Abstract
We previously reported that laminar flow activates peroxisome proliferator-activated receptor γ (PPARγ) in vascular endothelial cells in a ligand-dependent manner that involves phospholipase A2 and cytochrome P450 epoxygenases. In this study, we investigated whether epoxyeicosatrienoic acids (EETs), the catalytic products of cytochrome P450 epoxygenases, are PPARγ ligands. Competition and direct binding assays revealed that EETs bind to the ligand-binding domain of PPARγ with Kd in the μM range. In the presence of adamantyl-ureido-dodecanoic acid (AUDA), a soluble epoxide hydrolase (sEH)-specific inhibitor, EETs increased PPARγ transcription activity in endothelial cells and 3T3-L1 preadipocytes. Inclusion of AUDA in the perfusing media enhanced, but overexpression of sEH reduced, the laminar flow-induced PPARγ activity. Furthermore, laminar flow augmented cellular levels of EETs but decreased sEH at the levels of mRNA, protein, and activity. Blocking PPARγ by GW9662 abolished the EET/AUDA-mediated antiinflammatory effect, which indicates that PPARγ is an effector of EETs.
Keywords: endothelial cells, shear stress
Atherosclerosis preferentially localizes in branches and curved regions of the arterial tree, where the blood flow is disturbed. In contrast, the straight parts of vessels exposed to nondisturbed laminar flow have few lesions (1). The focal distribution of atherosclerotic lesions has been proposed to be related to the proinflammatory effect of disturbed flow imposed on the endothelium vs. the antiinflammatory effect of laminar flow. In vitro studies using flow channels with cultured endothelial cells (ECs) revealed that disturbed flow induces a number of molecules involved in inflammation, including chemoattractants, adhesion molecules, and cytokines (2, 3). However, prolonged exposure to laminar flow suppresses the cytokine-stimulated or oxidized low-density lipoprotein (LDL)-stimulated inflammatory response in ECs (4).
Recent studies showed that the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) is involved in antiinflammatory effects in the artery wall (5, 6). The activation of PPARγ in cultured ECs suppresses the NF-κB-mediated expression of molecules such as vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and endothelin 1 that are involved in the inflammatory response (7, 8). Troglitazone, a synthetic PPARγ ligand, attenuates the formation of lesions in both apolipoprotein E- and low-density lipoprotein receptor-deficient mice (7, 9), due in part to the reduction of monocytes/macrophages homing to the plaques.
We previously demonstrated that laminar flow activates PPARγ in a ligand-dependent manner, which exerts an antiinflammatory effect in ECs. Furthermore, we showed that such induction of PPARγ ligands involves phospholipase A2 and cytochrome P450 epoxygenases (CYPs) (10). Epoxyeicosatrienoic acids (EETs), the main products of arachidonic acid catalyzed by CYPs, have been reported to dilate coronary arteries by hyperpolarizing vascular smooth muscles (11) and to exert antiinflammatory effects in ECs (12). The cellular levels of EETs depend not only on their production by CYPs but also on hydrolysis to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH). Thus, inhibiting the expression of sEH to prevent EET hydrolysis has been used to increase EETs in vivo (13). For example, either ablation of the sEH gene in mice or administration of sEH inhibitors to angiotensin II-infused rats greatly increased the level of EETs and decreased the systolic blood pressure in these animal models (13, 14).
Because of the involvement of CYPs in laminar flow-activated PPARγ, we hypothesized that flow-increased EETs can bind PPARγ, thus providing an antiinflammatory effect for ECs. Results of our flow channel experiments show that laminar flow activates a system of sEH, EETs, and PPARγ that contributes to a major antiinflammatory effect in ECs.
Materials and Methods
Cell Cultures. Bovine aortic ECs (BAECs) were cultured in DMEM supplemented with 10% FBS and 1 mM sodium pyruvate. Human umbilical vein ECs (HUVECs) isolated from umbilical cords were cultured in M199 medium supplemented with 20 mM Hepes (pH 7.4), 5% FBS, 5 ng/ml recombinant human fibroblast growth factor, and 90 μg/ml heparin. HUVEC cultures were within passage 5. 3T3-L1 preadipocytes were cultured in DMEM supplied with 10% FBS. All cultures were supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin. All reagents for cell culture were purchased from GIBCO/BRL except FBS and recombinant human fibroblast growth factor, which were obtained from HyClone and Sigma, respectively.
