Abstract
Burkholderia pseudomallei is the causative agent of melioidosis, an infectious disease with protean clinical manifestations. The major route of infection is thought to be through subcutaneous inoculation of contaminated soil and water, although ingestion and inhalation of contaminated aerosols are also possible. This study examines infection through the intranasal route in a murine model to mimic infection through inhalation. Two strains of mice, C57BL/6 and BALB/c, exhibit differential susceptibilities to the infection, with the C57BL/6 mice being considerably more resistant. To examine host factors that could contribute to this difference, bacterial loads and cytokine profiles in the two strains of mice were compared. We found that infected BALB/c mice exhibited higher bacterial loads in the lung and spleen and that they produced significantly higher levels of gamma interferon (IFN-γ) in the serum than C57BL/6 mice. Although tumor necrosis factor alpha and interleukin-1 could be detected in the nasal washes and sera of both strains of mice, the production in serum was transient and much lower than that of IFN-γ. C57BL/6 mice also exhibited memory responses to bacteria upon reinfection, with the production of serum immunoglobulin G (IgG) and mucosal IgA antibodies. Thus, it is possible that the production of systemic and mucosal antibodies is important for protection against disease in C57BL/6 mice.
Melioidosis is an infectious disease caused by the gram-negative bacterium Burkholderia pseudomallei. The disease is endemic in southeast Asia and northern Australia, and there is evidence that it may be endemic in Africa, the Indian subcontinent, and Central and South America (11). The clinical manifestations of the disease vary and include an asymptomatic state, benign pneumonitis, acute or chronic pneumonia, or fulminant septicemias (10). Severe septic melioidosis is usually associated with underlying diseases such as diabetes and chronic renal failure, although it sometimes occurs in previously healthy individuals (8). Asymptomatic seroconversion has been observed in areas of endemicity such as northeast Thailand, where antibodies were found in about 80% of children by the time they were 4 years old (15). In most cases, infection is thought to occur through subcutaneous inoculation of contaminated soil and water, although it is thought to also occur via ingestion or inhalation of contaminated aerosols (17). B. pseudomallei can cause infection in many organs, although pathology occurs mainly in the lung, spleen, and liver (20, 25).
In order to understand the pathogenesis of the disease and the mechanism of host resistance, a murine model that mimics the acute and chronic forms of human melioidosis has been established (16). It was demonstrated that BALB/c mice were highly susceptible to infection with virulent B. pseudomallei, while C57BL/6 mice were relatively resistant. Following intravenous infection with as few as 37 CFU of virulent bacteria, substantial bacterial growth occurred in the livers and spleens of BALB/c mice, followed by overwhelming bacteremia to which the mice succumbed within 72 to 96 h. In contrast, C57BL/6 mice did not develop bacteremia, although they finally succumbed with apparent bacterial growth in the liver and spleen, displaying incomplete resistance (16).
The intraperitoneal and intravenous routes of infections used in animal models are thought to mimic systemic melioidosis. However, the first exposure to many microorganisms is often through the mucosal surfaces in the nasal passages and the gut. The route of infection can lead to different disease outcomes due to differences in the local immune environment. As melioidosis has such a wide spectrum of clinical presentations, it is possible that the route of infection is one of several factors that influence disease outcome. In this study, we examined the outcome of intranasal infection of BALB/c and C57BL/6 mice with virulent B. pseudomallei to simulate natural infection through inhalation and to determine if the murine model of differential susceptibility is still valid. The establishment of an intranasal infection model with differential outcomes would allow us to investigate the factors that could potentially contribute to host resistance and to our understanding of mucosal immunity. The examination of correlates of immunity in animals that were protected from infection would contribute to our understanding of protective immunity and to vaccine development.
MATERIALS AND METHODS
Animals.
Female 5- to 6-week-old C57BL/6 and BALB/c mice were purchased from the Laboratory Animals Center of the National University of Singapore. They were housed in polypropylene cages with a bedding of wood shavings and were fed on a diet of commercial pellets (Glenforrest Stock Feeders, Perth, Australia) and potable water ad libitum. The cages were maintained in an isolated animal room with a class II biological safety cabinet. All experimental procedures with animals and infection were approved by the Animal Ethics Research Committee of the Faculty of Medicine, National University of Singapore.
Bacteria.
