Abstract
T-cell immunity is critical for survival of hosts infected with Toxoplasma gondii. Among the cells in the T-cell population, CD8+ T cells are considered the major effector cells against this parasite. It is believed that CD4+ T cells may be crucial for induction of the CD8+-T-cell response against T. gondii. In the present study, CD4−/− mice were used to evaluate the role of conventional CD4+ T cells in the immune response against T. gondii infection. CD4−/− mice infected with T. gondii exhibited lower gamma interferon (IFN-γ) messages in the majority of their tissues. As a result, mortality due to a hyperinflammatory response was prevented in these animals. Interestingly, T. gondii infection induced a normal antigen-specific CD8+-T-cell immune response in CD4−/− mice. No difference in generation of precursor cytotoxic T lymphocytes (pCTL) or in IFN-γ production by the CD8+-T-cell populations from the knockout and wild-type animals was observed. However, the mutant mice were not able to sustain CD8+-T-cell immunity. At 180 days after infection, the CD8+-T-cell response in the knockout mice was depressed, as determined by pCTL and IFN-γ assays. Loss of CD8+-T-cell immunity at this time was confirmed by adoptive transfer experiments. Purified CD8+ T cells from CD4−/− donors that had been immunized 180 days earlier failed to protect the recipient mice against a lethal infection. Our study demonstrated that although CD8+-T-cell immunity can be induced in the absence of conventional CD4+ T cells, it cannot be maintained without such cells.
Toxoplasma gondii induces a strong humoral and cellular immune response in an infected host (25, 36, 42). However, it has been demonstrated that cell-mediated immunity is a major factor responsible for resistance against this parasite (13). The importance of cellular immunity against T. gondii is shown by the high incidence of toxoplasmosis in the human immunodeficiency virus-infected population before highly active antiretrovirus therapy was introduced (29). Similarly, nude mice that lack T cells do not develop resistance against T. gondii, and transfer of immune T cells to these animals protects them from infection (11).
During acute toxoplasma infection, before adaptive immunity is established, NK cells are important for restraining the infection (7, 15). However, antigen-specific T cells are critical for long-term survival of the host (16). Among the cells in the T-cell population, CD8+ T cells are considered the major effector cells against T. gondii infection, and CD4+ T cells play a synergistic role (12, 20). This has been confirmed by adoptive transfer studies, which have demonstrated that CD8+ T cells are the principal mediators of protective immunity against T. gondii (20, 39).
Although CD4+ T cells are considered important, their role in the maintenance of CD8+-T-cell effector immunity against T. gondii is not well defined. Various studies have suggested that CD4+ T cells may be essential for priming of CD8+-T-cell effector immunity against T. gondii (4, 13). In the present study, we used gene knockout mice to evaluate the importance of CD4+ T cells in induction and maintenance of the CD8+-T-cell response against T. gondii infection. Although primary CD8+-T-cell immunity in CD4−/− mice after oral infection could be induced, these animals did not sustain a long-term memory CD8+-T-cell response. Over time, CD8+-T-cell immunity in CD4−/− mice was dampened, and the mice became susceptible to T. gondii challenge.
MATERIALS AND METHODS
Mice, infection, and challenge.
CD4−/− mice with a C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, Maine). Age- and sex-matched parental wild-type mice were used as controls. Mice were challenged perorally with 20 to 100 cysts of T. gondii strain 76K (kindly provided by Daniel Bout, Tours, France). This strain is maintained by continuous oral passage of cysts. Tachyzoites of T. gondii strain PLK were used for the challenge experiments.
Quantitation of parasite burden.
Tissues (guts, spleens, livers, lungs, and brains) were recovered from mice that had been infected 7 days previously with T. gondii. DNA was extracted from tissues with a QIAamp tissue kit (Qiagen, Chatsworth, Calif.), and 400 ng of each sample was analyzed by PCR. Parasite DNA was amplified by using primers specific for the toxoplasma B1 gene (5′-GGAACTGCATCCGTTCATGAG-3′ and 5′-TCTTTAAAGCTTCGTGGTC-3′), a 35-fold repetitive sequence found in all known parasite strains (2). A 134-bp competitive internal standard containing the same primer template sequences as the 194-bp B1 PCR fragment was generated and used (23). These two segments were amplified in a 50-μl reaction mixture containing 1.25 U of Amplitaq DNA polymerase, 1× buffer (Perkin-Elmer), 0.2 mM (each) dGTP, dATP, dTTP, and dCTP, and each of the B1 primers at a concentration of 0.4 μM. For each reaction, a known amount of DNA from tissue was amplified with different amounts of the internal standard. The parasite load was estimated by comparison with the internal control. To determine the parasite loads for the infected tissues, PCR was performed by using the same conditions and a known number of parasites. The level of the internal control was calculated per parasite (23).
Determination of T. gondii cysts in mouse brains.
Groups of five CD4−/− and wild-type mice were challenged orally with 15 cysts of T. gondii 76K. The animals were sacrificed on day 45 postinfection (p.i.), and the brains were removed. Each entire brain was ground in 2 ml of phosphate-buffered saline (PBS) by using a Dounce homogenizer. The mean number of cysts was then determined by counting the numbers of cysts in six samples (10 μl each) by light microscopy.
Histopathological analysis.
Tissues (guts and livers) from the infected CD4−/− and parental control animals were fixed in 10% buffered formalin and processed to obtain 5-μm histological sections, which were placed on slides and stained with hematoxylin and eosin stain. The slides were examined and photographed by using an Olympus Van Ox microscope and Kodak Elite 100 film. Images were digitized with a Polaroid Sprint scanner, and a figure was assembled with Adobe Photoshop software.
Detection of IFN-γ.