Plasmids and Transient Transfection. PPAR-responsive element (PPRE)×3-TK-Luc, MH100×4-TK-Luc, and GAL-mPPARγ-LBD (15) were transiently transfected into ECs by the use of Lipofectamine (Invitrogen). CMV-Renilla-Luc was cotransfected as a transfection control. After various treatments, ECs were lysed, and the cell lysates were collected for luciferase activity assays. The results were normalized to those of Renilla luciferase.
Ligand-Binding Assays. For competition assays, [3H]rosiglitazone (American Radiolabeled Chemicals, St. Louis) at 50 Ci/mmol (1 Ci = 37 GBq), together with 5,6-, 8,9-, 11,12-, or 14,15-EETs (Cayman Chemical, Ann Arbor, MI) were incubated with the recombinant protein of the PPARγ-ligand-binding domain (i.e., GST-mPPARγ-LBD) in a buffer containing 10 mM Tris·HCl (pH 8.0), 50 mM KCl, 10 mM DTT, and 200 ng/μl ovalbumin (15). To determine Kd, the various [3H]EETs at 0.94 Ci/mmol (16) were incubated with GST-mPPARγ-LBD in the same buffer without ovalbumin. After being incubated at 25°C for 30 min and chilled on ice for 15 min, the free and bound ligands were separated by Sephadex G-25 (Sigma) columns in a buffer containing 15% glycerol, 25 mM Tris·HCl (pH 7.8), 0.05% Triton X-100, 0.5 mM EDTA, and 75 mM KCl. The bound ligands were then determined by liquid scintillation counting.
Flow Experiments. A parallel plate flow system (17) was used to impose a laminar shear stress of 12 dyne/cm2 (1 dyne = 10 μN) by perfusing the culture media through a confluent monolayer of ECs seeded on glass slides. The flow system was kept at 37°C and ventilated with 95% humidified air with 5% CO2.
Quantitative Real-Time RT-PCR. Total RNA was isolated from cells with TRIzol reagent (Invitrogen). One microgram of the isolated RNA was converted into cDNA by reverse transcriptase (Promega) with oligo(dT) as the primer. The obtained cDNAs were then used as the templates for quantitative RT-PCR with the use of the Brilliant SYBR Green QPCR Master Mix (Stratagene). The nucleotide sequences of the primers were as follows: fatty acid-binding protein 4, 5′-TACTGGGCCAGGAATTTGAC-3′ and 5′-AATGCGAACTTCAGTCCAGG-3′; adipocyte P2, 5′-TGGAAGCTTGTCTCCAGTGA-3′ and 5′-TCGACTTTCCATCCCACT TC-3′; sEH, 5′-GAATGGTCAAACCTGAACCTCA-3′ and 5′-CTCGGGAAATCCATGGCAGA-3′; β-actin, 5′-TGACCGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′.
sEH Activity Assays. The enzyme activity of sEH was assayed as described in ref. 18. Briefly, cells were scraped and homogenized in 0.25 M sucrose, and the cytosolic supernatants were obtained by centrifugation. Fifty micrograms of the samples in 100 μl were then incubated in 0.1 M Na2HPO4 (pH 7.4) at 37°C for 1 min, and trans-[3H]stilbene oxide was added to a final concentration of 50 μM. After incubation at 37°C for another 10 min, the reactions were terminated by extracting the mixtures with 2 vol of n-dodecane. The hydrolyzed product of trans-[3H]stilbene oxide in the aqueous phase was determined by liquid scintillation counting.
Measurement of EETs. The cellular content of EETs was analyzed as described in refs. 19 and 20. Briefly, total lipids were extracted three times with CHCl3/MeOH/H2O (2:1:1 vol/vol) and dried under nitrogen. The extracted lipids were then incubated in 1 M NaOH at 25°C for 2 h, extracted with ethyl acetate, dried under nitrogen, and dissolved in 25 μl of MeOH/water (75:25) containing internal standards. Oxylipids in extracts were separated by reverse-phase HPLC on an XTerra MS C18 column [30 × 2.1 mm i.d., 3.5 μm (Waters, Milford, MA)]. EETs were quantified by using a Quattro Ultima tandem quadrupole mass spectrometer (Micromass, Manchester, U.K.) with negative mode electrospray ionization and multiple reaction monitoring.
