Abstract
Human monocytic ehrlichiosis is an emerging tick-borne disease caused by the rickettsia Ehrlichia chaffeensis. We investigated the impact of two genes that control macrophage and T-cell function on murine resistance to E. chaffeensis. Congenic pairs of wild-type and toll-like receptor 4 (tlr4)- or major histocompatibility complex class II (MHC-II)-deficient mice were used for these studies. Wild-type mice cleared the infection within 2 weeks, and the response included macrophage activation and the synthesis of E. chaffeensis-specific Th1-type immunoglobulin G response. The absence of a functional tlr4 gene depressed nitric oxide and interleukin 6 secretion by macrophages and resulted in short-term persistent infections for ≥30 days. In the absence of MHC-II alleles, E. chaffeensis infections persisted throughout the entire 3-month evaluation period. Together, these data suggest that macrophage activation and cell-mediated immunity, orchestrated by CD4+ T cells, are critical for conferring resistance to E. chaffeensis.
Human monocytic ehrlichiosis (HME) is a recently reported tick-borne disease caused by the rickettsia Ehrlichia chaffeensis (14, 20, 36). This pathogen also infects white-tailed deer, dogs, goats, and coyotes (8, 15–17, 29, 30). Early symptoms of HME include fever, headache, malaise, muscle aches, vomiting, diarrhea, cough, joint pains, and confusion (61). If untreated, ehrlichiosis can cause severe, potentially fatal illness in immune compromised and elderly people (43, 44). Severe manifestations of the disease include prolonged fever, renal failure, respiratory distress, seizures, and coma.
Members of the class Ehrlichiales, including E. chaffeensis, persist in their vertebrate hosts for long periods of time, despite active host immune responses (1, 18, 45, 66). The relationship between E. chaffeensis and its targeted host cells, macrophages and monocytes, is critical because contrary to their natural function, these cells fail to clear E. chaffeensis, allowing them to establish persistence by undefined evasion mechanisms. Thus, studies to determine host immune responses to E. chaffeensis in an experimental host are useful to understand the immune evasion strategies used by this and other rickettsiales and by other macrophage-tropic pathogens.
The mouse has been utilized to determine the impact of the host response on resistance to E. chaffeensis infections (55, 62, 63). Wild-type immunocompetent mice clear infections in 16 days (62), while the absence of the macrophage-regulating tlr4 gene results in persistence of up to 28 days (55). E. chaffeensis infections in severely immunocompromised SCID mice (deficient for T and B cells) results in severe multiorgan infections, and the infected animals become moribund around 24 days postinfection (62). The role of T cells for conferring protective immunity to Cowdria ruminantium, a closely related pathogen of E. chaffeensis, has been reported recently (10). An important role for T cells also is implicated by the T-cell response against a major surface protein of the genogroup II Ehrlichia, Anaplasma marginale (9).
Because macrophages and T cells appear important in the control of this macrophage-tropic rickettsia, we proposed the hypothesis that gene disruptions in important macrophage and T-cell regulatory genes would impact the course of E. chaffeensis infection. We tested the hypothesis by following the course of infection and measuring several immunological and pathological responses in mice with genetic backgrounds ranging from wild type to mutants for tlr4 and major histomcompatibility complex class II (MHC-II) genes that impact macrophage and T-cell function. The tlr4 gene product, responsible for the stimulatory effects of gram-negative bacterial lipopolysaccharide (LPS), is an important regulator of macrophage responsiveness (58). The MHC-II gene complex encodes heterodimeric molecules that bind antigenic peptides for presentation to T cells and serve as the signal transduction molecules to regulate macrophage function (26, 27, 38, 39, 41, 56). The expression of the MHC-II molecules is also necessary for the T-cell maturation to CD4+ T cells (25).
MATERIALS AND METHODS
Mouse strains. (i) Mice used for analysis of tlr4 gene impact.
To evaluate the impact of the tlr4 gene on E. chaffeensis infection, two congenic sets of mice were utilized. (i) Infections in FeJ (C3HeB/FeJ) mice were compared with those in HeJ (C3H/HeJ) mice, and (ii) infections in B6 (C57BL/6J) mice were compared with those in B10 (C57BL/10ScN) mice. FeJ mice were embryo derived from HeJ mice and have the same genetic background (19) (http://www.informatics.jax.org/external/festing/mouse/docs/C3H.shtml). The subsequent spontaneous mutation of the tlr4 gene (58) that occurred at Jackson Labs between 1960 and 1965 allowed for routine congenic comparisons between mice that express functional tlr4 genes in FeJ mice and HeJ mice that do not carry functional tlr4 gene alleles in HeJ mice (24, 33) (http://www.informatics.jax.org/external/festing/mouse/docs/C3H.shtml). B6 (C57BL/6J) and B10 (C57BL/10ScN) mice differ only at the H9, Igh2, and Lv loci (19) (http://www.informatics.jax.org/external/festing/mouse/docs/C57BL.shtml). In addition, the B10 mice carry the additional deletion of the tlr4 gene (60) but do not carry the interleukin 12 (IL-12) defect recently reported in C57BL/10 ScCr mice (40). Since tlr4 functions normally in B6 mice, comparison between B6 and B10 mice on E. chaffeensis growth was chosen to further evaluate the impact of tlr4 gene.
(ii) Mice used for analysis of MHC-II impact.
The C2D mouse (B6.129-Abbtm1 N5F20) has the MHC-II genes deleted (25). It has been backcrossed five times at Taconic to the B6 (C57BL/6) mouse background. The C2D mouse has been brother-sister mated for more than 20 generations over the last 8 years at Kansas State University (KSU). Because the B6 mouse strain is the only inbred strain that the C2D mice have been backcrossed to, this incipient congenic mouse pair is routinely used for congenic comparisons to determine the impact of MHC-II genes (5, 57).
The recently created hybrid mouse strain FeJ × C2D also carries mutations for MHC-II genes (MHC-II−/−), while the HeJ × C2D strain is a mutant for both tlr4 and MHC-II genes (12, 63). These hybrid mouse strains were included in the study to understand the impact of these genes in outbred populations like humans. All mice were bred in the rodent facility of the Division of Biology, Kansas State University, and housed in isolators under specific-pathogen-free conditions. Recombinant breeder mice were treated with sulfamethoxazole and trimethoprim (Sulfatrim, 1 ml/100 ml of H2O) for 1 week once per month to inhibit infections. Weaned mice did not receive antibiotics. All mouse experiments were approved by the institutional animal care and use committee.
E. chaffeensis mouse infections.
E. chaffeensis Arkansas (14) isolates obtained from the Centers for Disease Control and Prevention (CDC&P), Atlanta, Ga., were cultivated in DH82 cells as described previously (13, 54). Cultured bacteria from T75 flasks were harvested (13) when 80 to 100% of the confluent DH82 cells were infected. The cell suspension was diluted 1:1 in phosphate-buffered saline (PBS), and 0.5 ml of the suspension (∼5 × 106 cells) was injected intraperitoneally (i.p.) per mouse as described previously (61). Control mice received 0.5-ml i.p. injections containing uninfected DH82 cell suspension. E. chaffeensis-infected mice and control mice were sacrificed and evaluated on days 3, 8, 16, 23, 30, 50, and 92 postinfection.