Messages for gamma interferon (IFN-γ) in the tissues of CD4−/− mice were analyzed by quantitative PCR. Tissues (guts, spleens, livers, lungs, and brains) from knockout and wild-type mice were collected on day 7 p.i. RNAs were collected from the tissues by using Trizol (Gibco BRL, Gaithersburg, Md.) as recommended by the manufacturer. Reverse transcription was performed by using Moloney murine leukemia virus reverse transcriptase (Gibco BRL) and random hexamer primers (Promega, Madison, Wis.). IFN-γ mRNA levels were determined by quantitative PCR using the PQRS quantitative method (35). The tissues from uninfected mice were used to establish a baseline value of 1.0, and this value was used to determine the levels of cytokine message in the test mice.
CD8+ T cells from infected animals were assayed for IFN-γ production at various times after T. gondii infection. CD8+ T cells from the infected animals were separated by positive selection by using antibody-coated microbeads (Miltenyl Biotech, Auburn, Calif.) as recommended by the manufacturer. The purity of the cells was >95%, as determined by fluorescence-activated cell sorter analysis. The purified CD8+ T cells were stimulated in vitro with toxoplasma lysate antigen (TLA) and irradiated feeder cells in a 24-well plate. TLA was prepared by using a previously described protocol (19). After 72 h of incubation, the cultures were harvested, and supernatants were collected, centrifuged, and stored at −80°C until they were used. The supernatants were assayed for IFN-γ production by an enzyme-linked immunosorbent assay. The assay was performed with cytokine-specific CytoSets according to instructions of the manufacturer (Biosource Int., Camarillo, Calif.).
Adoptive transfer of CD8+ T cells.
Parental C57BL/6 mice and CD4−/− mice were infected orally with 20 cysts of T. gondii. At 90 and 180 days p.i. the mice were sacrificed and splenectomized, and CD8+ T cells (>95% pure) were adoptively transferred to naïve C57BL/6 mice via intravenous tail vein inoculation. Then 24 h after the adoptive transfer of immune cells, each of the mice was challenged with 5 ×104 tachyzoites of strain PLK.
Estimation of pCTL frequency.
The frequency of antigen-specific CD8+ T cells was determined by performing a precursor cytotoxic T lymphocyte (pCTL) analysis by a standard technique (9). CD8+ T cells from the infected animals were separated as described above. Purified CD8+ T cells were cultured by the limiting dilution assay method in 96-well round-bottom plates. The cells were grown in RPMI 1640 medium containing appropriate growth factors, including 15 U of recombinant interleukin-2 (IL-2) (R&D Chemicals, Minneapolis, Minn.) per ml, and irradiated strain PLK tachyzoites (5 × 103 tachyzoites/well, irradiated at 15,000 rads). A total of 2 × 105 syngeneic splenocytes irradiated at 3,000 rads were added to each well as feeder cells. Purified CD8+ T cells were serially diluted to obtain concentrations ranging from 3,125 to 50,000 cells/well. Control wells contained only irradiated parasites and feeder cells. After 1 week, the cells were harvested and incubated with 51Cr-labeled parasite-infected and uninfected macrophages. The macrophages were collected and labeled as described elsewhere (20). Briefly, mouse peritoneal macrophages were obtained by lavage 2 days after intraperitoneal inoculation with 1 ml of thioglycolate. The macrophages were washed three times in PBS and dispensed at a concentration of 2 × 104 cells/well into 96-well U-bottom tissue culture plates. After overnight incubation, they were radiolabeled with 51Cr (0.5 μCi/well; New England Nuclear Research Products, Boston, Mass.) for 3 h at 37°C. After several washes in PBS, the macrophages were infected with 2 × 104 freshly obtained PLK parasites. The next morning, the spontaneous lysis caused by overnight parasite infection was measured, and all wells exhibiting >250 cpm in the supernatant were excluded from the experiment. Macrophages were washed in PBS and incubated with CD8+-T-cell cultures. The amount of radioisotope released was measured following 4 h of incubation. Wells were considered positive for lytic activity if the total amount of radioactivity released by effector cells plus target cells was more than 3 standard deviations greater than the amount observed for the control wells (the mean amount of radioactivity released by the target cells incubated with antigen-presenting cells and irradiated parasites alone). The pCTL frequencies were calculated by using χ2 analysis as described by Taswell (41) and a computer program kindly provided by William R. Green of Dartmouth Medical School.
Statistical analysis.
Statistical analysis of the data was performed by using Student’s t test (32).
RESULTS
CD4−/− mice survived oral infection with T. gondii.
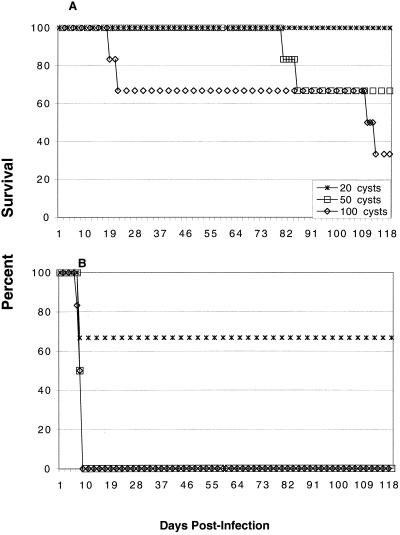
To determine if a lack of CD4+ T cells can alter the outcome of acute toxoplasma infection, CD4−/− and parental controls were infected with 20 to 100 cysts of T. gondii 76K. As shown in Fig. 1A, CD4−/− animals survived a high dose of T. gondii. No early mortality was observed in such animals that received infective doses of up to 50 cysts. When the inoculum was increased to 100 cysts, two of the six mice died between days 19 and 21 p.i. In comparison, all of the wild-type mice died by day 9 p.i when they were infected orally with 50 to 100 cysts (Fig. 1B). The level of mortality observed with wild-type mice infected with 20 cysts was 33%, while none of the knockout mice that received this dose died. However, two of six CD4−/− mice that were infected with 50 cysts died between 80 and 100 days p.i. (Fig. 1A). Similarly, two animals belonging to the knockout group that received a challenge dose of 100 cysts died between 100 and 110 days p.i. (Fig. 1A). The level of mortality remained the same until 180 days p.i., when the experiments were terminated.
