1) strain grown in the absence and presence of 200 μg of CMP-NeuNAc/ml, respectively; lanes 3 and 3A, strain M986-NCV (L3,7); lanes 4 and 4A, strain A1 (A:L8); lanes 5 and 5A, strain 7880 (A:L10); lanes 6 and 6A, strain M978 (B:L8). The arrows and asterisks are as described in the legend of Fig. 2.
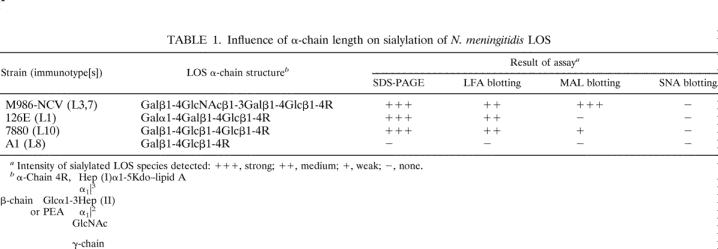
No difference in results was observed between sialylation of LOS in N. meningitidis in culture and sialylation of LOS in vitro using STase extracted from the organism and an LOS acceptor either in purified form or in OMV. The accessibility of the terminal Gal in the four LOS acceptors to STase for sialylation and the influence of their α-chain structures on sialylation are summarized in Table 1. Sialylation of LOS occurred only when the α chain was a trisaccharide or a longer oligosaccharide extended out from Hep (I) moiety, as in 126E (L1), M986-NCV (L3,7), and 7880 (L10) LOSs. Lactose, a disaccharide, when present in the α chain, could not function as an acceptor for sialylation, as shown for A1 (A:L8) and M978 (B:L8) LOSs. The inaccessibility of the terminal Gal of lactose in the L8 LOS to STase is probably due to steric hindrance caused by phosphorylethanolamine or other group extending out from Hep (II), as depicted in Fig. 1. In N. meningitidis, the expression of an L8 LOS phenotype increased the organism’s sensitivity to serum bactericidal activity compared to that of the organism expressing an L3 LOS (17). Sialylation of the LNnT-containing L3 LOS but not L8 LOS on the organism is a possible explanation or contributing factor for this observation, since LOS sialylation on N. meningitidis and N. gonorrhoeae inhibited complement-mediated serum bactericidal activity (3, 31).
Table 1.
In this study, all STases extracted from N. meningitidis with Triton X-100, including that of strain 126E, formed an α2,3-linked NeuNAc by using an LNnT-containing LOS as the acceptor. The LOS isolated from strain 126E was reported to have an α2,6-linked NeuNAc (28). The SNA lectin, which was expected to bind to the NeuNAcα2-6Gal sequence, bound to sialylated glycoproteins (transferrin and fetuin) but did not bind to the sialylated native 126E LOS. Thus, the binding of SNA apparently requires the presence of an antepenultimate sugar with β1,4-linked GlcNAc as in the glycoproteins, and there is no binding if this sugar is α1,4-linked Gal as in the native 126E LOS. Alternatively, the lack of binding of 126E LOS by SNA may be the result of steric hindrance by a neighboring group surrounding the NeuNAcα2-6Galα1-4Galβ1-4Glc region of the LOS. We do not know the linkage of sialic acid formed in 126E LOS by the addition of exogenous CMP-NeuNAc. It has been reported that the 126E STase has bifunctional properties and forms α2,3-linked NeuNAc as well as α2,6-linked NeuNAc (29). The possibility exists that the L1 LOS acceptor causes the 126E STase to form only α2,6-linked sialic acid. Further studies are needed to determine the sialic acid linkage formed in the 126E LOS acceptor by the STases from different strains in the presence of CMP-NeuNAc.
In summary, the STases extracted from four N. meningitidis strains with Triton X-100 were characterized as α2,3-STases when an LNnT-containing LOS was used as an acceptor. Sialylation of N. meningitidis LOS in vitro and in vivo was influenced by the length of the α chain in an LOS. The α chain of an LOS acceptor required a trisaccharide or a longer oligosaccharide that possesses a terminal Gal for the attachment of NeuNAc by the enzyme. Although free lactose was reported to serve as an acceptor, the disaccharide (i.e., lactose) in the α chain of L8 LOS could not function as an acceptor for the STase, presumably due to steric hindrance by the Hep (II) and its phosphorylethanolamine attachment in the LOS. Lack of LOS sialylation in N. meningitidis expressing the L8 phenotype may increase its sensitivity to serum bactericidal activity (17).
Acknowledgments
We thank Irene Hodge for her assistance in preparing materials used in this study.
Editor: E. I. Tuomanen
REFERENCES
- 1.Apicella, M. A., R. E. Mandrell, M. Shero, et al. 1990. Modification of sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162: 506–512. [DOI] [PubMed] [Google Scholar]
- 2.Estabrook, M. M., N. C. Christopher, J. M. Griffiss, C. J. Baker, and R. E. Mandrell. 1992. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J. Infect. Dis. 166: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 3.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65: 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert, M., A.-M. Cunningham, D. C. Watson, A. Martin, C. J. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis α-2,3-sialyltransferase and its acceptor affinity. Eur. J. Biochem. 249: 187–194. [DOI] [PubMed] [Google Scholar]
- 5.Gotschlich, E. C. 1990. Neisseriae, p. 551–560. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 4th ed. J. B. Lippincott, Philadelphia, Pa.