Blood collection.
Mice were anesthetized i.p. with ketamine (80 mg/kg of body weight) and xylazine (10 mg/kg). Blood was collected from the retro-orbital sinus using a Pasteur pipet containing approximately 25 μl of 14% EDTA. Collected blood (∼300 μl per mouse) was transferred to microcentrifuge tubes containing 50 μl of 14% EDTA and stored at 4°C. Within 2 to 4 h after collection, blood samples were centrifuged at 3,000 × g and plasma was collected and stored at −70°C. Plasma samples were assayed for the presence of E. chaffeensis-specific immunoglobulin G (IgG) antibodies and selected cytokines.
Peritoneal macrophage collection.
Peritoneal cells containing predominantly macrophages were collected from infected and control mice by peritoneal lavage with 20 ml of ice-cold, sterile PBS. Approximately 2 × 107 cells per sample were seeded into wells of 24-well culture plates in 2-ml volumes. Cells were incubated for 18 h, culture supernatants were collected, and cell supernatant was stored at −20°C until cytokine assays were performed. Two milliliters of peritoneal exudate cells was also used to determine the presence of viable rickettsiae in infected and control mice by in vitro culture assay (described below).
Culture isolation of E. chaffeensis from peritoneal cells of infected mice.
Cells from 2 ml of peritoneal exudate were harvested by centrifugation at 3,000 × g for 5 min in a SERO-FUGE centrifuge (Becton Dickinson, Franklin Lakes, N.J.). The cell pellet was resuspended in 1 ml of culture medium containing DH82 cells, transferred to 24-well, sterile culture plates, and incubated with 5% CO2 at 37°C under humidified conditions. Cultures were monitored for growth every three to four days until the recovery of viable organisms, or up to 8 weeks. Monitoring was done by light-microscopic examination of culture fluid transferred onto a Cytospin slide (Shadon Southern Products Ltd., Chessire, England) stained with Hema 3 stain (Biochemical sciences, Inc., Swedesboro, N.J.). Detection of viable rickettsiae by culture usually required a two week incubation period. Culture positives were verified by PCR assay (described below) using the genomic DNA isolated from the cultured organisms. All culture-negative samples were monitored for up to 8 weeks before being classified as negatives.
Genomic DNA isolation and E. chaffeensis rRNA gene-specific PCR.
Genomic DNA from liver and spleen tissue samples was extracted by the sodium dodecyl sulfate-proteinase K-phenol-chloroform-isoamyl alcohol method (37). PCR assay was performed using ∼1 μg of genomic DNA and E. chaffeensis rRNA gene-specific primer pair (E. chaffeensis species-specific forward primer, RRG3: 5′CAATTGCTTATAACCTTTTGGTTATAAAT; Ehrlichia genus-specific reverse primer, RRG2: 5′CTATAGGTACCGTCATTATCTTCCC). The primers were designed based on the published sequence available in the GenBank (under accession no. U60476). Amplicons of 0.39 kb were identified by hybridization with an rRNA gene-specific probe. The hybridization step was included to rule out the false positives resulting from nonspecifically amplified, predicted size products. DNA isolation and PCR setup were performed in a DNA isolation laboratory, while PCRs and the product analyses were done in a separate, PCR analysis laboratory. PCRs were prepared in a Clean Spot PCR UV work station (Coy Laboratory Products, Grass Lake, Mich.). A master mix containing all PCR ingredients except Taq DNA polymerase and DNA template was prepared, divided into multiple aliquots, and stored at −20°C for use in the assays. PCRs were performed after adding Taq DNA polymerase (Applied Biosystems, Foster City, Calif.) to freshly thawed aliquots. All PCR assays included a negative control containing no template DNA and a positive control containing purified, culture-derived E. chaffeensis genomic DNA. PCR cycles were performed in a GenAmp9700 (Applied Biosystems) using one initial denaturing cycle of 94°C for 4 min followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min and one cycle of 72°C for 5 min. PCR products were stored briefly at 4°C and transferred to −20°C until use. Twenty percent of the PCR products were resolved on a 1.5% agarose gel, transferred to a nylon membrane, and hybridized with a random primer 32P-labeled ribosomal DNA probe generated from culture-derived E. chaffeensis DNA. The conditions for hybridization and washes were as previously described (47). Kodak X-Omat film was exposed to the hybridized membranes for 2 to 3 h at −70°C. Whenever a strong hybridization signal was noted, the data were verified by exposing the blot for shorter exposure times.
Histopathology and immunohistochemistry.
Liver and spleen samples were collected from infected and control mice and stored at −20°C or used immediately for isolating total genomic DNA. Sections of liver were also placed in formalin or Histochoice for histopathological and immunohistochemical analysis, respectively. Formalin-fixed samples were processed routinely, embedded in paraffin, cut at a 5-μm thickness, and stained with hematoxylin and eosin (H&E). Two cross-sectional areas of liver obtained from each mouse were evaluated for inflammatory foci, and the average of the two slides was used to assign a score for the inflammatory lesion as follows: score 0, no inflammatory foci; score 1, neutrophilic foci associated with rare apoptosis; score 2, 2 to 6 neutrophilic foci with apoptosis; score 3, 6 to 15 foci containing neutrophils with apoptotic hepatocytes; score 4, >15 neutrophilic foci with apoptosis; score 5, 1 to 15 foci of principally epithelioid macrophages with few mononuclear cells and neutrophils; score 6, >15 principally epithelioid macrophages with few mononuclear cells and neutrophils.
Immunohistochemical analysis was performed as described (61) with minor changes. Primary E. chaffeensis-specific antiserum was obtained from an HME patient (courtesy of Chris Paddock, CDC&P). E. chaffeensis-specific antibody-antigen complexes were detected using biotinylated goat anti-human secondary antibodies and avidin-biotin-alkaline phosphatase. Fast Red TR/napthol AS-MX (Sigma Chemical Co., St Louis, Mo.) was used to detect the alkaline phosphatase reaction. Normal human serum was used as the primary antibody for a negative control. Counterstaining of hepatocytes was performed with hematoxylin II.
Western blot analysis.