Immunoblotting Analysis. To assess the cellular level of sEH and IκBα, cell lysates were resolved by 10% SDS/PAGE and transferred to a nitrocellulose membrane. The sEH and IκBα proteins were detected with the use of rabbit anti-sEH (21) and anti-IκBα polyclonal antibody, respectively (Pharmingen). The bound primary antibody was then recognized by a goat anti-rabbit IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology) and visualized by the ECL detection system (Amersham Pharmacia).
Statistical Analyses. The data were analyzed by two-tailed Student's t test. P < 0.05 was considered statistically significant.
Results
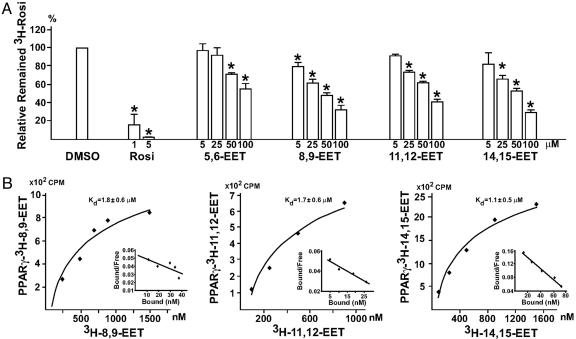
EETs Are PPARγ Ligands. Given that CYPs are involved in the activation of PPARγ by laminar flow (10) and EETs are formed by these CYPs, we first investigated whether any of the four EET isoforms (i.e., 5,6-, 8,9-, 11,12-, and 14,15-EET) could bind to the ligand binding domain of PPARγ. A competition binding assay was performed to assess the replacement of mPPARγ-LBD-bound [3H]rosiglitazone with various EETs. As shown in Fig. 1A, nonlabeled rosiglitazone, serving as a control, at 1 and 5 μM greatly reduced the binding of [3H]rosiglitazone to mPPARγ-LBD. At higher concentrations, EETs could replace the binding of [3H]rosiglitazone to mPPARγ-LBD. To determine the Kd of various EETs, we used their 3H-labeled counterparts for Scatchard analysis. Fig. 1B shows the Kd for 8,9-, 11,12- and 14,15-EET as 1.8 ± 0.6, 1.7 ± 0.6, and 1.1 ± 0.5 μM, respectively. The Kd of 5,6-EET was not determined because of the lack of labeled substrate.
Fig. 1.
EETs are PPARγ ligands. (A) One microgram of GST-PPARγ-LBD fusion protein was incubated with 100 nM [3H]rosiglitazone (Rosi) in a total volume of 50 μl in the presence of vehicle (DMSO), unlabeled rosiglitazone, or various EETs at the indicated concentrations. (B) For each data point, 1 μg of GST-PPARγ-LBD was incubated with various amounts of [3H]EETs in a total volume of 50 μl. The free and bound ligands in A and B were separated on a Sephadex G-25 column, and the amount of bound [3H]rosiglitazone or [3H]EETs was then determined by liquid scintillation counting. The results in A are presented as mean ± SD from three sets of experiments. *, P < 0.05 compared with DMSO controls. The plot of the binding curve in B represents three independent experiments, and Scatchard analysis was performed by replotting the data shown in Insets. Kd was presented as mean ± SD of Scatchard analysis results from three independent experiments.