The virulent strain KHW of B. pseudomallei used in this study was isolated from a member of the Singapore national service who died from melioidosis. The isolate was identified as B. pseudomallei based on colonial morphology, API20 NE tests (BioMerieux, Marcy l’Etoile, France), and 16S RNA sequence. For inoculation into mice, bacteria were cultured on Trypticase soy agar (TSA) (Difco Laboratories, Detroit, Mich.) for 24 h at 37°C, colonies were picked and resuspended in sterile phosphate-buffered saline (PBS), and the bacterial suspension was adjusted to a density equivalent to that of a no. 0.5 McFarland nephelometer standard (approximately 1.5 × 108 bacteria/ml). The suspension was then diluted to the appropriate concentration in PBS for inoculation, and aliquots were plated on TSA in duplicate to determine the actual number of bacteria inoculated.
Determination of 50% lethal dose (LD50).
Mice were divided into groups of six, and each group was inoculated with a different dose (Table 1). The doses reported reflect the actual dose of the inoculum as determined by colony counts on TSA plates. After being anesthetized with a combination of Hypnorm and midazolam, the mice were inoculated intranasally, through one nostril with a yellow pipette tip, with the appropriate dose of B. pseudomallei in 20 μl of PBS. Mortality was scored over 10 days.
TABLE 1.
Ten-day LD50s for C57BL/6 and BALB/c mice intranasally infected with B. pseudomallei strain KHW
| Mouse strain | No. dead/total at 10 days after intranasal infection with the following inoculation dose (CFU)a:
|
LD50 (CFU) | ||||
|---|---|---|---|---|---|---|
| 5/150 | 15/450 | 45/1,350 | 135/4,050 | 405/12,150 | ||
| BALB/c | 0/6 | 0/6 | 3/4b | 4/6 | 6/6 | 45 |
| C57BL/6 | 0/6 | 1/6 | 1/6 | 3/6 | 3/6 | 4,854 |
Dose for BALB/c mice/dose for C57BL/6 mice.
Two mice in this group died from the anesthetization procedure.
Infection of mice and preparation of organs.
After being anesthetized with a combination of Hypnorm and midazolam, the mice were inoculated intranasally, through one nostril with a yellow pipette tip, with 50 CFU of B. pseudomallei in 20 μl of PBS. Control mice were inoculated with PBS only. At 24, 48, and 72 h after infection, three infected mice and one control mouse were euthanized. The lungs, livers, and spleens were aseptically removed, placed in 3 ml of ice-cold medium separately, and immediately homogenized on ice. A small aliquot was removed for serial dilution in PBS and plated on Ashdown agar (3) in duplicate for each dilution. The colonies on plates were counted after 2 days of incubation at 37°C, and the bacterial load per organ was calculated from the average count taken from plates of appropriate dilutions giving countable colonies. The rest of the homogenates were centrifuged at 4°C and resuspended in Trizol reagent (Gibco-BRL, Grand Island, N.Y.) for immediate RNA isolation or stored at −80°C until use.
RNA isolation and reverse transcription-PCR (RT-PCR).
Total RNA was extracted using Trizol reagent according to the manufacturer’s instructions. RNA was resuspended in diethyl pyrocarbonate-treated water, and the quantity was determined using a spectrophotometer at 260 nm. Five micrograms of RNA was used for reverse transcription in a final mixture of 0.16 μM oligo(dT), 0.25 mM deoxynucleoside triphosphates, 20 U of Moloney murine leukemia virus reverse transcriptase (Biotools-Biotechnological and Medical Laboratories, Madrid, Spain), and 12 U of RNasin (Promega, Madison, Wis.). The reaction was performed for 1 h at 37°C, and the resulting cDNA was readjusted to 150 μl with Tris-EDTA buffer. The final PCR mixture contained 5 μl of cDNA, 1× PCR buffer (Biotools), 0.25 mM deoxynucleoside triphosphates, 0.6 U of Taq DNA polymerase (Biotools), and 0.4 μM sense and antisense primers. The thermal cycling parameters were as follows: 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 1 to 2°C below the melting temperature of the primers for 1 min, and extension at 72°C for 2 min. PCR was performed using primers to amplify various cytokines of interest, including gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-10, granulocyte-macrophage colony-stimulating factor, IL-4 (12), IL-12 p40 (7), IL-1β, and IL-6 (23). Gene-specific primers for all of the cytokines are designed to span introns so that PCR products would allow discrimination of amplification from cDNA or genomic DNA, with the exception of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (19). One of the primers for tumor necrosis factor alpha (TNF-α) is located on an intron-exon junction to prevent coamplification of genomic DNA (sense, 5′GGA TGA GAA GTT CCC AA3′; antisense, 5′ CAG ATT GAC CTC AGC GC3′). All PCRs were performed in an automated DNA thermal cycler (MJ Research, Watertown, Mass.). PCR products were size fractionated through a 1% agarose gel.
Determination of cytokine production by ELISA.