Plasma from E. chaffeensis-infected and control mice was assayed for the presence of E. chaffeensis 28-kDa outer membrane protein-specific antibody. Antigen for the assay was an expressed copy of the E. chaffeensis Arkansas isolate 28-kDa outer membrane protein (Omp) gene, open reading frame 5 (47) (also known as the Omp-1g [42] or p28-19 [64]). The recombinant antigen was synthesized and purified as previously described (46). The blot assay was performed using the ECL Western blotting system according to manufacturer protocols (Amersham/Pharmacia Biotech Inc., Piscataway, N.J.). Briefly, equal amounts of purified recombinant protein (∼3 μg/well) were resolved on a sodium dodecyl sulfate-10% polyacrylamide gel (37), transferred to a Hybond-N nitrocellulose membrane and blocked with 5% nonfat dairy milk. Membranes were assembled in a multichannel Western blot apparatus (Immunogenetics, Cambridge, Mass.) and incubated with 1:128-diluted mouse plasma for 2 h at room temperature. Following three washes for 10 min each in Tris-buffered saline solution containing 0.1% Tween 20 at room temperature, blots were incubated with 1:2,000-diluted horseradish peroxidase (HRPO)-conjugated antimouse IgG (Caltag, Burlingame, Calif.). Antigen-antibody complexes were detected by exposing X-ray film (Amersham Hyperfilm) for ∼2 min using the HRPO chemiluminescent substrate, Luminol (Amersham/Pharmacia Biotech., Inc.).
Quantitative ELISA to measure IgG subclasses.
Quantitative enzyme-linked immunosorbent assays (ELISA) were performed to measure the concentrations of E. chaffeensis 28-kDa Omp-specific IgG subclass antibodies by following the protocol described previously (48) with some modifications. Briefly, the 96-well ELISA plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with the purified recombinant 28-kDa Omp antigen (46) at a concentration of 20 ng/well using 50 mM sodium carbonate buffer, pH 9.6. One-hundred microliters of each plasma sample diluted 1:64 was added to antigen-coated wells and incubated for 2 h at room temperature. The wells were washed thrice with PBS containing 0.05% Tween 20 (PBST) and incubated with HRPO-conjugated goat anti-mouse IgG subclass antibodies, IgG1, Ig2a, IgG2b, and IgG3 (Caltag), at a dilution of 1:2,000. Unbound secondary antibodies were removed by washing with PBST, and the color was developed using TMB (3,3′,5,5′-tetramethyl benzidine) (Calbiochem, San Diego, Calif.) as a substrate. After 10 min of incubation with the substrate, the reaction was stopped by the addition of phosphoric acid to a final concentration of 333 mM, and the color developed was measured by using an ELISA plate reader at 450 nm. For determining the concentrations of IgG subclasses, serial dilutions of purified mouse IgG1, IgG2a, IgG2b, and IgG3 were added to plates coated with anti-mouse immunoglobulins. Color developed from the standards incubated with anti-goat-IgGs and TMB were used to determine concentration of each of the four IgG subclasses by linear regression analysis. All assays, including the serial dilution standards, were performed in triplicate wells and the average values were used for analysis.
Nitric oxide assessment.
As a measure of NO production, we measured peritoneal macrophage secretion of NO2, a stable end product of the NO synthesis pathway (51), by using the Griess reaction (53). The sensitivity of the assay was approximately 200 nM. IL-6 and IL-10 were assayed using a capture ELISA in 96-well polyvinyl chloride plates (11). Tumor necrosis factor alpha (TNF-α) concentrations were determined using TNF-α-sensitive LM-929 cells as described previously, except MTT was used to quantitate cell death (21).
Statistical analysis.
Mann-Whitney U tests and chi-square analyses were done as indicated to determine differences between mouse groups. To determine the impact of individual gene deficiencies, animals with similar MHC-II and tlr4 genotypes were pooled and assessed for differences using nonparametric statistics tests which did not depend on normal distribution of the pooled data. Statistical analyses were performed using the StatMost Statistical Software Package (Data Most, Salt Lake City, Utah).
RESULTS
Evaluation of E. chaffeensis infections in mice.
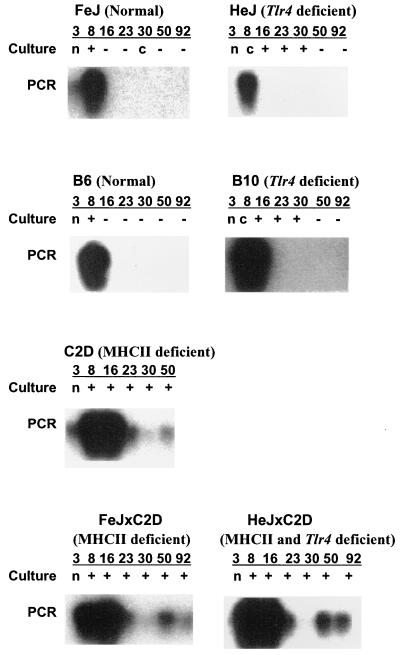
To test if disruptions in important macrophage and T-cell regulatory genes would impact the course of E. chaffeensis infection, wild-type mice and mutants for tlr4 and/or MHC-II genes were infected i.p. with cultured E. chaffeensis organisms. Mouse strain pairs that allow comparisons between congenic strains for tl4 gene impact are HeJ-FeJ and B6-B10. B6 and C2D mice allow congenic comparisons for evaluating the impact of MHC-II. The courses of the infections were monitored for 3 months. Peritoneal exudate cells from control mice were negative for rickettsiae by in vitro culture detection assay, while the samples from all infected mouse strains were positive for E. chaffeensis (Fig. 1, culture data). Wild-type mouse strains FeJ and B6 were culture negative for the pathogen beginning at 16 days after infection. The tlr4 mutant strains HeJ and B10 were culture positive for E. chaffeensis up to 30 days postinfection. All mice deficient for MHC-II genes remained E. chaffeensis culture positive throughout the 92 days postinfection.
FIG. 1.
Results of in vitro culture isolation and PCR assays for E. chaffeensis-infected mice. Typical results obtained for each mouse strain were presented at each time point postinfection for all 7 mouse strains. Numbers 3, 8, 16, 23, 30, 50, and 92 refer to sample analysis days postinfection. Ninety-two days postinfection samples for C2D mice were not available. All control mice were negative for E. chaffeensis as determined by culture isolation and PCR assay. Culture, culture positives of E. chaffeensis from peritoneal exudate cells (+, positive; −, negative; c, contaminated; n, data not available); PCR, hybridization data from PCR assay for E. chaffeensis-specific ribosomal gene in genomic DNA isolated from liver samples.
Trafficking of E. chaffeensis from the inoculation site.
To determine if the rickettsiae introduced into peritoneal cavity are filtered through the reticuloendothelial system, liver DNAs were tested for the presence of E. chaffeensis by PCR assay targeted to the E. chaffeensis rRNA gene. The control mice were negative throughout the 92-day study. All E. chaffeensis-infected mice were PCR positive up to 8 days postinfection (Fig. 1, PCR data), but the mouse strains having functional MHC-II genes tested negative after day 8. In contrast, all MHC-II mutant mouse strains were E. chaffeensis PCR positive for up to 92 days postinfection. Similarly, PCR analysis of spleen DNA from all infected animals also identified E. chaffeensis DNA (data not shown). These data demonstrate the trafficking of E. chaffeensis from the inoculation site to liver and spleen in all mouse strains and the rapid decrease of rickettsiae in mice having functional MHC-II genes.