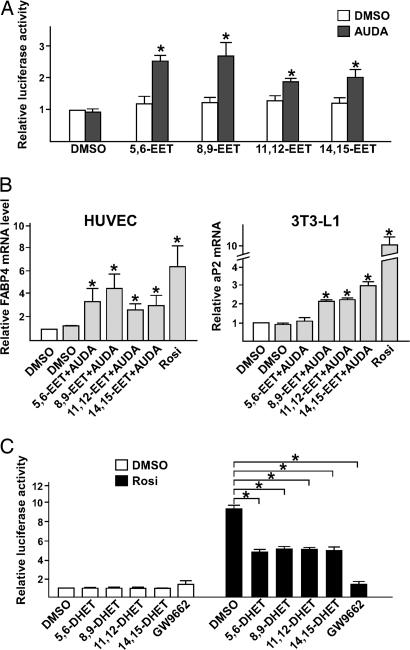
To determine whether the binding of EETs to PPARγ is associated with its activation, we transiently transfected BAECs with a mammalian two-hybrid system that includes the GAL4 reporter construct (i.e., MH100×4-TK-Luc) and an expression plasmid encoding GAL-mPPARγ-LBD, a fusion protein in which the DNA binding domain of GAL4 is fused to mPPARγ-LBD (15). Compared with DMSO controls, cells incubated with the four EET isoforms showed only marginally increased luciferase activity (Fig. 2A). However, if the transfected cells were incubated with adamantyl-ureido-dodecanoic acid (AUDA), an sEH-specific inhibitor (19), luciferase activity was increased by 2.5 ± 0.2, 2.7 ± 0.4, 1.9 ± 0.1, and 2.0 ± 0.3 times for 5,6-, 8,9-, 11,12-, and 14,15-EET, respectively. As shown in Fig. 2B, treating HUVECs with EETs and AUDA also increased the mRNA level of fatty acid-binding protein 4, a PPARγ-targeted gene (22). A similar induction of adipocyte P2, the mouse homologue of fatty acid-binding protein 4, was found in 3T3-L1 preadipocytes treated with EETs and AUDA. These results suggest that exogenous EET activates PPARγ, with the ensuing activation of the PPARγ-targeted genes in ECs if the expression of endogenous sEH is inhibited. DHETs, the hydrolyzed products of EETs, attenuated ≈50% induction of GAL-mPPARγ-LBD by rosiglitazone, whereas GW9662, a synthetic antagonist of PPARγ, abolished the induction by rosiglitazone (Fig. 2C). This result suggests that DHETs may act as the natural antagonists on PPARγ.
Fig. 2.
EETs, together with AUDA, activate the PPARγ-regulated transcription. (A) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc (0.25 μg), GAL-mPPARγ-LBD (0.25 μg), and CMV-Renilla-Luc (0.05 μg). The transfected cells were then incubated with EETs (1 μM) in the presence or absence of AUDA (1 μM), and the media were replaced every 2 h. After 8 h, cells were lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO controls. (B) Total RNAs were isolated from HUVECs or 3T3-L1 cells incubated with EETs (1 μM) in the presence of AUDA (1 μM) for 12 h. The levels of fatty acid-binding protein 4 mRNA in HUVECs or adipocyte P2 mRNA in 3T3-L1 cells were determined by quantitative RT-PCR in which β-actin was an internal control. The relative mRNA level is defined as the levels in cells treated with various EETs in reference to that of DMSO set as 1. (C) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc, GAL-mPPARγ-LBD, and CMV-Renilla-Luc. The transfected cells were then incubated with DHETs or GW9662 (5 μM) in the presence or absence of rosiglitazone (1 μM) for 8 h and lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO controls as 1. *, P < 0.05.
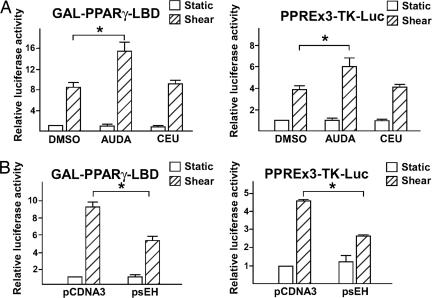
sEH Is Involved in the Activation of PPARγ by Laminar Flow. The inhibition of sEH expression has been reported to increase EET levels in hypertensive rat models (13). We applied laminar flow to ECs in the presence of AUDA to investigate whether suppressing sEH expression could further activate PPARγ. As shown in Fig. 3A, the inclusion of AUDA in the perfusing media doubled the laminar flow-induced GAL-mPPARγ-LBD activity. Similarly, AUDA also augmented the flow-induced luciferase reporter activity driven by three copies of PPAR-responsive element (PPRE; i.e., PPRE×3-TK-Luc in Fig. 3A). However, 1-cyclohexyl-3-ethyl urea, an AUDA analogue that is not an sEH inhibitor, had no effect on flow-induced GAL-mPPARγ-LBD and PPRE×3-TK-Luc activity. Given the positive effect of sEH inhibition on the induction of PPARγ-LBD and PPRE, we examined the consequences of elevated sEH expression in ECs exposed to flow. As shown in Fig. 3B, the overexpression of sEH attenuated the induction of GAL-mPPARγ-LBD and PPRE×3-TK-Luc by laminar flow.