FIG. 1.
Survival of CD4−/− (A) and wild-type (B) mice infected with different doses of T. gondii. Female CD4−/− mice and parental C57BL/6 mice that were 5 to 6 weeks old were challenged perorally with 20, 50, or 100 cysts of T. gondii 76K. Survival of animals was monitored daily. There were six animals per group, and the experiment was performed twice with similar results. The data shown are the data from one of the two experiments.
Analysis of the cytokine message in response to T. gondii infection.
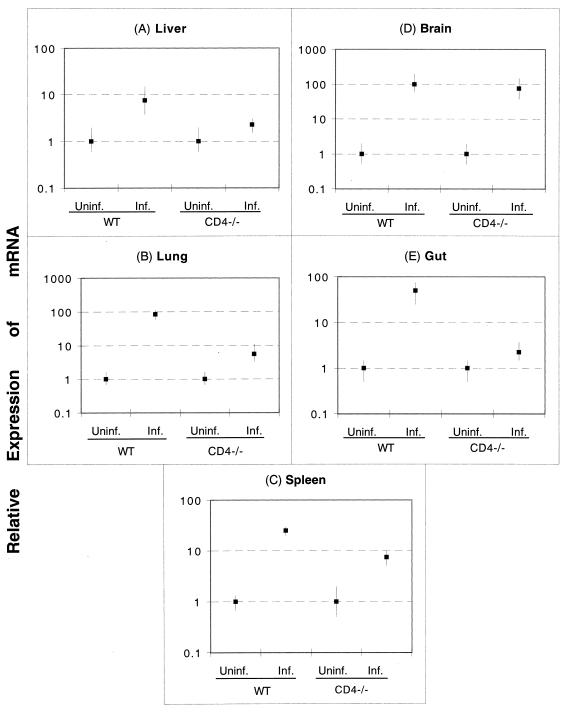
As CD4+ T cells have been reported to be an important source of IFN-γ production during T. gondii infection (6), the levels of the cytokine message in the various tissues of the infected animals were determined by quantitative PCR. All of the tissues from infected CD4−/− mice except the brain tissue (Fig. 2D) showed decreased upregulation of the IFN-γ message compared to the tissues from the control wild-type animals. It has been suggested recently that non-T cells are involved in the production of IFN-γ in the brains of T. gondii-infected mice (18). As shown in Fig. 2, 10- to 20-fold decreases in the IFN-γ message were observed for the lungs (Fig. 2B) and the guts (Fig. 2E) of CD4−/− mice. Similarly, the livers (Fig. 2A) and spleens (Fig. 2C) of CD4−/− mice expressed three- to fivefold-lower IFN-γ messages than the livers and spleens of the parental C57BL/6 mice expressed.
FIG. 2.
IFN-γ mRNA expression following T. gondii infection in CD4−/− and parental wild-type (WT) mice. Mice were infected orally with 20 cysts of T. gondii as described in the text. On day 7 p.i., tissues from both infected (Inf.) and uninfected (Uninf.) controls (three mice per group) were harvested and pooled. Expression of mRNA for IFN-γ was assayed by reverse transcriptase PCR. The transcriptional levels for the genes are expressed relative to the transcriptional level in uninfected mice (defined as 1). The cDNA concentration examined at each time was standardized to the hypoxanthine phosphoribosyltransferase mRNA level (data not shown).
Histological analysis.
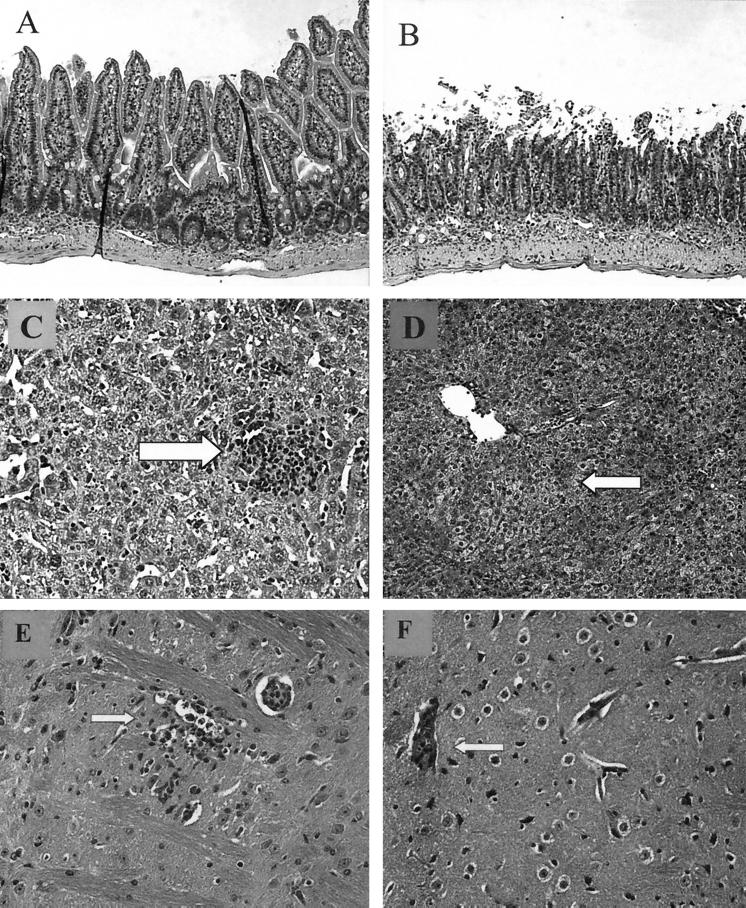
Sections of small intestines and livers from CD4−/− and parental C57BL/6 mice that had been infected perorally with 50 cysts of T. gondii 76K were subjected to a histopathological analysis on day 7 p.i. The distal small bowels from the C57BL/6 mice were extensively necrotic, with full thickness inflammation and ulceration (Fig. 3B). In contrast, no necrosis was observed in the distal small bowels of CD4−/− mice (Fig. 3A). Similarly, the livers of wild-type mice exhibited extensive fatty cell degeneration (Fig. 3D) compared to the livers of CD4−/− mice (Fig. 3C). The analysis of brains showed that CD4−/− mice had necrotic encephalitis with moderate severity in the cerebral cortex together with a small number of T. gondii cysts and tachyzoites scattered in the lesions (Fig. 3E). Additionally, there was mild infiltration of lymphocytic cells in the perivascular space and thickening of the small blood vessel walls. The meninges exhibited moderate hypercellularity consisting mainly of mononuclear cells in some affected areas. The lesions in a wild-type C57BL/6 mouse were much milder, and no focal necrosis or T. gondii organisms were observed (Fig. 3F)
FIG. 3.