Six BALB/c mice and six C57BL/6 mice were inoculated intranasally with 50 CFU of bacteria. At days 2 and 4 after infection, blood was collected from the lateral tail veins of three infected mice from each mouse strain and pooled. Mice were sacrificed, and the mandibles were removed. The nasal cavity from the posterior opening of the nose was flushed with PBS, and the nasal wash was collected from the anterior opening of the nose and pooled from three mice. Blood was allowed to clot overnight at 4°C and centrifuged to obtain the serum. Samples were assayed for IFN-γ, IL-1β, and TNF-α using enzyme-linked immunosorbent assay (ELISA) kits (Pharmingen, San Diego, Calif.) according to the manufacturer’s instructions. The optical densities were measured at wavelengths of 490 and 750 nm in an MR5000 system (Dynatech, Chantilly, Va.). Pooled samples from three uninfected BALB/c mice and three C57BL/6 mice were also tested as negative controls to obtain cutoff values. The ELISAs were performed in duplicate. The standard deviations between all duplicates were below 10% of the average values, except for four samples for which the standard deviations were 11.1, 12.7, 13.1, and 14% of their average values. This experiment was repeated once with the same number of animals and the same setup and yielded similar results. Thus, results from only one set of experiments are shown in Results.
Antibody response against B. pseudomallei.
C57BL/6 mice were divided into three groups, in which 12 mice were inoculated intranasally with 50 CFU of live bacteria, another 12 animals with were inoculated with 50,000 CFU of heat-killed bacteria, and 9 mice were inoculated with PBS alone. They were inoculated again 14 days later with 100 CFU of live bacteria, 100,000 CFU of heat-killed bacteria, or PBS alone. On day 28, all mice were challenged with 10,000 CFU of live bacteria intranasally. At days 0, 7, 14, 21, 28, 33, and 38 from the start of the first inoculation, blood was collected from the lateral tail veins of mice from each group and pooled. On day 28 before the challenge and 5 (day 33) and 10 (day 38) days after the challenge, three mice from each group were sacrificed and the nasal washes were pooled.
Detection of IgG and IgA by ELISA.
Ninety-six-well plates were coated overnight at room temperature with heat-inactivated B. pseudomallei strain KHW at a concentration of 5 × 107/ml. Plates were subsequently washed extensively with PBS-Tween and blocked with 1% bovine serum albumin for 2 h at 37°C. The plates were washed, and the appropriate dilutions of serum and nasal washes were added and incubated for 2 h at 37°C. After further washings, goat anti-mouse immunoglobulin G (IgG) peroxidase conjugate or goat anti-mouse IgA peroxidase conjugate (Sigma, St. Louis, Mo.) was added at the appropriate dilution and left for 1 h at 37°C. The plates were developed by the addition of o-phenylenediamine (Sigma) as a substrate, and the A492 was read in a Dynatech MR5000 system. The reactions were performed in triplicate.
RESULTS
Intranasal infection with B. pseudomallei.
The 10-day LD50s for the virulent B. pseudomallei strain KHW, determined using the method of Reed and Muench (21), are 45 and 4,854 CFU for intranasal infection of BALB/c and C57BL/6 mice, respectively (Table 1). The three remaining C57BL/6 mice infected with the highest dose of 12,150 CFU at day 10 were lethargic, had ruffled fur, and died within 2 weeks after infection.
Differential susceptibility of C57BL/6 and BALB/c mice to infection.