The PCR analyses revealed the presence of E. chaffeensis less frequently than the culture recovery of the organism from peritoneal wash cells (comparison of culture and PCR data in Fig. 1). These differences may be explained as a result of lower sensitivity of the detection by PCR assay or may reflect the lower abundance of E. chaffeensis in the liver and spleen compared to the peritoneum. To address this issue, and to verify the results from the culture isolation method, genomic DNA was isolated from peritoneal exudate cells collected 50 and 92 days after infection and was evaluated for the presence of E. chaffeensis by the PCR assay. All samples that were culture positive for rickettsiae were also positive by PCR assay (data not shown), suggesting that the peritoneal cavity had significantly more E. chaffeensis-infected macrophages than the liver or the spleen.
Impact of individual gene mutations on clearance of E. chaffeensis.
The assessment of individual mouse strains implicated an important role for both MHC-II and tlr4 in murine resistance to E. chaffeensis in comparing congenic strains B6 and C2D for the MHC-II gene and FeJ and HeJ or B6 and B10 for the tlr4 gene (Fig. 1). To confirm the impact of individual gene mutations on the clearance of E. chaffeensis, mouse infections were analyzed according to their individual MHC-II or tlr4 genotypes independently of other gene expression (Tables 1 and 2). All mice that carried functional MHC-II alleles cleared E. chaffeensis infections, while the mice lacking functional MHC-II genes had persistent infections throughout the study period (Table 1). Mice carrying mutations only in the tlr4 gene (tlr4d/d) had persistent infections for ≥30 days and subsequently cleared the infections (Table 2). The presence of functional tlr4 genes also had no influence in overcoming the effects of MHC-II mutation in clearing E. chaffeensis infections (Table 1).
TABLE 1.
E. chaffeensis infection in mice lacking MHC-II genes results in persistent infectiona
| Day | No. positive/no. tested (%)
|
Pb | |
|---|---|---|---|
| MHC-II+/+ mice | MHC-II−/− mice | ||
| 8 | 4/4 (100) | 7/7 (100) | NS |
| 16 | 4/8 (50) | 12/12 (100) | 0.004 |
| 23 | 2/5 (40) | 11/12 (92) | 0.007 |
| 30 | 2/7 (29) | 8/11 (73) | 0.04 |
| 50 | 0/8 (0) | 10/12 (83) | 0.0002 |
| 92 | 0/8 (0) | 7/8 (88) | 0.0004 |
Peritoneal exudate cells were recovered from mice at the indicated times after i.p. infection with E. chaffeensis and were used to recover E. chaffeensis by culture in DH82 cells. Cultures were maintained for up to 8 weeks and examined twice a week for the presence of organisms. Cultures were monitored for growth every three to four days until the recovery of viable organisms. Note: MHC-II+/+ mice included both the tlr4n/n and tlr4d/d mice.
Statistical analysis was performed by the chi-square test. NS, not statistically significant.
TABLE 2.
E. chaffeensis infections in mice lacking functional tlr4 gene results in the short-term persistencea
| Day | No. positive/no. tested (%)
|
Pb | |
|---|---|---|---|
| tlr4n/n and MHC-II+/+ mice | tlr4d/d and MHC-II+/+ mice | ||
| 8 | 4/4 (100) | 4/4 (100) | NS |
| 16 | 0/4 (0) | 4/4 (100) | 0.005 |
| 23 | 0/3 (0) | 2/2 (100) | 0.025 |
| 30 | 0/3 (0) | 2/4 (50) | 0.138 |
| 50 | 0/4 (0) | 0/4 (0) | NS |
| 92 | 0/4 (0) | 0/4 (0) | NS |
Peritoneal exudate cells were recovered from mice at the indicated times after i.p. infection with E. chaffeensis and were used to recover E. chaffeensis by culture in DH82 cells. Cultures were maintained for up to 8 weeks and examined twice a week for the presence of organisms. Cultures were monitored for growth every three to four days until the recovery of viable organisms.
Statistical analysis was performed by the chi-square test. NS, not statistically significant.
Inflammatory response in E. chaffeensis-infected mice.
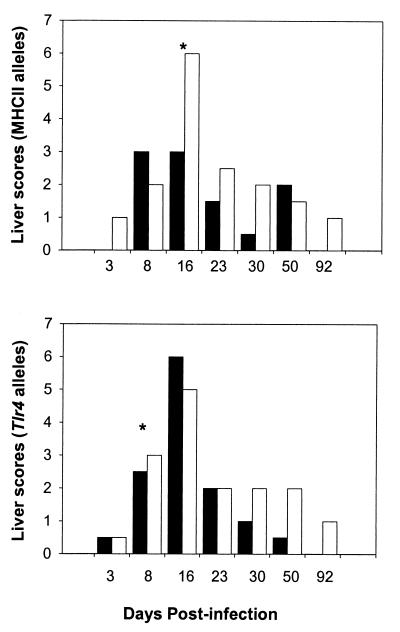
To further evaluate the impact of individual gene mutations on the trafficking of E. chaffensis and the subsequent inflammatory response, histopathological analysis was performed on the liver sections of infected and uninfected mice. Independent of the allelic background, E. chaffeensis-infected mice had liver lesions with lesion scores ranging from 2 to 4 for samples analyzed from 8 days after infection, while uninfected controls had no lesions (Fig. 2 and 3A to C). The lesions in infected mice consisted of neutrophil infiltrates associated with one or more apoptotic hepatocytes (Fig. 2 and 3A and B). The lesions peaked at 16 days postinfection (scores, 4 to 6) and began to resolve thereafter. By day 16, the lesions were composed of epithelioid macrophages surrounded by few mononuclear cells, defined as granulomas, and were most numerous in the MHC-II−/− mice (Fig. 2 and 3C). The inflammatory foci were found in mid-zonal and periportal areas of the hepatic lobule, indicating entry into the liver via the portal vein.
FIG. 2.
Liver lesion severity in mice infected with E. chaffeensis Arkansas isolate. Lesions were evaluated at various time intervals and assigned lesion scores (described in Materials and Methods). Data are presented as the median scores based on the presence (black bars) or absence (white bars) of MHC-II and tlr4 gene alleles. MHC-II+/+ data included 8 mice each, while tlr4n/n data are from 10 mice each. The MHC-II−/− data included 12 mice each, and data for tlr4d/d mice were compiled from 10 mice. An asterisk indicates significant differences between mice with functional and mutant alleles (P < 0.01 using a Mann-Whitney rank sum test).
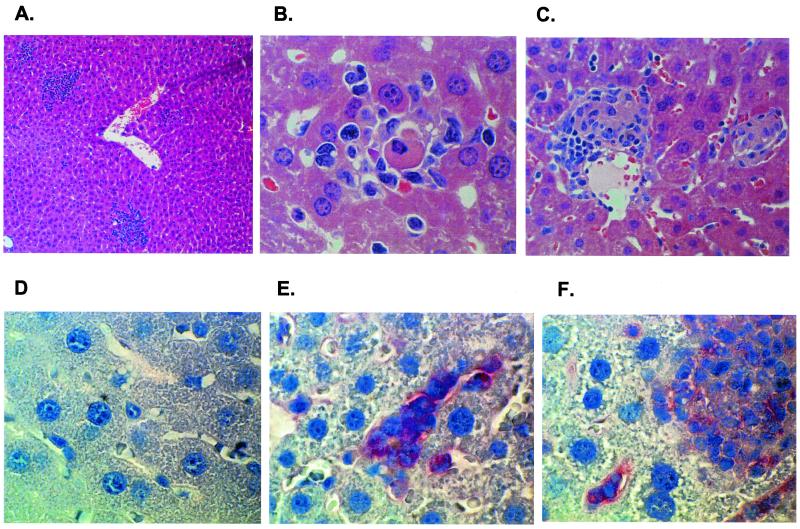
FIG. 3.