(A and B) Ilea from CD4−/− (A) and wild-type control (B) mice on day 7 p.i. In the CD4−/− ileum (magnification, ×10) the architecture of the villi was normal for the most part, although there was increased cellular infiltrate in the lamina propria. In the wild-type control infected mouse there was severe blunting and necrosis of the villi and a high level of lymphocyte infiltration. (C and D) Livers from CD4−/− (C) and wild-type control (D) mice on day 7 p.i. In the CD4−/− liver there was remodeled hepatocyte architecture with little fatty cell degeneration, but focal lymphocytic nodules (arrow) provided evidence of acute toxoplasma infection. In the wild-type control infected mouse there was extensive fatty cell degeneration (left of arrow). (E and F) Brains from CD4−/− (E) and wild-type control (F) mice on day 7 p.i. In the CD4−/− mouse there was necrotic encephalitis associated with T. gondii tachyzoites (arrow). In the wild-type C57BL/6 mice there were occasional focal cellular infiltrates (arrow).
CD4−/− mice had a higher parasite burden in their tissues.
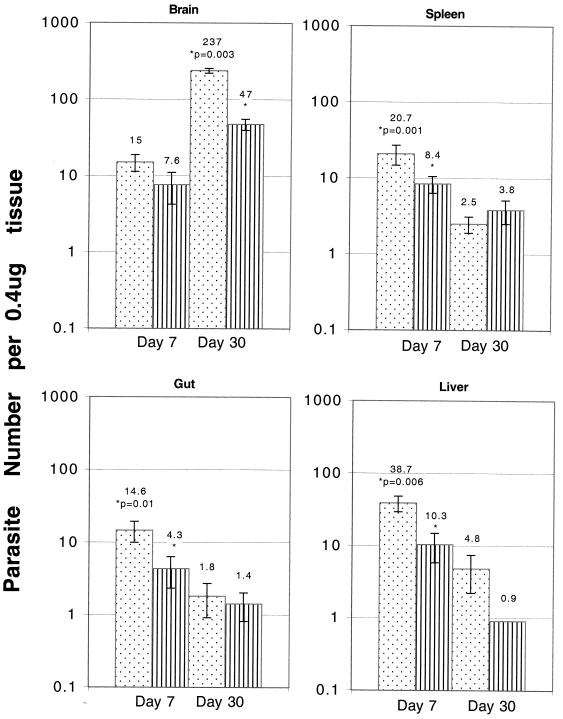
The parasite loads in tissues from both CD4−/− and parental C57BL/6 mice that had been infected with 20 cysts of the 76K stain were evaluated to determine the levels of parasite multiplication in the knockout animals. The relative abundance of the B1 gene, a genetic marker for T. gondii, was determined on days 7 and 30 p.i. On day 7 p.i., all of the organs (spleens, livers, guts, and brains) from knockout mice exhibited two- to fourfold greater parasite burdens than the organs of the parental C57BL/6 mice (Fig. 4). By day 30 p.i., however, the parasite loads in the spleens, livers, and guts of the knockout mice were similar to those in the tissues of the C57BL/6 mice at the same time. Compared to the livers of the parental mice, there was a minor increase in the parasite numbers in the livers of knockout animals. The brains of the CD4−/− mice did have significantly greater parasite loads than the brains of the parental C57BL/6 mice (P = 0.003). These findings were confirmed by determining the numbers of cysts in the brains of the knockout and wild-type mice on day 45 p.i. The average number of cysts in the brains of wild-type C57BL/6 mice was 560 ± 104, and the number of cysts in the brains of knockout mice was fourfold higher (2,233 ± 516). While the number of cysts in the wild-type animals decreased 6 months p.i. (230 ± 87), the number of cysts in the CD4−/− mice increased at this time (5,336 ± 976). Interestingly, this increase coincided with the decrease in the immune CD8+-T-cell function in the infected hosts.
FIG. 4.
Numbers of parasites per 0.4 microgram of tissue DNA in the organs of CD4−/− mice (dotted bars) and parental C57BL/6 mice (striped bars) infected with T. gondii. Mice (three mice per group) were infected orally with 20 cysts of T. gondii. On days 7 and 30 p.i., the organs were collected from both wild-type and knockout mice, and the parasite loads in the tissues were determined by competitive DNA PCR. This experiment was performed twice, and similar results were obtained both times.
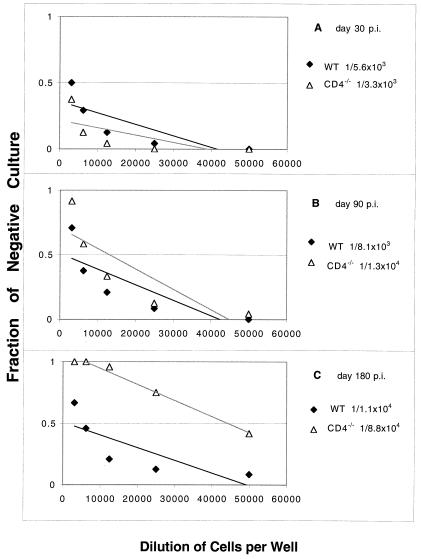
CD4−/− mice exhibited a decreased long-term CD8+ pCTL response.