Fig. 3.
Inhibition of sEH in ECs enhances the response of PPARγ to laminar flow. (A) BAECs transfected with GAL-mPPARγ-LBD and MH100×4-Luc or PPRE×3-TK-Luc were kept as static controls or subjected to laminar flow for 8 h in the presence or absence of AUDA (1 μM), an sEH-specific inhibitor, or 1-cyclohexyl-3-ethyl urea (CEU, 1 μM), an AUDA analogue that does not inhibit sEH. (B) BAECs on glass slides were cotransfected with pCDNA3 (0.3 μg) or an expression plasmid encoding sEH (psEH, 0.3 μg) with GAL-mPPARγ-LBD (0.3 μg) and MH100×4-Luc (0.3 μg) or PPRE×3-TK-Luc (0.6 μg). The transfected cells were then kept as static controls or subjected to laminar flow for 8 h. Cells in both A and B were then lysed for luciferase activity assays. The bars represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO-treated static cells in A or pCDNA3-transfected cells under static conditions in B. *, P < 0.05.
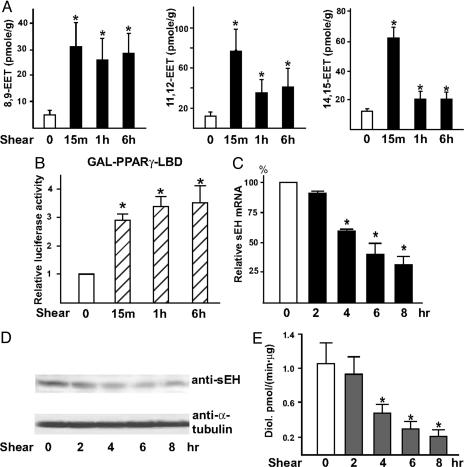
Laminar Flow Augments the Levels of EETs but Down-Regulates sEH in ECs. The cellular levels of EETs are determined by the balance between production and degradation, which are catalyzed by CYPs and sEH, respectively. Because phospholipase A2 is activated by flow (23) and CYPs are involved in the flow activation of PPARγ (10), we investigated whether laminar flow increases the level of EETs in ECs. As shown in Fig. 4A, the cellular levels of 8,9-, 11,12-, and 14,15-EET were increased in BAECs exposed to laminar flow for 15 min or 1 or 6 h compared with static controls (31 ± 9, 26 ± 8, and 28 ± 8 vs. 5 ± 2; 77 ± 26, 36 ± 13, and 41 ± 19 vs. 12 ± 4; 63 ± 7, 20 ± 6, and 20 ± 5 vs. 13 ± 1 pmol/g, respectively). We were unable to determine the level of 5,6-EET because of the lack of valid methods. The condition media collected from each time point in the flow experiments (15 min, 1 h, and 6 h) induced GAL-mPPARγ-LBD 2.9 ± 0.2-, 3.4 ± 0.3-, and 3.5 ± 0.6-fold, respectively, compared with the static media (Fig. 4B).
Fig. 4.
Laminar flow increases EETs and down-regulates sEH in ECs. BAECs in A, D, and E and HUVECs in C were kept as static controls or subjected to a laminar flow at 12 dyne/cm2 for the indicated times. (A) Total lipids were extracted from static BAECs or those exposed to laminar flow in the absence of FBS. EETs were quantified by LC/MS/MS. The amounts of EETs were normalized to the mass of the cell pellets (in grams). (B) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc, GAL-mPPARγ-LBD, and CMV-Renilla-Luc. The transfected cells were then incubated with the condition media collected from the flow experiments in A for 8 h and lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of static medium controls as 1. (C) Total RNA was isolated from HUVECs, and the level of sEH mRNA was determined by quantitative RT-PCR with β-actin used as an internal control. (D) BAECs were lysed for immunoblotting with the use of polyclonal anti-sEH Ab. (E) Cytosolic supernatants obtained from BAECs were incubated with trans-[3H]stilbene oxide for sEH activity assays. *, P < 0.05, compared with static controls.