Histopathological (A to C) and immunohistochemical (D to F) analyses of liver. Histology sections were stained with hematoxylin II and eosin, while specific staining of E. chaffeensis-infected mononuclear cells was achieved using alkaline phosphatase-conjugated secondary antibodies with Fast Red TR/napthol AS-MX as the substrate (Sigma Chemical Co.). (A) Representative inflammatory response at day 8 postinfection. Several inflammatory cells and lesions are present (magnification, ×10; H&E staining). (B) Inflammatory response focus containing neutrophils and an apoptotic body (magnification, ×100; H&E staining). (C) Granulomatous inflammation on day 16 (magnification, ×40, H&E staining). (D) Control mouse liver; there is no immunostaining (magnification, ×100; hematoxylin staining). (E) E. chaffeensis-positive red staining in the cytoplasm of inflammation cells and mononuclear cells in hepatic sinusoids (magnification, ×100; hematoxylin staining). (F) E. chaffeensis-positive red staining in macrophage foci and mononuclear cells in hepatic sinusoids (magnification, ×100; hematoxylin staining).
To examine if the liver lesions contained E. chaffeensis organisms, immunohistochemical analyses were performed on liver sections of mouse strain HeJ × C2D, which carries mutations for both the tlr4 and MHC-II genes (Fig. 3D to F). The analysis revealed the presence of E. chaffeensis in inflammatory foci on days 8 and 16 postinfection (Fig. 3E and F). Several mononuclear cells (Kupffer cells) in the hepatic sinusoids also stained positive for E. chaffeensis (Fig. 3E and F). These data demonstrate the macrophage-tropic nature of the infection and verified the PCR detection of E. chaffeensis in liver tissue samples. Livers from control mice were negative for the immunostaining (Fig. 3D).
Wild-type and tlr4 mutant mice induced IgG response against E. chaffeensis.
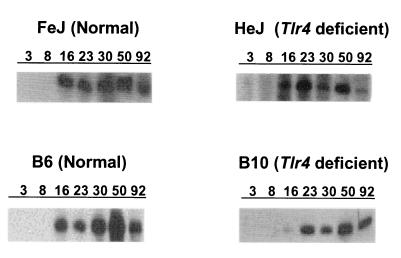
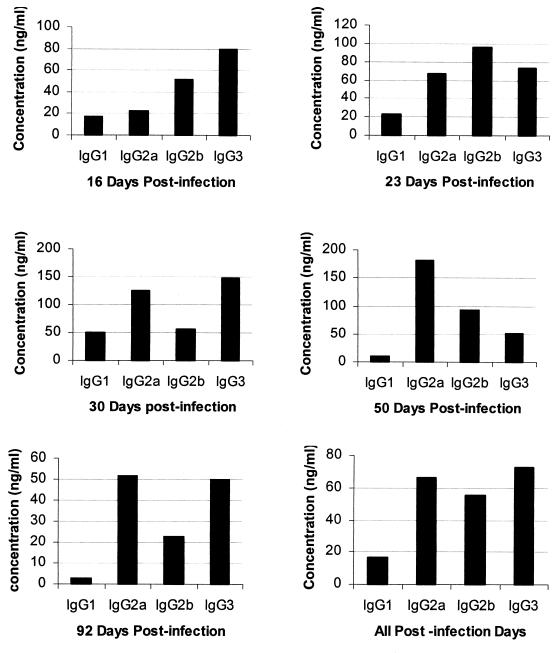
Previous studies found that antibody has the ability to reduce the rickettsial load but not clear E. chaffeensis infections completely (34, 62). Because our histopathological assessment revealed that all mice, including MHC-II mutants, ameliorated the hepatic infection after 16 days, we assayed for the E. chaffeensis-specific IgG response. IgG antibodies specific for an expressed E. chaffeensis 28-kDa outer membrane protein were detected in plasma from all MHC-II+/+ mice beginning 16 days postinfection (Fig. 4). Plasma samples from all MHC-II−/− mice, independent of the presence or absence of functional tlr4 genes, were negative for IgG. The concentrations of individual IgG subclass antibodies in MHC-II+/+ mice were estimated by quantitative ELISA (Fig. 5). The data for all mice were presented for 16 to 92 days postinfection. In addition, the average values for all postinfection days from 16 to 92 days were presented (Fig. 5). Consistent with the Western blot data, Ehrlichia-specific IgG concentrations were low or undetectable in samples collected at 3 and 7 days postinfection (not shown). Independent of the time after infection or the presence of a functional tlr4 gene, only low levels of IgG1 (5 to 8% of total IgG) were made by any mouse strain. The predominant expression of complement-fixing IgG molecules IgG2a, -2b, and -3 (22, 31) constituted 92 to 95% of the total IgG made.
FIG. 4.
Western blot profile showing response to an expressed E. chaffeensis 28-kDa recombinant outer membrane protein. Antibody data are not presented for mice that did not induce the IgG response. Typical results obtained for each mouse strain were presented. Numbers 3, 8, 16, 23, 30, 50, and 92 refer to sample analysis days postinfection.
FIG. 5.
IgG subclass distribution in mice that had an IgG response to the E. chaffeensis infection. Plasma samples from all mice beginning 16 days to 92 days postinfection were analyzed by quantitative ELISA. The data for all mice are presented by days postinfection and the pooled data for mice from all postinfection dates from days 16 to 92 are also presented. Each bar represents a median IgG concentration determined from samples analyzed from eight mice.
Macrophages from E. chaffeensis-infected mice produce NO.
Nitric oxide is an important macrophage mediator involved in destroying intracellular pathogens (6). Because E. chaffeensis grows in macrophages and the peritoneum was the site of infection, we assessed NO2 (a stable end product of NO) production in peritoneal macrophages ex vivo at various times after infection. Peritoneal macrophages from uninfected mice produced low to undetectable concentrations of NO2. Macrophages from infected mice of all strains produced significant concentrations of NO2 by 8 days postinfection. Although subtle differences in the secretion patterns by macrophages from different mouse strains were evident, there was no clear association between NO2 detected and E. chaffeensis clearance (data not shown). However, when the data were sorted by the presence or absence of functional alleles for the Tlr4 and MHC-II genes, there was a significant decrease in the NO2 concentrations generated by macrophages from tlr4-deficient mice compared to those from mice having a functional tlr4 gene (Table 3). Comparisons among the congenic mice with functional or mutant tlr4 genes (FeJ versus HeJ or B6 versus B10) also showed significantly more NO2 made by FeJ and B6 mice at days 8, 16, 23, and 30 (P < 0.02 as assessed by the Mann-Whitney test [data not shown]) compared to HeJ and B10 mice. There were no significant differences noted between MHC-II−/− and MHC-II+/+ mice (Table 3). As seen for the NO2 secretion, there was also a strong impact of the tlr4 gene on macrophages secretion of IL-6, while IL-10 and TNF-α were not made in notable concentrations (data not shown).