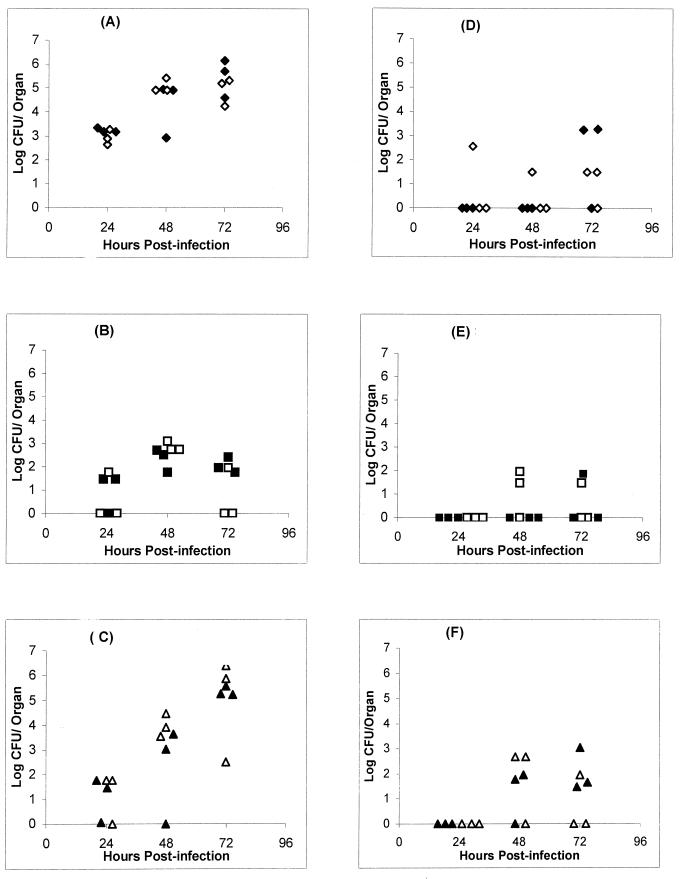
In agreement with other studies showing increased resistance of C57BL/6 mice to intravenous B. pseudomallei infection compared to BALB/c mice, the C57BL/6 mice were 100-fold more resistant to intranasal infection than BALB/c mice. In order to investigate the factors that could contribute to the differential resistance to the infection in the two mouse strains, the animals were infected with 50 CFU, a dose that was lethal for BALB/c mice but had no apparent effect on C57BL/6 mice. To examine the kinetics of infection, the animals were sacrificed at 1, 2, or 3 days after infection, and the bacterial loads in lung, liver, and spleen were determined. The results clearly show that the BALB/c mice developed very high bacterial loads in the lung by the second day after infection (Fig. 1). The spleens also contained high numbers of bacteria, indicating the spread of bacteria into the bloodstream. However, the livers contained considerably fewer bacteria, which were usually cleared by day 3. In contrast, C57BL/6 mice had very few bacteria, if any, in all of the organs. Therefore, the bacterial burden in the organs directly correlated with disease in the animals.
FIG. 1.
Bacterial loads in BALB/c and C57BL/6 mice. The bacterial loads in the lungs (A and D), livers (B and E), and spleens (C and F) of BALB/c (A, B, and C) and C57BL/6 (D, E, and F) mice at different time points after intranasal infection with virulent B. pseudomallei are shown. The logarithm of the number of CFU was plotted against time. Each symbol represents one mouse. The control mice are not represented, since no colonies were found in any of their organs. Filled symbols represent mice from one experiment, and open symbols represent animals from another experiment. Results from the two experiments performed under the same conditions are superimposed.
Cytokine gene expression in C57BL/6 and BALB/c mice.
To investigate the possibility that polarization of cytokine expression and lack of an inflammatory cytokine response contribute to susceptibility to infection in the mice, we examined cytokine gene expression in the lungs, spleens, and livers of the mice by RT-PCR (Fig. 2; Table 2). Both mouse strains expressed high levels of IFN-γ and proinflammatory cytokines after infection, with the peak expression on day 2 for BALB/c mice. IL-4 was not detected and IL-12 was found at low levels in the spleens of C57BL/6 mice. IL-10 was largely absent in BALB/c mice and was not detected consistently in C57BL/6 mice. Therefore, B. pseudomallei infection in BALB/c mice was capable of inducing an inflammatory cytokine response, and the differential resistance to B. pseudomallei in the mouse strains is not due to a Th1- and Th2-type polarization of cytokine responses.
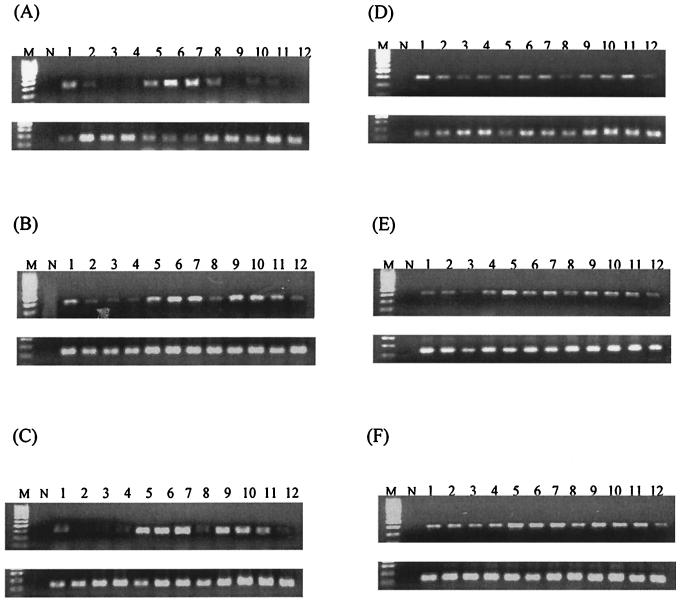
FIG. 2.