T. gondii is known to generate an antigen-specific CD8+-T-cell response in an infected host (14, 38). No difference in the absolute number of CD8+ T cells in the spleens of wild-type and knockout mice was observed on day 7 p.i (data not shown). Based on our experience, the sizes of the spleens of infected animals return to normal by day 14 to 21 p.i. Hence, the differences in the antigen-specific CD8+ T cells at later times were estimated by the pCTL assay. The frequencies of pCTLs in the knockout and C57BL/6 mice were determined on days 30, 90, and 180 p.i. There was not a significant difference in the number of antigen-specific CD8+ T cells based on pCTL analysis between the wild-type and CD4−/− mice on days 30 and 90 p.i (Fig. 5A and B). However, on day 180 p.i. (Fig. 5C) the pCTL frequency for the knockout mice (1/88,453) was less than the pCTL frequency for the wild-type C57BL/6 mice (1/10,814) (P = 0.08).
FIG. 5.
pCTL frequency in CD4−/− mice infected orally with T. gondii. CD4−/− and wild-type (WT) C57BL/6 mice were infected orally with 20 cysts of T. gondii. At different times (days 30, 60, and 180 p.i.) the total spleen cell populations from infected animals (three mice per group) were collected, and CD8+ T cells were isolated and cultured by a limiting dilution assay method as described in Materials and Methods. After 1 week, the pCTL frequency of the CD8+ effector cells cultured in the presence of antigen was determined based on a comparison with the results obtained with negative cultures to which no effector cells were added. The data are representative of the data obtained in one of the two experiments performed.
In addition to their cytotoxicity, CD8+ T cells are also known to be an important source of IFN-γ during certain microbial infections (1, 3, 24). IFN-γ production after antigenic restimulation is an important characteristic of memory/effector T cells (40). To further evaluate the long-term CD8+-T-cell immunity against T. gondii in the CD4−/− mice, the kinetics of IFN-γ production by CD8+ T cells from the knockout mice at different times p.i. were determined. As shown in Table 1, CD8+ T cells from both CD4−/− and wild-type mice, isolated on days 30 and 90 p.i., released almost equal amounts of IFN-γ when they were stimulated with TLA. However, IFN-γ production by CD8+ T cells from knockout mice started to decline on day 135 p.i. (Table 1). Similar to the results of the pCTL assays, on day 180 p.i. CD8+ T cells from the mutant mice produced significantly less (P = 0.02) IFN-γ than control CD8+ T cells from the wild-type mice produced.
TABLE 1.
IFN-γ production by CD8+ T cells
| Days p.i. | IFN-γ production (pg/ml)a
|
|||
|---|---|---|---|---|
| Wild-type cells
|
CD4−/− cells
|
|||
| Unstimulated | Stimulated | Unstimulated | Stimulated | |
| 0 | ND | 13 ± 2.6 | ND | 9.7 ± 1.9 |
| 30 | 164 ± 14 | 1,348 ± 82.5 | 164 ± 18 | 1291 ± 96 |
| 120 | 130.5 ± 18.5 | 1,322 ± 47 | 117 ± 19 | 1,143 ± 105 |
| 135 | 90.3 ± 17.9 | 1,185.7 ± 65.1b | 90.3 ± 18.9 | 602 ± 147.4b |
| 180 | 122 ± 16 | 1,268 ± 116b | 65.5 ± 20.5 | 190.5 ± 54.5b |
CD4−/− and parental control mice were infected perorally with 20 cysts of T. gondii 76K. On days 30, 60, 90, and 180 p.i., the mice (three mice per group) were sacrificed, and splenocytes were isolated and pooled. CD8+ T cells were purified as described in Materials and Methods. A total of 1 × 106 purified CD8+ T cells (>95% pure) were cultured in 24-well plates and stimulated with 15 μg of TLA per ml in the presence of 5 × 105 irradiated feeder cells. After 72 h of incubation the supernatants were collected, centrifuged, and stored at −70°C. The supernatants were assayed for IFN-γ production by an enzyme-linked immunosorbent assay. ND not detected.
P < 0.02, as determined by Student’s t test. (Comparison is between antigen-stimulated CD8+ T cells from CD4−/− mice and those from wild-type mice.)
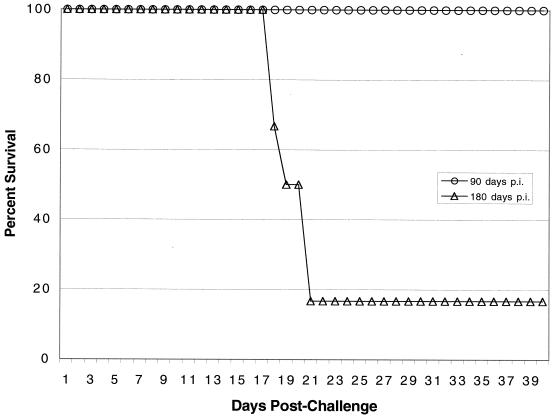
CD4−/− immune mice were not able to prevent T. gondii challenge in the long term.
The experiments described above demonstrated that CD8+-T-cell immunity in the CD4−/− mice was significantly compromised after 180 days of infection. Next, the protective immunity of CD4−/− mice against lethal T. gondii challenge was evaluated. CD4−/− and wild-type C57BL/6 mice were challenged 90 and 180 days p.i. All of the mice challenged on day 90 p.i. were able to resolve the infection, and none of them died until termination of the experiment (Fig. 6). In contrast, when the mice were challenged with T. gondii 180 days p.i., the majority of the mice (five of six mice) succumbed to infection. No mortality was observed in the immune wild-type controls at any of the times tested (data not shown).