TABLE 3.
Influence of tlr4 and MHC-II genes on peritoneal macrophage nitrite secretion after infection with E. chaffeensisa
| Day | Nitrite secretionb (μM) in mice with tlr4 genotype
|
Nitrite secretionb (μM) in mice with MHC-II genotype
|
||||
|---|---|---|---|---|---|---|
| n/n (n = 10) | d/d (n = 10) | P | +/+ (n = 8) | −/− (n = 12) | P | |
| 3 | 10 (0, 29) | 0 (0, 0) | <0.06 | 0 (0, 5) | 0 (0, 23) | NS |
| 8 | 87 (65, 100) | 55 (37, 77) | <0.05 | 90 (71, 99) | 57 (36, 79) | <0.06 |
| 16 | 143 (120, 155) | 24 (16, 64) | <0.01 | 90 (50, 132) | 66 (20, 144) | NS |
| 23 | 105 (55, 120) | 15 (7, 24) | <0.01 | 37 (22, 66) | 41 (10, 107) | NS |
| 30 | 40 (25, 77) | 23 (0, 43) | <0.1 | 13 (0, 38) | 41 (30, 58) | NS |
| 51 | 48 (2, 83) | 16 (0, 54) | NS | 0 (0, 9) | 68 (8, 84) | NS |
| 93 | 32 (0, 92) | 0 (0, 7) | NS | 0 (0, 8) | 54 (0, 112) | NS |
Peritoneal exudate cells were recovered from mice at the indicated times after i.p. infection with E. chaffeensis and were cultured in vitro for 20 h. Supernatants were assayed for nitrite using the Greiss reagent.
Values are median nitrite concentrations. First and last quartile values are presented in parentheses. Statistical comparisons between functional and nonfunctional alleles were performed at each gene using the Mann-Whitney test. NS, not statistically significant, P > 0.1, except where indicated.
DISCUSSION
In this study, we have established that two genes that contribute to cell-mediated immunity, tlr4 and the MHC-II gene, are important for protection against E. chaffeensis. The importance of these genes is demonstrated using congenic mouse strains. In addition, the use of two hybrid strains led to the same conclusions. Therefore, our observations should be valuable to understanding ehrlichiosis in outbred populations such as humans. Assuming that the negatives for culture and PCR assays indicate clearance, wild-type mouse strains clear E. chaffeensis infections within 16 days after infection. In tlr4d/d mutants, the response to E. chaffeensis infection is similar to that observed in wild-type mice, except that E. chaffeensis clearance is delayed for at least 2 additional weeks. The short-term persistence observed in tlr4-deficient mice confirmed the earlier findings of Telford and Dawson (55). These data suggest that in the absence of the tlr4 receptor, the macrophage stimulatory effect of Ehrlichia LPS may have been abolished. This hypothesis is supported by the lower NO and IL-6 responses by tlr4 mutant mice. However, macrophage activation may have been compensated for by one or more alternative pathways allowing mice to clear the infection after a short delay.
Persistence of E. chaffeensis for the entire 3-month study period in MHC-II−/− mice suggests that functional MHC-II molecules are essential for clearance of the organisms from the murine host. Consistent with recent reports that passively acquired E. chaffeensis-specific antibodies transiently protect SCID mice from fatal infection (34, 62), our data support the hypothesis that complete suppression of this intracellular rickettsia infection requires the presence of functional CD4+ T cells.
Mice with functional MHC-II genes had predominant expression of IgG2a, -2b, and -3 (92% of total IgG made). These three IgG subclasses are TH1-type, complement-fixing antibodies (22, 31). The fluctuations in the type of IgGs made with time postinfection may have resulted from differences in the gamma interferon (IFN-γ) concentrations (49, 50). Because the expansion of IgG2a-, IgG2b-, and IgG3-secreting clones is IFN-γ dependent (50, 52), a protective immune response against E. chaffeensis may be associated with IFN-γ secretion and a TH1 type cellular response. This hypothesis is supported by the earlier observation by Barnewall and Rikihisa (4) that the activation with IFN-γ inhibits monocyte infections with E. chaffeensis in vitro. The lower abundance of IgG1, a TH2 antibody (23), further supports this hypothesis. A recent study of Li et al. (34), using monoclonal IgG2a or IgG3 antibodies specific to the 28-kDa outer membrane protein of E. chaffeensis, reported that the IgG2a subclass has a greater efficacy in rescuing SCID mice from fatal infection than IgG3, suggesting that IgG2a may be a more effective in promoting host resistance. In the present study, we did not detect the differences in the clearance of the organisms relative to IgG subclasses expression in MHC-II+/+ mice.
Our cytokine analysis did not reveal changes in the concentrations of TNF-α and IL-10 secreted by macrophages between controls and E. chaffeensis-infected mice, which would be consistent with the previous studies of Lee and Rikihisa (32). Although there are minor fluctuations in the nitric oxide concentrations after E. chaffeensis infection, there is no correlation between NO production and subsequent long-term persistence of infection observed in some mouse strains. This is contrary to the important role NO plays in eliminating other macrophage-tropic pathogens (7, 28, 35). A recent study presented by Banerjee et al. (2) demonstrated a short-term delay in the clearance of the human granulocytic ehrlichiosis (HGE) agent in nitric oxide synthase (NOS2) gene knockout mice. The lowered NO response in tlr4d/d mutant mice may reflect the overall lack of macrophage activation in our mouse strains and may also be consistent with the data for the HGE agent (2). The tlr4d/d mutants ultimately cleared the E. chaffeensis infection, as the NOS2 knockout mice cleared the HGE agent (2), suggesting that NO is one of many possible mediators that macrophages can use to help clear rickettsiae. Banerjee et al. (3) also reported that HGE agent infections repress respiratory burst. Although persistent infection did not inhibit the NO response in our studies, there may be differences in the impact of these rickettsiae on the O2− pathway and the NO pathway. Cell tropism differences between E. chaffeensis and the HGE agent also may explain the contrast between our two studies. These hypotheses remain to be tested. While macrophage activation may contribute to clearance of E. chaffeensis, it alone is not sufficient for the pathogen clearance, and CD4+ T cells appear to be the critical factor. This hypothesis is supported by the fact that all mice having functional tlr4 but lacking MHC-II genes failed to clear the infection.