- 6.Griffiss, J. M., B. L. Brandt, N. B. Saunders, and W. D. Zollinger. 2000. Structural relationships and sialylation among meningococcal L1, L8, and L3,7 lipooligosaccharide serotypes. J. Biol. Chem. 275: 9716–9724. [DOI] [PubMed] [Google Scholar]
- 7.Gu, X.-X., and C.-M. Tsai. 1991. Purification of rough-type lipopolysaccharides of Neisseria meningitidis from cells and outer membrane vesicles in spent medium. Anal. Biochem. 196: 311–318. [DOI] [PubMed] [Google Scholar]
- 8.Jones, D. M., R. Borrow, A. J. Fox, S. Gray, K. A. Cartwright, and J. T. Poolman. 1992. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb. Pathog. 13: 219–224. [DOI] [PubMed] [Google Scholar]
- 9.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66: 5939–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and fuction of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24(4): 281–334. [DOI] [PubMed] [Google Scholar]
- 11.Kim, J. J., N. J. Phillips, B. W. Gibson, J. M. Griffiss, and R. Yamasaki. 1994. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect. Immun. 62: 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. J. Biol. Chem. 266: 83–88. [PubMed] [Google Scholar]
- 13.Knibbs, R. N., S. E. Osborne, G. D. Glick, and I. J. Goldstein. 1993. Binding determinants of the sialic acid-specific lectin from the slug Limax flavus. J. Biol. Chem. 268: 18524–18531. [PubMed] [Google Scholar]
- 14.Kogan, G., D. Uhrin, J.-R. Brisson, and H. J. Jennings. 1997. Structural basis of Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr. Res. 298: 191–199. [DOI] [PubMed] [Google Scholar]
- 15.Mandrell, R. E., J. M. Griffiss, H. Smith, and J. A. Cole. 1993. Distribution of a lipooligosaccharide-specific sialyltransferase in pathogenic and non-pathogenic Neisseria. Microb. Pathog. 14: 315–327. [DOI] [PubMed] [Google Scholar]
- 16.Mandrell, R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173: 2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran, E. E., B. L. Brandt, and W. D. Zollinger. 1994. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect. Immun. 62: 5290–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons, N. J., J. R. Andrade, P. V. Patel, J. A. Cole, and H. Smith. 1989. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetylneuraminic acid. Microb. Pathog. 7: 63–72. [DOI] [PubMed] [Google Scholar]
- 19.Pavliak, V., J.-R. Brisson, F. Michon, D. Uhrin, and H. J. Jennings. 1993. Structure of the sialylated L3 lipopolysaccharide of Neisseria meningitidis. J. Biol. Chem. 268: 14146–14152. [PubMed] [Google Scholar]
- 20.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22(3): 139–180. [DOI] [PubMed] [Google Scholar]
- 21.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the NeuNAc(α2-6)Gal/GalNAc sequence. J. Biol. Chem. 262: 1596–1601. [PubMed] [Google Scholar]
- 22.Smith, H., N. J. Parsons, and J. A. Cole. 1995. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb. Pathog. 19: 365–377. [DOI] [PubMed] [Google Scholar]
- 23.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119: 115–119. [DOI] [PubMed] [Google Scholar]
- 24.Tsai, C.-M., R. Boykins, and C. E. Frasch. 1983. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J. Bacteriol. 155: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai, C.-M., and C. I. Civin. 1991. Eight lipooligosaccharides of Neisseria meningitidis react with a monoclonal antibody which binds lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc). Infect. Immun. 59: 3604–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, C.-M., W. Chen, and P. A. Balakonis. 1998. Characterization of terminal NeuNAcα2-3Galβ1-4GlcNAc sequence in lipooligosaccharides of Neisseria meningitidis. Glycobiology 8: 359–365. [DOI] [PubMed] [Google Scholar]
- 27.Tsai, C.-M. 2000. Characterization of sialylated and nonsialylated lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins. Adv. Exp. Med. Biol. 491: 525–542. [DOI] [PubMed] [Google Scholar]
- 28.Wakarchuk, W. W., M. Gilbert, A. Martin, Y. Wu, J.-R. Brisson, P. Thibault, and J. C. Richards. 1998. Structure of an α-2,6-sialylated lipooligosaccharide from Neisseria meningitidis immunotype L1. Eur. J. Biochem. 254: 626–633. [DOI] [PubMed] [Google Scholar]
- 29.Wakarchuk, W. W., D. Watson, F. St. Michael, J. Li, Y. Wu, J.-R. Brisson, N. M. Young, and M. Gilbert. 2001. Dependence of the bi-functional nature of a sialyltransferase from Neisseria meningitidis on a single amino acid substitution. J. Biol. Chem. 276: 12785–12790. [DOI] [PubMed] [Google Scholar]
- 30.Ward, M. E., P. J. Watt, and A. A. Glynn. 1970. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature 227: 382–384. [DOI] [PubMed] [Google Scholar]
- 31.Wetzler, L. M., K. Barry, M. S. Blake, and E. C. Gotschlich. 1992. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 60: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]