Because laminar flow increased the level of EETs in ECs, we examined whether sEH could be down-regulated or deactivated as well. As revealed by quantitative RT-PCR and immunoblotting, the mRNA and protein levels of sEH were decreased in ECs by laminar flow in a time-dependent manner (Fig. 4 C and D). Consistent with the decreased transcription and translation of sEH, the enzyme activity of sEH in ECs decreased after exposure to laminar flow (Fig. 4E). Taken together, these results show that laminar flow increases the level of cellular EETs not only by enhanced generation but also by attenuated degradation.
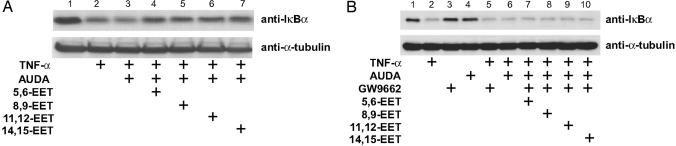
PPARγ Is Involved in the Antiinflammatory Effect of EETs. Both PPARγ and EETs have been reported to exert their antiinflammatory effect by inhibiting NF-κB-mediated transcription (8, 12). Thus, we used TNF-α-induced IκBα degradation (lane 2 in Fig. 5A) as an inflammatory response to investigate whether PPARγ mediates the antiinflammatory effect of EETs. The inclusion of 5,6-, 8,9-, 11,12-, or 14,15-EET, together with AUDA, in culture media prevented the TNF-α-induced IκBα degradation (Fig. 5A, lanes 4-7). However, treating cells with various EETs in the absence of AUDA for 8 h had little effect (data not shown), which indicates the rapid degradation of EETs by sEH in ECs. GW9662, an antagonist of PPARγ, attenuated the antiinflammatory effect of EETs by preventing IκBα degradation (Fig 5B, lane 2 vs. lanes 7-10). These results suggest that the antiinflammatory effect of EETs is mediated, at least in part, by PPARγ.
Fig. 5.
PPARγ mediates the antiinflammatory effect of EETs. BAECs were pretreated with AUDA (1 μM) for 2 h and then incubated with various EETs (1 μM) for 8 h. (B) Cells were cultured as in A, except that GW9662 (1 μM) was included in the indicated experiments during the 8-h incubation. All cells were then stimulated with TNF-α (10 ng/ml) for 30 min. The collected cell lysates were immunoblotted with anti-IκBα antibody. α-Tubulin was used as a loading control.
Discussion
Laminar flow is atheroprotective, in part because of its antiinflammatory effect. Flow-channel experiments showed that laminar flow inhibits the NF-κB-mediated expression of vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 in ECs (4). In vivo, NF-κB activation and vascular cell adhesion molecule 1 expression are enhanced in the endothelium in regions prone to atherosclerosis in low-density lipoprotein receptor-null mice fed an atherogenic diet (24, 25). We previously demonstrated that laminar flow activates PPARγ in ECs in a ligand-dependent manner and exhibits an antiinflammatory effect (10). In the current study, aimed at elucidating the molecular mechanisms underlying such activation, we found that EETs are PPARγ ligands, which contributes to the antiinflammatory effect of EETs; that laminar flow down-regulates sEH; and that increased levels of EETs lead to an antiinflammatory effect.