FIG. 6.
Long-term survival of immune CD4−/− mice challenged with a lethal dose of T. gondii. Female CD4−/− mice and wild-type C57BL/6 animals that were 5 to 6 weeks old were infected orally with 20 cysts of T. gondii 76K. The immune animals were challenged intraperitoneally with 1 × 104 tachyzoites of strain PLK at 90 or 180 days p.i. Survival of the challenged animals was monitored daily until the end of the experiment. The data are representative of the data obtained in one of the two experiments performed.
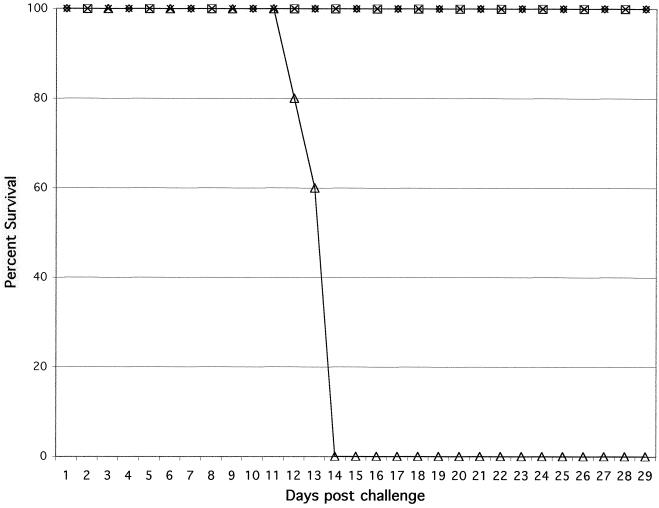
CD8+ T cells from CD4−/− mice that had been infected 180 days earlier were not able to protect naïve animals.
To confirm the loss of long-term CD8+-T-cell immunity in the CD4−/− mice, adoptive transfer studies were performed. Affinity-purified CD8+ T cells were transferred to naïve C57BL/6 mice, which were subsequently challenged with a lethal toxoplasma infection. CD8+ T cells from both CD4−/− and parental C57BL/6 mice that had been infected 90 days earlier were able to protect the naïve animals against T. gondii infection. (Fig. 7). However, when the adoptive transfer was performed 180 days p.i., the CD8+ T cells from the CD4−/− mice were not able to protect the naïve mice (Fig. 7). In contrast, the CD8+ T cells from the wild type continued to protect the nonimmune animals against a lethal toxoplasma infection.
FIG. 7.
Adoptive transfer of immune CD8+ T cells from CD4−/− mice to naïve recipients. CD4−/− and wild-type C57BL/6 mice were infected orally with 15 cysts of T. gondii. The infected mice (four mice per group) were sacrificed on days 90 and 180 p.i., and the spleens were collected and pooled. CD8+ T cells were separated from the spleen cells by affinity purification. The purified CD8+ T cells (5 × 106 cells/mouse) were injected into naïve C57BL/6 mice (five mice per group) intravenously. The recipients were challenged intraperitoneally with 5 × 104 tachyzoites 24 h after transfer, and survival was monitored daily. Symbols: ⋄, knockout mice 90 days p.i.; □, wild-type mice 90 days p.i.; ▵, knockout mice 180 days p.i.; ×, wild-type mice 180 days p.i.
DISCUSSION
In the present study, the role of CD4+ T cells during acute and long-term T. gondii infections was studied. Our data demonstrate that CD4+ T cells are important for early IFN-γ production during T. gondii infection and that a lack of such cells leads to increased parasite multiplication in the tissues. However, no mortality as a result of an acute T. gondii infection was observed in the knockout mice. On the contrary, CD4−/− mice survived higher doses of toxoplasma than wild-type mice, which died from a hyperimmune inflammatory response. These observations confirm previous findings obtained with major histocompatibility complex class II-deficient mice (26), which lack conventional CD4+ T cells. As expected, tissues from CD4−/− mice did not show gut necrosis or acute liver pathology like the tissues from the parental C57BL/6 animals. Nevertheless, as indicated above, CD4−/− mice did exhibit increased parasite burdens in the tissues, especially the brain, which could have been a result of an overall decrease in IFN-γ production. These findings are in agreement with previous studies, which demonstrated that among the cells in the T-cell population, CD4+ T cells are the major source of IFN-γ production during T. gondii infection (4, 12). Although the majority of CD4−/− mice survived the early T. gondii infection due to a decreased inflammatory response, some of these animals did succumb to infection later (80 to 100 days p.i.). This finding is somewhat different from the findings obtained in previous studies, in which combined antibody depletion of CD4+ and CD8+ T cells resulted in 100% mortality in chronically infected animals (12). The reason for this could be that although the long-term CD8+-T-cell response in the CD4-deficient mice is weakened, it might be still enough to limit the chronic infection. Alternatively, knockout mice may contain chronic toxoplasma infections by some unidentified redundant mechanism(s).
The interesting feature of the present findings is that they demonstrate that CD4+ T cells have an important role in the maintenance of CD8+-T-cell immunity against T. gondii. As in parental C57BL/6 mice, primary CD8+-T-cell immunity in the CD4−/− mice could be induced, but unlike the parental controls, long-term CD8+-T-cell immunity in the absence of CD4+ T cells was not sustained. Compared to CD8+ T cells from the wild-type animals, CD8+ T cells from the CD4−/− mice exhibited lower pCTL frequency and decreased IFN-γ production in response to antigenic restimulation 180 days after the primary infection.