Expression of IFN-γ mRNA in the lungs (A and D), livers (B and E), and spleens (C and F) of BALB/c (A, B, and C) and C57BL/6 (D, E, and F) mice as determined by RT-PCR. In each set of gels, the top panel depicts IFN-γ and the bottom panel depicts the corresponding GAPDH. Lanes M, 100-bp molecular size marker; lanes N, no-template control; lanes 1 to 3, three infected mice at 24 h postinfection; lanes 4, control mock-infected mouse at 24 h postinfection; lanes 5 to 7, three infected mice at 48 h postinfection; lanes 8, control mock-infected mouse at 48 h postinfection; lanes 9 to 11, three infected mice at 72 h postinfection; lanes 12, control mock-infected mouse at 72 h postinfection.
TABLE 2.
Cytokine gene expression in infected mice
| Cytokine | mRNA expressiona at the indicated h postinfection in:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C57BL/6 mice
|
BALB/c mice
|
|||||||||||||||||
| Lung
|
Liver
|
Spleen
|
Lung
|
Liver
|
Spleen
|
|||||||||||||
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| IFN-γ | +++ | +++ | +++ | ++− | +++ | +++ | +++ | +++ | +++ | ++− | +++ | ++− | +++ | +++ | +++ | +−− | +++ | +++ |
| TNF-α | −−− | ++− | +−− | ++− | +++ | ++− | +++ | +++ | +++ | −−− | −−− | −−− | +−− | +−− | +−− | +− | ++− | ++− |
| IL-1β | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| IL-4 | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− |
| IL-6 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++− | +++ | ++− | +++ | +++ | +++ | +++ |
| IL-10 | −−− | −−− | +++ | +++ | ++− | +++ | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− |
| IL-12 | −−− | −−− | −−− | −−− | −−− | −−− | +−− | +++ | +++ | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− | −−− |
+, presence of mRNA expression; −, absence of mRNA expression. Each symbol represents the result from one mouse.
Cytokines in the nasal washes and sera of C57BL/6 and BALB/c mice.
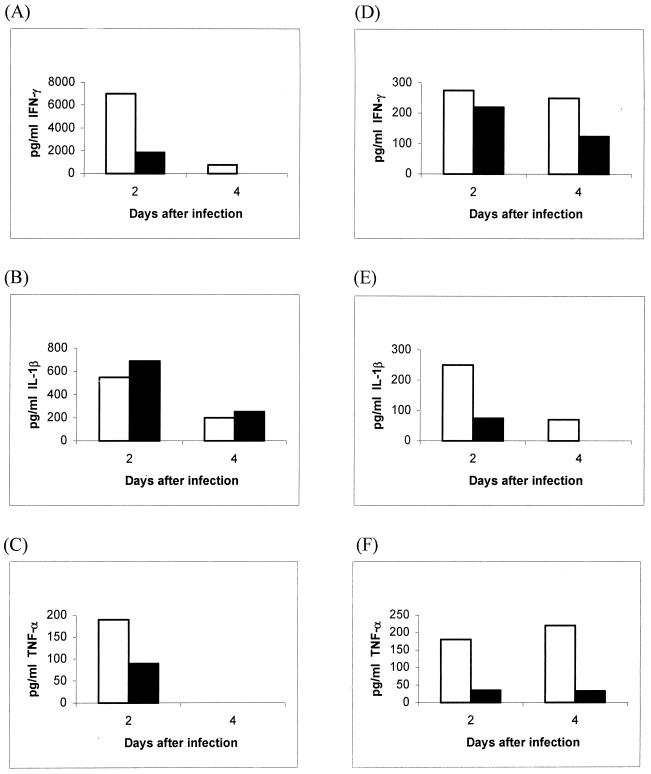
Based on data on the cytokine gene expression profiles in the various organs, we selected IFN-γ and the proinflammatory cytokines TNF-α and IL-1 for quantification in the nasal washes and sera of the mice. High levels of IFN-γ were detected in the sera of BALB/c mice; these were at least fivefold higher than the levels in the C57BL/6 mice (Fig. 3). However, TNF-α levels were low and were undetectable by day 4 for both strains, and IL-1β levels were still low compared to IFN-γ levels and were similar in the two strains of mice. It is thus unlikely that BALB/c mice were dying due to TNF-α- and IL-1β-mediated septic shock. However, local production of these cytokines in the nasal wash showed a clear elevation of TNF-α and IL-1β in BALB/c mice over C57BL/6 mice, even when the IFN-γ levels in BALB/c mice were higher than those in C57BL/6 mice by day 4 although they were comparable on day 2.