A variety of eicosanoids derived from arachidonic acid have been found to be ligands of PPARγ. These compounds include the products generated by cyclooxygenases [i.e., 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2)] and lipoxygenases [i.e., 9- and 13-hydroxy-(S)-10,12-octadecadienoic acid]. Laminar flow has been shown to induce the expression of cyclooxygenases (COX) 2 and lipocalin-type prostaglandin D2 synthase in ECs, resulting in increased 15d-PGJ2 production (26). Such induction of 15d-PGJ2 may contribute to the activation of PPARγ by flow. However, our previous study demonstrated that inhibition of CYP450 but not COXs or lipoxygenases attenuates the flow activation of PPARγ (10). These results suggest that metabolic products of CYP450 rather than those of COXs are likely physiological ligands for PPARγ in response to laminar flow. Data in Figs. 1 and 2 reveal that EETs generated by CYPs, another major pathway involved in arachidonic acid metabolism, can also bind and activate PPARγ. The binding of EETs to PPARγ with Kd in the low micromolar range (Fig. 1B) is comparable with that of other PPAR ligands. For example, 15d-PGJ2 and 8(S)-hydroxyeicosatetraenoic acid bind to PPARγ and PPARα with a Kd of 2.5 and 1 μM, respectively (15, 27). With Kd in the micromolar range, EETs may not be potent PPARγ ligands. Such a weak binding raises the question of whether laminar flow would generate a sufficient amount of EETs to activate PPARγ. The concentrations of EETs in ECs are ≈10-6 to 10-7 M. This estimation is based on ECs' having a volume of 4 × 103 μm3 (C.-C. Wu and S. Chien, personal communication), and 5 × 107 cells result in1gof cell pellets. Although the plasma level of EETs is ≈30 nM (28), our estimation of cellular concentrations of EETs is supported by results of a previous study showing that ECs can uptake 14,15-EET up to 5 μM (29). Results from competition assays indicate that EETs can replace 50% of the bound rosiglitazone at concentrations between 50 and 100 μM. However, 15d-PGJ2 at a similar concentration can displace 90% of the PPARγ-bound rosiglitazone (15). Such a discrepancy suggests that the binding site(s) may vary among EETs, rosiglitazone, and 15d-PGJ2. Thus, EETs may cause PPARγ conformational changes different from those of other ligands, signifying a distinct biological consequence.
In addition to their role in vessel dilation, EETs have been shown to exert antiinflammatory effects in ECs. Incubating ECs with 11,12-EET at nanomolecular concentrations or overexpression of CYP2J2, an endothelial-abundant CYP family member, prevented the cytokine- or LPS-induced vascular cell adhesion molecule 1 and E-selectin expression through NF-κB inhibition (12). The inhibition of PPARγ by GW9662 abolished the EET-mediated antiinflammatory effect, namely, by preventing IκB degradation (Fig. 5). These results indicate that the antiinflammatory effect of EETs is, at least in part, through PPARγ. In contrast to the finding that 11,12-EET has the best antiinflammatory capability (12), our data do not show regioisomeric specificity among EETs (Fig. 5). This discrepancy may be due to the longer time courses between the studies and the inclusion of AUDA in our experiments.
The temporal changes of cellular contents of EETs (Fig. 4A) are possibly regulated by different mechanisms. Being a key enzyme to hydrolyze membrane phospholipids, phospholipase A2 in ECs has been shown to be activated by flow to increase the cellular level of arachidonic acid (23). Human ECs express CYP family members 2C and 2J, which are epoxygenases producing EETs from arachidonic acid. The rapid production of EETs by laminar flow (0-15 min in Fig. 4A) suggests that the flow-generated increase in EETs mainly results from the increase of arachidonic acid as the available substrate for CYP450s but not the change of CYP450 enzyme levels. We confirmed this assumption by use of regular RT-PCR to show that laminar flow does not change the mRNA encoding 2C8, 2C9, or 2J2 in ECs subjected to flow for 4 h (data not shown). Compared with that at 15 min, the flow-produced EETs are reduced greatly at 1 h. Such a reduction is postulated to be mediated by the activity of sEH, the primary enzyme for the conversion of EETs to DHETs. At 6 h, the levels of EETs were similar to those at 1 h. The involved mechanism was probably the inhibition of sEH expression at both mRNA and protein levels, as suggested in Fig. 4 C-E. The reduced sEH expression by flow thereby maintained the EET level in ECs. Thus, the increased activity of EETs likely results from the flow-activated phospholipase A2, synergistic with down-regulated sEH, pertaining to a basal level of CYPs.
Even in the presence of AUDA, EET did not induce GAL-mPPARγ-LBD to the same level as that by laminar flow (Fig. 2 A vs. Fig. 3). Several possibilities may account for this scenario. First, laminar flow exerted a greater inhibition of sEH than AUDA did (data not shown), which suggests that laminar flow may maintain a higher level of EETs in ECs. Moreover, laminar flow may increase the level of EETs in ECs in a sustained manner, whereas EETs added to culture media may be degraded by remaining sEH that was not inhibited by AUDA. Second, we found that DHETs could inhibit rosiglitazone-induced GAL-mPPARγ-LBD (Fig. 2C), which indicates that DHETs may act as PPARγ antagonists. Thus, the activation of PPARγ by laminar flow is possibly enhanced by the up-regulated agonists (i.e., EETs) in conjunction with the down-regulated antagonists (i.e., DHETs). Third, laminar flow may generate other PPARγ ligands that contribute to the higher induction in cells under flow.