The role of T-cell immunity in T. gondii infections has been studied very well (6, 16, 39). The majority of the studies have suggested that both CD4+- and CD8+-T-cell subtypes are important for protection against T. gondii infection, with the CD8+ T cells playing a primary effector role and the CD4+ T cells having a synergistic effect (12, 34). This hypothesis is supported by the findings of Gazzinelli et al., who demonstrated that depletion of CD4+ T cells prior to vaccination did not protect the mice against a lethal T. gondii challenge (13). However, in our study, we found that a normal CD8+-T-cell response can be induced in the absence of CD4+ T cells. The difference between our findings and those of Gazzinelli et al. could be due to the fact that CD8+-T-cell priming in the CD4−/− mice may occur via redundant mechanisms known to exist in the knockout animals (5). Another major difference between the findings of Gazzinelli et al. and our findings is that depletion of the CD4+ T cells after vaccination in the study of Gazzinelli et al. had no effect on the survival of the immune animals. In contrast, in our study CD8+-T-cell immunity could not be maintained in the absence of CD4+ T cells. These differences could be attributed to a number of things. While the study of Gazzinelli et al. was carried out with a mutant parasite, we used a natural cyst-forming strain of T. gondii. Moreover, the effect of CD4+ depletion in the study of Gazzinelli et al. was determined for only 30 days after vaccination, and the long-term consequences of the lack of CD4+ T cells were not evaluated.
The role of conventional CD4+ T cells in induction of the CD8+-T-cell response has been studied in other infectious disease models (30, 44). During an infection with lymphocytic choriomeningitis virus, mice lacking CD4+ T cells exhibited a significantly lower pCTL response than wild-type controls (30). As a result, CD4+-T-cell-deficient mice were not able to clear the virus. Coordinated interaction between CD4+ and CD8+ T cells was required to resolve an infection with the intracellular bacterium Listeria monocytogenes (44). However, the absence of CD4+ T cells did not affect the CD8+-T-cell response against an Encephalitozoon cuniculi infection (31). Similar to these findings, our findings showed that a CD8+-T-cell immune response against T. gondii could be induced in CD4−/− mice. Recent studies with Plasmodium yoelii showed that priming of the CD8+-T-cell response against the parasite was dependent on IL-12 and NK cells (8). Very strong induction of an IL-12-dependent NK cell response during acute T. gondii infection has also been reported (13, 22). Thus, it is possible that as in P. yoelli infection, the early CD8+-T-cell response against T. gondii is regulated by IFN-γ-producing NK cells. This possibility is supported by recent reports from our laboratory which showed that induction of the CD8+-T-cell response against T. gondii in p40−/− mice, which are not able to produce IL-12, was significantly compromised due to a severe defect in IFN-γ production in these animals (9). Moreover, recent studies performed in our laboratory have demonstrated that spleen cells of CD4−/− mice contain higher numbers of NK cells during T. gondii infection than spleen cells of parental controls (Duvand and Khan, unpublished observations).
Based on our observations, we hypothesize that T. gondii infection results in early IL-12 production in an infected host. Release of IL-12 causes NK cell proliferation and subsequent polarization of CD4+ T cells toward IFN-γ production. IFN-γ release by both NK cells and CD4+ T cells is important for reduction of the parasite burden in the host. An absence of CD4+ T cells causes a decline in IFN-γ production, which results in increased parasite numbers in the tissues of knockout animals. At the same time, a decrease in IFN-γ production protects the knockout mice from mortality due to a hyperimmune response, without any obvious effect on induction of the CD8+-T-cell response against the parasite. However, our findings raise two very important questions. First, how does optimal CD8+-T-cell priming take place in the absence of IL-2-producing CD4+ T cells? This question gains further importance because of observations of Denkers et al., who reported that IL-2 release by CD4+ T cells is important for induction of a CD8+-T-cell response in T. gondii-infected hosts (4). This could happen through one of two mechanisms. First, in the knockout mice certain other cell types, like CD4− CD8− double-negative T cells or γδ T cells, may compensate for regular CD4+ T cells in priming of effector CD8+-T-cell immunity. Development of functional double-negative helper T cells against Leishmania major infection in CD4−/− mice has been reported (27). The role of γδ T cells in the regulation of both CD4+- and CD8+-T-cell immune responses in microbial infections has been described previously (10, 33). However, neither of these T-cell subsets is known to be a major source of IL-2 production (17, 43). Alternatively, CD8+-T-cell priming against T. gondii infection in CD4−/− mice may take place via an IL-2-independent mechanism. Generation of normal CD8+-T-cell responses in IL-2-deficient mice against tumor cells has been reported (37). The second and most important question is, why are CD4+ T cells required for sustaining the long-term CD8+-T-cell response? It is possible that IL-2 may be important for maintenance of an expanded pool of memory cells against recurrent or challenge T. gondii infections. However, recent studies have suggested that maintenance of memory CD8+ T cells is dependent on IL-15 (21, 28, 46), which is produced by a wide variety of cell types, such as macrophages and dendritic cells (45). It is very likely that IFN-γ-producing CD4+ T cells are required for keeping the antigen-presenting cells in an activated state so that they constantly restimulate memory CD8+ T cells. Ongoing studies in our laboratory should resolve these issues.
Acknowledgments
We thank Wangxue Chen, Trudeau Institute, New York, N.Y., for his help with the histopathological analysis.