FIG. 3.
Concentrations of IFN-γ (A and D), IL-1β (B and E), and TNF-α (C and F) in sera (A, B, and C) and nasal washes (D, E, and F) of BALB/c (white bars) and C57BL/6 (black bars) mice after intranasal infection with 50 CFU of virulent B. pseudomallei. Each bar represents pooled sera or nasal washes from three mice. The optical densities in the ELISA are expressed as picograms per milliliter corresponding to the linear portion of the standard curves.
Antibody response in C57BL/6 mice reinfected with bacteria.
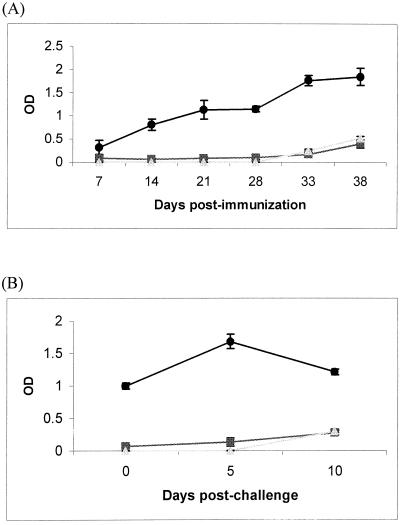
C57BL/6 mice infected intranasally with 50 CFU of B. pseudomallei remained symptom free and did not succumb to disease. When they were sacrificed at 2 weeks after infection, two out of six mice had 40 and 20 CFU of bacteria in their lungs, while one out of six mice had 60 CFU in the liver. The rest of the mice had no detectable bacteria in all organs (data not shown). It is possible that these mice had developed immunity and could withstand a normally lethal dose of bacteria upon reinfection. To examine this possibility, animals were immunized twice with either a low dose of live bacteria, a high dose of dead bacteria, or PBS, followed by reinfection with 10,000 CFU of live bacteria as described in Materials and Methods. We found that mice previously immunized with a low dose of live bacteria were protected from the challenge, while control mice inoculated with 50,000 CFU of heat-killed bacteria or PBS succumbed to disease. The antibody response to the bacteria was examined to see whether it correlated with protection. Only mice previously immunized with a low dose of live bacteria produced serum IgG and mucosal IgA to the bacteria in the nasal wash (Fig. 4). The IgG responses increased after the second immunization at day 14 and the high-dose challenge at day 28 (Fig. 4A), demonstrating secondary immune responses.
FIG. 4.
Serum IgG (A) and mucosal IgA (B) responses in C57BL/6 mice after intranasal inoculation with 50 CFU of virulent B. pseudomallei (•), 50,000 CFU of heat-killed B. pseudomallei (▪), or PBS (▴). Each symbol represents a 1:10 dilution of serum at the indicated days after the first inoculation at day 0, the second inoculation at day 14, and reinfection with 10,000 CFU of live bacteria at day 28 (A), or represents undiluted nasal wash at the indicated days after reinfection with 10,000 CFU live bacteria at day 28 (B), from three mice pooled together. Results represent means ± standard deviations of triplicates. OD, optical density.
DISCUSSION
Besides acute septicemia, one of the most common manifestations of B. pseudomallei infection in humans is pulmonary involvement. This could reflect the organotropism of the bacteria or the physiological relevance of infection through the intranasal route. To date, little is known about the immunopathogenesis of B. pseudomallei, and even less is known for mucosal infection. Based on the murine model of differential susceptibility to intravenous infection of B. pseudomallei (16), we demonstrated that the relative susceptibility of BALB/c mice compared to C57BL/6 mice was also observed with intranasal infection. In fact, BALB/c mice were exquisitely susceptible to intranasal infection, with an LD50 of only 45 CFU, while C57BL/6 mice were at least 100-fold more resistant. An important difference between our study and the previous study was that intranasally infected C57BL/6 mice did not succumb to disease at 50 CFU, a dose lethal for BALB/c mice, even when they were observed for up to 3 months (data not shown), whereas they developed chronic infection reflecting incomplete resistance during intravenous infection (16). The establishment of a murine model demonstrating differential susceptibility through intranasal infection allows us to examine the host factors that correlate with resistance.