The hypothesis that an effective cell-mediated immune response is necessary for ehrlichial resistance is also supported by the formation of granulomatous inflammation in the livers of infected mice. Peak inflammation is observed 16 days postinfection, with inflammation decreasing over time. Granulomatous inflammation found in infected mice having functional MHC-II genes is expected as part of a cell-mediated immune response necessary for the clearance of intracellular organisms. However, we did observe a similar inflammation in all mice independent of their MHC-II gene alleles. The response is transient, with a peak inflammation at 16 days after infection. Similar granuloma formation was reported earlier in E. chaffeensis-infected SCID mice that lack both T and B cells (61). Mice in that study became moribund within 24 days postinfection (61). In contrast, MHC-II and tlr4 mutants in our study did not become ill or die. The granuloma formation in MHC-II mutant mice likely consists of macrophages, CD4-negative T cells (such as CD8+ or CD8− natural killer cells), and possibly B cells. Further studies are needed to determine the composition of the infiltrates. The establishment of persistent infections in MHC-II−/− mice suggests that the CD4+ T cells are essential for the complete clearance. However, the decline of liver inflammation and survival despite persistent infection in these mutants suggest that other immune cells and mechanisms also contribute to the host response against E. chaffeensis.
The results from this study support the hypothesis that the macrophage and T-cell regulatory genes impact the course of E. chaffeensis infection. We provided evidence that E. chaffeensis stimulates an effective cell-mediated immune response. The response is associated with B-cell activation leading to the synthesis of a TH1-type IgG response and macrophage activation. tlr4 mutation suppresses the early clearance of the pathogen but does not compromise the subsequent resistence. Likewise, macrophages from E. chaffeensis-infected mice produced high levels of NO, but it was not sufficient for complete rickettsial clearance. Delayed clearance of E. chaffeensis in tlr4 mutants, the rickettsial persistence in MHC-II−/− mice, and the production of a TH1-type, complement-fixing, IgG antibody response suggest that CD4+ T cells orchestrate the complete clearance of E. chaffeensis.
Acknowledgments
This study was supported by the USDA Animal Health Funds Section 1433 grants 4-81315 and 4-81833; NASA grants NAGW-1197, NAG2-1274, and NCC5-168; and by the College of Veterinary Medicine, Kansas State University, Manhattan.
E. chaffeensis-specific antisera obtained from an HME patient and E. chaffeensis isolates were provided by Christopher Paddock, CDC&P.
Editor: J. T. Barbieri
Footnotes
This work is contribution 01-279-5-J of the Agricultural Experimental Station.
REFERENCES
- 1.Andrew, H. R., and R. A. Norval. 1989. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet. Parasitol. 34: 261–266. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, R., J. Anguita, and E. Fikrig. 2000. Granulocytic ehrlichiosis in mice deficient in phagocyte oxidase or inducible nitric oxide synthase. Infect. Immun. 68: 4361–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164: 3946–3949. [DOI] [PubMed] [Google Scholar]
- 4.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 62: 4804–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beharka, A. A., J. W. Armstrong, J. J. Iandolo, and S. K. Chapes. 1994. Binding and activation of major histocompatibility complex class II-deficient macrophages by staphylococcal exotoxins. Infect. Immun. 62: 3907–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12: 64–76. [DOI] [PubMed] [Google Scholar]
- 7.Boockvar, K. S., D. L. Granger, R. M. Poston, M. Maybodi, M. K. Washington, J. B. Hibbs, and R. L. Kurlander. 1994. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 62: 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36: 2645–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 10.Byrom, B., M. Obwolo, A. F. Barbet, and S. M. Mahan. 2000. A polarized Th1 type immune response to Cowdria ruminantium infection is detected in immune DBA/2 mice. J. Parasitol. 86: 983–992. [DOI] [PubMed] [Google Scholar]
- 11.Chapes, S. K., and A. A. Beharka. 1995. Lipopolysaccharide is required for the lethal effects of enterotoxin B after D-galactosamine sensitization. J. Endotox. Res. 2: 263–271. [Google Scholar]
- 12.Chapes, S. K., D. A. Mosier, A. D. Wright, and M. L. Hart. 2001. MHCII, Tlr4 and Nramp1 genes control host pulmonary resistance against the opportunistic bacterium Pasteurella pneumotropica. J. Leukoc. Biol. 69: 381–386. [PubMed] [Google Scholar]
- 13.Chen, S. M., V. L. Popov, H. M. Feng, and D. H. Walker. 1996. Analysis and ultrastructural localization of Ehrlichia chaffeensis proteins with monoclonal antibodies. Am. J. Trop. Med. Hyg. 54: 405–412. [DOI] [PubMed] [Google Scholar]
- 14.Dawson, J. E., B. E. Anderson, D. B. Fishbein, C. Y. Sanchez, C. Y. Goldsmith, K. H. Wilson, and C. W. Duntley. 1991. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 29: 2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson, J. E., K. L. Biggie, C. K. Warner, K. Cookson, S. Jenkins, J. F. Levine, and J. G. Olson. 1996. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human erlichiosis, in dogs from southeast Virginia. Am. J. Vet. Res. 57: 1175–1179. [PubMed] [Google Scholar]
- 16.Dawson, J. E., J. E. Childs, K. L. Biggie, C. Moore, D. Stallknecht, J. Shaddock, J. Bouseman, E. Hofmeister, and J. G. Olson. 1994. White-tailed deer as a potential reservoir of Ehrlichia spp. J. Wildl. Dis. 30: 162–168. [DOI] [PubMed] [Google Scholar]
- 17.Dugan, V. G., S. E. Little, D. E. Stallknecht, and A. D. Beall. 2000. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 38: 448–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumler, J. S., W. L. Sutker, and D. H. Walker. 1993. Persistent infection with Ehrlichia chaffeensis. Clin. Infect. Dis. 17: 903–905. [DOI] [PubMed] [Google Scholar]
- 19.Festing, M. F. W. 1994. Inbred strains of mice (updated 3rd Aug. 1994). Mouse Genome 92(Suppl.): 373–374. [Google Scholar]
- 20.Fishbein, D., L. Sawyer, C. Holland, E. Hayes, W. Okoroanyanwu, B. Williams, R. Sikes, M. Ristic, and J. McDade. 1987. Unexplained febrile illnesses after exposure to ticks: infection with an Ehrlichia?. JAMA 257: 3100–3104. [PubMed] [Google Scholar]
- 21.Fleming, S. D., J. J. Iandolo, and S. K. Chapes. 1991. Murine macrophage activation by staphylococcal exotoxins. Infect. Immun. 59: 4049–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germann, T., M. Bongartz, H. Dlugonska, H. Hess, E. Schmitt, L. Kolbe, E. Kolsch, F. J. Podlaski, M. K. Gately, and E. Rude. 1995. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 25: 823–829. [DOI] [PubMed] [Google Scholar]
- 23.Goldsby, R. A., T. J. Kindt, and B. A. Osborne. 2000. Kuby-immunology. W. H. Freeman and Company, New York, N.Y.