National Institutes of Health grant AI33325 supported this work.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Andersen, H., D. Dempsey, R. Chervenak, and S. R. Jennings. 2000. Expression of intracellular IFN-gamma in HSV-1-specific CD8+ T cells identifies distinct responding subpopulations during the primary response to infection. J. Immunol. 165:2101–2107. [DOI] [PubMed] [Google Scholar]
- 2.Burg, J. L., C. M. Grover, P. Pouletty, and J. C. Boothroyd. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R. J. Mazzaccaro, J. L. Flynn, P. F. Barnes, S. Southwood, E. Celis, B. R. Bloom, R. L. Modlin, and A. Sette. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA 97:12210–12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkers, E. Y., R. T. Gazzinelli, D. Martin, and A. Sher. 1993. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J. Exp. Med. 178:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denkers, E. Y., T. Scharton-Kersten, S. Barbieri, P. Caspar, and A. Sher. 1996. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J. Exp. Med. 184:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkers, E. Y., A. Sher, and R. T. Gazzinelli. 1993. CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res. Immunol. 144:51–57. [DOI] [PubMed] [Google Scholar]
- 7.Diez, B., R. Nicolas, A. Galdeano, R. Cisterna, and M. L. Canavate. 1991. Kinetics and regulation of NK activity by interleukin-2 and interferon in acute toxoplasmosis. Scand. J. Immunol. 34:673–677. [DOI] [PubMed] [Google Scholar]
- 8.Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884–892. [PubMed] [Google Scholar]
- 9.Ely, K. H., L. H. Kasper, and I. A. Khan. 1999. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J. Immunol. 162:5449–5454. [PubMed] [Google Scholar]
- 10.Ferrick, D. A., M. D. Schrenzel, T. Mulvania, B. Hsieh, W. G. Ferlin, and H. Lepper. 1995. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature 373:255–257. [DOI] [PubMed] [Google Scholar]
- 11.Frenkel, J. K., and D. W. Taylor. 1982. Toxoplasmosis in immunoglobulin M-suppressed mice. Infect. Immun. 38:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180. [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292. [PubMed] [Google Scholar]
- 14.Hakim, F. T., R. T. Gazzinelli, E. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310–2316. [PubMed] [Google Scholar]
- 15.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, L. L. 1992. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect. Immun. 60:3719–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadena, T., G. Matsuzaki, S. Fujise, K. Kishihara, H. Takimoto, M. Sasaki, M. Beppu, S. Nakamura, and K. Nomoto. 1997. TCR alpha beta+ CD4− CD8− T cells differentiate extrathymically in an lck-independent manner and participate in early response against Listeria monocytogenes infection through interferon-gamma production. Immunology 91:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, H., and Y. Suzuki. 2001. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun. 69:2920–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan, I. A., and L. Casciotti. 1999. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 163:4503–4509. [PubMed] [Google Scholar]
- 20.Khan, I. A., K. H. Ely, and L. H. Kasper. 1994. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 152:1856–1860. [PubMed] [Google Scholar]
- 21.Khan, I. A., and L. H. Kasper. 1996. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J. Immunol. 157:2103–2108. [PubMed] [Google Scholar]
- 22.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirisits, M., E. Mui, and R. McLeod. 2000. A new method to study the efficacy of vaccines and antimicrobial therapy against toxoplasmosis. Int. J. Parasitol. 30:149–155. [DOI] [PubMed] [Google Scholar]
- 24.Kolls, J. K., S. Habetz, M. K. Shean, C. Vazquez, J. A. Brown, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. R. Summer, and J. E. Shellito. 1999. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 162:2890–2894. [PubMed] [Google Scholar]
- 25.Li, S., G. Maine, Y. Suzuki, F. G. Araujo, G. Galvan, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J. Clin. Microbiol. 38:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locksley, R. M., S. L. Reiner, F. Hatam, D. R. Littman, and N. Killeen. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261:1448–1451. [DOI] [PubMed] [Google Scholar]
- 28.Lodolce, J. P., D. L. Boone, S. Chai, R. E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9:669–676. [DOI] [PubMed] [Google Scholar]
- 29.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211–222. [DOI] [PubMed] [Google Scholar]
- 30.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretto, M., L. Casciotti, B. Durell, and I. A. Khan. 2000. Lack of CD4+ T cells does not affect induction of CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect. Immun. 68:6223–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neter, J., W. Wasserman, and M. H. Kuter. 1985. Applied linear statistical models, 2nd ed. Irwin, Homewood, Ill.
- 33.Nomura, A., G. Matsuzaki, H. Takada, K. Hiromatsu, S. Nabeshima, T. Nakamura, K. Kishihara, and K. Nomoto. 1998. The role of gamma delta T cells in induction of bacterial antigen-specific protective CD8+ cytotoxic T cells in immune response against the intracellular bacteria Listeria monocytogenes. Immunology 95:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp Immunol. 84:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiner, S. L., S. Zheng, D. B. Corry, and R. M. Locksley. 1993. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods 165:37–46. (Errata, 173:133, 1994; 175:275, 1994.). [DOI] [PubMed] [Google Scholar]
- 36.Sher, A., E. Y. Denkers, and R. T. Gazzinelli. 1995. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii. Ciba Found. Symp. 195:95–104. [DOI] [PubMed] [Google Scholar]
- 37.Steiger, J., P. W. Nickerson, W. Steurer, M. Moscovitch-Lopatin, and T. B. Strom. 1995. IL-2 knockout recipient mice reject islet cell allografts. J. Immunol. 155:489–498. [PubMed] [Google Scholar]
- 38.Subauste, C. S., A. H. Koniaris, and J. S. Remington. 1991. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J. Immunol. 147:3955–3959. [PubMed] [Google Scholar]
- 39.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946. [PubMed] [Google Scholar]
- 40.Swain, S. L., M. Croft, C. Dubey, L. Haynes, P. Rogers, X. Zhang, and L. M. Bradley. 1996. From naive to memory T cells. Immunol. Rev. 150:143–167. [DOI] [PubMed] [Google Scholar]
- 41.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614–1619. [PubMed] [Google Scholar]
- 42.Taylor, M. R., B. Lennon, C. V. Holland, and M. Cafferkey. 1997. Community study of toxoplasma antibodies in urban and rural schoolchildren aged 4 to 18 years. Arch. Dis. Child. 77:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukaguchi, K., K. N. Balaji, and W. H. Boom. 1995. CD4+ alpha beta T cell and gamma delta T cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J. Immunol. 154:1786–1796. [PubMed] [Google Scholar]
- 44.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11–25. [DOI] [PubMed] [Google Scholar]
- 45.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19–49. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591–599. [DOI] [PubMed] [Google Scholar]