One of the most obvious differences between the two strains of mice after infection was the bacterial loads in their organs. The bacterial burden in C57BL/6 mice was very low throughout the 3 days postinfection in all three organs examined, and bacteria were not detectable by 2 weeks in most animals. In contrast, BALB/c mice showed increasing bacterial burdens in lungs and spleens. Interestingly, the bacterial load in livers up to 3 days postinfection was very low, indicating an efficient clearance mechanism at the early stage of infection. Hoppe et al. had reported lower bacterial burdens in livers than in spleens during intravenous infection and attributed it to the degradation of bacteria within phagolysosomes of hepatocytes (14). The low or absent bacterial burden in C57BL/6 mice is likely due to the inability of bacteria to establish infection in these mice, which suggests the presence of an effective innate immune response. In fact, peritoneal cell cultures from C57BL/6 but not BALB/c mice were shown to control and eliminate bacterial growth in vitro, and the presence of both macrophages and lymphocytes in the peritoneal cell cultures was necessary for this to occur (16, 24). Thus, it is possible that the resistance in C57BL/6 mice is in part due to the efficient early control of bacterial growth by tissue macrophages or other cell types present at the sites of infection.
The innate resistance of C57BL/6 compared to BALB/c mice to several intracellular infections had been attributed to polarized T-helper responses, where C57BL/6 mice developed a T-helper 1 (Th1) response effective against intracellular pathogens while BALB/c mice developed a Th2 response, which is important in the development of humoral immunity. Studies on a broad range of intracellular bacteria, such as Yersinia (4, 6) and mycobacteria (1, 2), have shown that innate host resistance correlated with the production of Th1-type cytokines such as IFN-γ, as well as the proinflammatory cytokines TNF-α, IL-1, and IL-6. However, the nature of the cytokine response induced early in infection not only affects polarization of T cells giving rise to different adaptive immune responses but also affects the activation of the innate immune response. For example, early production of IL-12 by macrophages or dendritic cells could activate natural killer cells to secrete IFN-γ, which could in turn activate macrophages to be microbicidal (5, 22). Proinflammatory cytokines also contribute to early recruitment and activation of phagocytes capable of killing bacteria. One could postulate that the susceptibility of the BALB/c mice could be due to an absent or inadequate inflammatory response to the bacteria. To investigate whether the differential susceptibility of C57BL/6 and BALB/c mice is due to differences in early cytokine expression which could translate into differential activation of the innate immune response, cytokine mRNAs in the lungs, spleens, and livers of the mice were examined. We found that both strains of mice made IFN-γ, TNF-α, IL-1β, and IL-6, confirming the results of Ulett et al. (23), who had found no cytokine polarization in the spleens and livers of C57BL/6 and BALB/c mice. Prominent induction of IFN-γ was observed in all three organs of BALB/c mice, and IL-6 was observed in their spleens and lungs. Previous studies with the murine models had not examined cytokine levels beyond their mRNA induction. As IL-1β mRNA is present constitutively at low levels and some cytokine mRNA may not be translated into proteins, it is necessary to examine the production of these cytokines in the mucosa, such as in the nasal wash, and systemically, such as in the serum, to better understand the roles of these cytokines in the immunopathogenesis of the disease. Both local and systemic production of IFN-γ was higher in BALB/c mice than in C57BL/6 mice. This is further evidence that hyperproduction of IFN-γ is not protective and may lead to septic shock (9, 13). Levels of TNF-α and IL-1β in serum were low and transient, and all three cytokines had decreased to low or undetectable levels by day 4. Local production of IFN-γ, IL-1β, and TNF-α in the nasal wash was higher and more sustained in BALB/c mice. This shows that B. pseudomallei infection in BALB/c mice promoted a strong local inflammatory response which spilled over to the systemic compartment, albeit transiently, and these responses not only did not translate into resistance but likely contributed to pathology through inappropriate tissue destruction. In contrast, C57BL/6 mice responded to the infection with a moderate and transient induction of an inflammatory response. The evidence that C57BL/6 mice did not succumb to disease suggests that these mice could mount an effective innate immune response to control infection. This outcome differs significantly from that of the intratracheal infection of Pseudomonas aeruginosa in mice, where BALB/c mice were more resistant than C57BL/6 mice (18). Thus, although until 1992 the two bacterial species had been considered to be closely related (26), differences in the susceptibilities of mice to the two strains lends support to their classification in distinct RNA homology groups.
In order to examine if C57BL/6 mice could mount a protective memory response to the bacteria, we examined antibody levels in sera and nasal washes and found that serum antibodies could be detected even at day 7 after infection. These levels increased with a second inoculation, and subsequently the mice were protected when reinfected a third time with 10,000 CFU of bacteria, which is a lethal dose for the mice. Protection correlated very well with antibody levels, as heat-killed bacteria were unable to protect mice and could not elicit an antibody response. Both the serum and mucosal antibody responses after infection with 10,000 CFU of bacteria were significantly higher in mice previously infected with the low dose of live bacteria. This finding demonstrated that protection against infection by B. pseudomallei correlated very well with the elicitation of a secondary systemic and mucosal antibody response.