- 24.Goodman, S. A., and D. C. Morrison. 1985. Lipopolysaccharide receptors on lymphocytes. I. Lack of immunologic recognition of a putative LPS receptor on LPS-responder lymphocytes by LPS-nonresponder mice. J. Immunol. 135: 1906–1910. [PubMed] [Google Scholar]
- 25.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253: 1417–1420. [DOI] [PubMed] [Google Scholar]
- 26.Hansen, T. H., B. M. Carreno, and D. H. Sachs. 1993. The major histocompatibility complex, p. 577–628. In W. E. Paul (ed.), Fundamental immunology. Raven Press, New York, N.Y.
- 27.Hauschildt, S., W. G. Bessler, and P. Scheipers. 1993. Engagement of major histocompatibility complex class II molecules leads to nitrite production in bone marrow-derived macrophages. Eur. J. Immunol. 23: 2988–2992. [DOI] [PubMed] [Google Scholar]
- 28.Kaushik, R. S., J. E. Uzonna, J. R. Gordon, and H. Tabel. 1999. Innate resistance to Trypanosoma congolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57B1/6 mice. Exp. Parasitol. 92: 131–143. [DOI] [PubMed] [Google Scholar]
- 29.Kocan, A. A., G. C. Levesque, L. C. Whitworth, G. L. Murphy, S. A. Ewing, and R. W. Barker. 2000. Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg. Infect. Dis. 6: 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. Mcpherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 37: 2631–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science 254: 707–710. [DOI] [PubMed] [Google Scholar]
- 32.Lee, E. H., and Y. Rikihisa. 1996. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect. Immun. 64: 4211–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei, M. G., and D. C. Morrison. 2000. Differential expression of caveolin-1 in lipopolysaccharide-activated murine macrophages. Infect. Immun. 68: 5084–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, J. S., E. Yager, A. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166: 1855. [DOI] [PubMed] [Google Scholar]
- 35.Liew, F. Y., S. Millott, C. Parkinson, R. M. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J. Immunol. 144: 4794–4797. [PubMed] [Google Scholar]
- 36.Maeda, K., N. Markowitz, R. C. Hawley, M. Ristic, D. Cox, and J. E. McDade. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316: 853–856. [DOI] [PubMed] [Google Scholar]
- 37.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Matsuyama, S., Y. Koide, and T. O. Yoshida. 1993. HLA class II molecule-mediated signal transduction mechanism responsible for the expression of interleukin-1 beta and tumor necrosis factor-alpha genes induced by a staphylococcal superantigen. Eur. J. Immunol. 23: 3194–3202. [DOI] [PubMed] [Google Scholar]
- 39.Mehindate, K., R. al-Daccak, F. Damdoumi, and W. Mourad. 1996. Synergistic effect between CD40 and class II signals overcome the requirement for class II dimerization in superantigen-induced cytokine gene expression. Eur. J. Immunol. 26: 2075–2080. [DOI] [PubMed] [Google Scholar]
- 40.Merlin, T., A. Sing, P. J. Nielsen, C. Galanos, and M. A. Freudenberg. 2001. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the Lps(d) C57BL/10ScCr mouse. J. Immunol. 166: 566–573. [DOI] [PubMed] [Google Scholar]
- 41.Mourad, W., K. Mehindate, T. J. Schall, and S. R. McColl. 1992. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J. Exp. Med. 175: 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohasi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paddock, C. D., D. P. Suchard, K. L. Grumbach, W. K. Hadley, R. L. Kerschmann, N. W. Abbey, J. E. Dawson, B. E. Anderson, K. G. Sims, J. S. Dumler, and B. G. Herndier. 1993. Brief report: fatal seronegative ehrlichiosis in a patient with HIV infection. N. Engl. J. Med. 329: 1164–1167. [DOI] [PubMed] [Google Scholar]
- 44.Paddock, C. D., J. W. Sumner, G. M. Shore, D. C. Bartley, R. C. Elie, J. G. McQuade, C. R. Martin, C. S. Goldsmith, and J. E. Childs. 1997. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J. Clin. Microbiol. 35: 2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reardon, M. J., and K. R. Pierce. 1981. Acute experimental canine ehrlichiosis: I. Sequential reaction of the hemic and lymphoreticular systems. Vet. Pathol. 18: 48–61. [DOI] [PubMed] [Google Scholar]
- 46.Reddy, G. R., and C. P. Streck. 1999. Variability in the 28-kDa surface antigen protein multigene locus of isolates of the emerging disease agent Ehrlichia chaffeensis suggests that it plays a role in immune evasion. Mol. Cell Biol. Res. Commun. 1: 167–175. [DOI] [PubMed] [Google Scholar]
- 47.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247: 636–643. [DOI] [PubMed] [Google Scholar]
- 48.Sha, Z., and R. W. Compans. 2000. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74: 4999–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. Pecanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175: 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947. [DOI] [PubMed] [Google Scholar]
- 51.Stamler, J. S., D. J. Singel, and J. Loscalzo. 1992. Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902. [DOI] [PubMed] [Google Scholar]
- 52.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334: 255–258. [DOI] [PubMed] [Google Scholar]
- 53.Stuehr, D. J., and C. F. Nathan. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169: 1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sumner, J. W., J. E. Childs, and C. D. Paddock. 1999. Molecular cloning and characterization of the Ehrlichia chaffeensis variable-length PCR target: an antigen-expressing gene that exhibits interstrain variation. J. Clin. Microbiol. 37: 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telford, S. R., and J. E. Dawson. 1996. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet. Microbiol. 52: 103–112. [DOI] [PubMed] [Google Scholar]
- 56.Unanue, E. R., and J. C. Cerottini. 1989. Antigen presentation. FASEB J. 3: 2496–2502. [DOI] [PubMed] [Google Scholar]
- 57.Vallance, B. A., F. Galeazzi, S. M. Collins, and D. P. Snider. 1999. CD4 T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infect. Immun. 67: 6090–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel, S. N. 1992. The Lps gene: insights into the genetic and molecular basis of LPS responsiveness and macrophage differentiation, p. 485–513. In B. Beutler (ed.), Tumor necrosis factors: the molecules and their emerging role in medicine. Raven Press, New York, N.Y.
- 59.Vogel, S. N., C. T. Hansen, and D. L. Rosenstreich. 1979. Characterization of a congenitally LPS-resistant, athymic mouse strain. J. Immunol. 122: 619–622. [PubMed] [Google Scholar]
- 60.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 121: 785–791. [PubMed] [Google Scholar]
- 61.Winslow, G. M., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66: 3892–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winslow, G. M., E. Yager, K. Shilo, E. Volk, A. Reilly, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68: 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright, A. D., and S. K. Chapes. 1999. LPS sensitivity in recombinant mice lacking functional alleles at MHCII, Lps, and Nramp1 genes. J. Endotoxin Res. 5: 297–305. [Google Scholar]
- 64.Yu, X., J. W. McBride, X. Zhang, and D. H. Walker. 2000. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene 248: 59–68. [DOI] [PubMed] [Google Scholar]
- 65.Zaugg, J. L., D. Stiller, M. E. Croan, and S. D. Lincoln. 1986. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field infected, chronic carrier cow. Am. J. Vet. Res. 47: 2269–2271. [PubMed] [Google Scholar]