In conclusion, this study is novel and important in defining and establishing a mucosal infection model of B. pseudomallei whose effect is not limited to the mucosa but is also systemic, as seen by the spread of bacteria systemically into other organs and the detection of proinflammatory cytokines in the blood. This could represent a physiologically relevant model of human infection where the route of infection is through inhalation, resulting in pulmonary involvement and even spread to the bloodstream, causing septicemia, if infection cannot be controlled early and effectively at the mucosa. Early host resistance correlates with moderate inflammation, while susceptibility correlates with hyperproduction of IFN-γ. Furthermore, secondary mucosal and serum antibodies against bacteria correlated with protection against a lethal dose of bacteria. Therefore, based on our study, we propose that the development of effective vaccines against B. pseudomallei should incorporate elements to promote moderate inflammation and to elicit appropriate mucosal IgA and systemic IgG antibody responses.
Acknowledgments
We thank Joseph Thong and Soh Chan Lim for technical assistance with this project.
This work was funded by grant R-183-000-030-112 from the Academic Research Fund of the National University of Singapore.
Editor: R. N. Moore
REFERENCES
- 1.Appelberg, R. 1994. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology 191:520–525. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, J. Pedrosa, R. A. Silva, I. M. Orme, and P. Minoprio. 1994. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect. Immun. 62:3962–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashdown, L. R. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293–297. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancroft, G. J., R. D. Schreiber, G. C. Bosma, M. J. Bosma, and E. R. Unanue. 1997. A T cell-independent mechanism of macrophage activation by interferon-γ. J. Immunol. 139:1104–1107. [PubMed] [Google Scholar]
- 6.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bost, K. L., and J. D. Clements. 1995. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect. Immun. 63:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brett, P. J., and D. E. Woods. 2000. Pathogenesis of and immunity to melioidosis. Acta Trop. 74:201–210. [DOI] [PubMed] [Google Scholar]
- 9.Car, B. D., V. M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaowagul, W., N. J. White, D. A. Dance, Y. Wattanagoon, P. Naigowit, T. M. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890–899. [DOI] [PubMed] [Google Scholar]
- 11.Dance, D. A. 1991. Melioidosis: the tip of the iceberg? Clin. Microbiol. Rev. 4:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan, Y. H., Y. Zhang, H. E. Khoo, and K. Esuvaranathan. 1999. Antitumour immunity of Bacillus Calmette-Guerin and interferon alpha in murine bladder cancer. Eur. J. Cancer 35:1123–1129. [DOI] [PubMed] [Google Scholar]
- 13.Heremans, H., J. Van Damme, C. Dillen, R. Dijkmans, and A. Billiau. 1990. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J. Exp. Med. 171:1853–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe, I., B. Brenneke, M. Rohde, A. Kreft, S. Haussler, A. Reganzerowski, and I. Steinmetz. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaphun, P., N. Thirawattanasuk, Y. Supputtamongkol, P. Naigowit, D. A. B. Dance, M. D. Smith, and N. J. White. 1993. Serology and carriage of Pseudomonas pseudomallei, a prospective study in 1000 hospitalized children in Northeast Thailand. J. Infect. Dis. 167:230–233. [DOI] [PubMed] [Google Scholar]
- 16.Leakey, A. K., G. C. Ulett, and R. G. Hirst. 1998. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 24:269–275. [DOI] [PubMed] [Google Scholar]
- 17.Leelarasamee, A., and S. Bovornkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413–425. [DOI] [PubMed] [Google Scholar]
- 18.Morissette, C., E. Skamene, and F. Gervais. 1995. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect. Immun. 63:1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305–312. [DOI] [PubMed] [Google Scholar]
- 20.Piggott, J. A., and L. Hochholzer. 1970. Human melioidosis: a histopathological study of acute and chronic melioidosis. Arch. Pathol. 90:101–111. [PubMed] [Google Scholar]
- 21.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg 27:493–497. [Google Scholar]
- 22.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon γ that drives differentiation of CD4 T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun 68:2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 1998. Macrophage-lymphocyte interactions mediate anti-Burkholderia pseudomallei activity. FEMS Immunol. Med. Microbiol. 21:283–286. [DOI] [PubMed] [Google Scholar]
- 25.Wong, K. T., S. D. Puthucheary, and J. Vadivelu. 1995. The histopathology of human melioidosis. Histopathology 26:51–55. [DOI] [PubMed] [Google Scholar]
- 26.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251–1275. [DOI] [PubMed] [Google Scholar]