In summary, the current study shows not only the putative effector (i.e., PPARγ) of EETs in their antiinflammatory effect but also that suppressing sEH expression is crucial for the EET-mediated antiinflammatory effect. Considering the role of EETs in vessel dilation (27) and antiinflammation (12), the suppressed expression of sEH may explain, in part, the beneficial effect of shear stress on vascular beds.
Acknowledgments
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL77448 (to J.Y.-J.S.) and HL72845 (to A.A.S.); Department of Defense/Small Business Innovation Research Grant 8045-020-0042 and National Institute of Environmental Health Sciences Grants ES02710, ES04699, and ES05707 (to B.D.H.); and American Heart Association Scientist Development Grant 0230096N (to X.F.).
Author contributions: Y.L., Y. Zhang, Y. Zhu, B.D.H., and J.Y.-J.S. designed research; Y.L., Y. Zhang, K.R.S., and T.-S.L. performed research; Y.L., Y. Zhang, K.R.S., X.F., A.A.S., S.G., C.M., and B.D.H. contributed new reagents/analytic tools; Y. Zhu, A.A.S., S.G., B.D.H., and J.Y.-J.S. analyzed data; and Y.L., Y. Zhang, and J.Y.-J.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AUDA, adamantyl-ureido-dodecanoic acid; EC, endothelial cell; BAEC, bovine aortic EC; CYP, cytochrome P450 epoxygenase; DHET, dihydroxyeicosatrienoic acid; EET, epoxyeicosatrienoic acid; HUVEC, human umbilical vein EC; LBD, ligand-binding domain; PPARγ, peroxisome proliferator-activated receptor γ; sEH, soluble epoxide hydrolase.
References
- 1.Wissler, R. W. (1995) Am. J. Med. Sci. 310, Suppl. 1, 29-36. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone, M. A., Jr., Nagel, T. & Topper, J. N. (1997) J. Clin. Invest. 99, 1809-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies, P. F. (1995) Physiol. Rev. 75, 519-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao, P. S., Buitrago, R., Chan, J. R. & Cooke, J. P. (1996) Circulation 94, 1682-1689. [DOI] [PubMed] [Google Scholar]
- 5.Plutzky, J. (2001) Curr. Opin. Lipidol. 12, 511-518. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh, W. A. & Law, R. E. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1891-1895. [DOI] [PubMed] [Google Scholar]
- 7.Pasceri, V., Wu, H. D., Willerson, J. T. & Yeh, E. T. (2000) Circulation 101, 235-238. [DOI] [PubMed] [Google Scholar]
- 8.Wang, N., Verna, L., Chen, N. G., Chen, J., Li, H., Forman, B. M. & Stemerman, M. B. (2002) J. Biol. Chem. 277, 34176-34181. [DOI] [PubMed] [Google Scholar]
- 9.Collins, A. R., Meehan, W. P., Kintscher, U., Jackson, S., Wakino, S., Noh, G., Palinski, W., Hsueh, W. A. & Law, R. E. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 365-371. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Y., Zhu, Y., Rannou, F., Lee, T. S., Formentin, K., Zeng, L., Yuan, X., Wang, N., Chien, S., Forman, B. M., et al. (2004) Circulation 110, 1128-1133. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, W. B., Gebremedhin, D., Pratt, P. F. & Harder, D. R. (1996) Circ. Res. 78, 415-423. [DOI] [PubMed] [Google Scholar]
- 12.Node, K., Huo, Y., Ruan, X., Yang, B., Spiecker, M., Ley, K., Zeldin, D. C. & Liao, J. K. (1999) Science 285, 1276-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imig, J. D., Zhao, X., Capdevila, J. H., Morisseau, C. & Hammock, B. D. (2002) Hypertension 39, 690-694. [DOI] [PubMed] [Google Scholar]
- 14.Sinal, C. J., Miyata, M., Tohkin, M., Nagata, K., Bend, J. R. & Gonzalez, F. J. (2000) J. Biol. Chem. 275, 40504-40510. [DOI] [PubMed] [Google Scholar]
- 15.Forman, B. M., Tontonoz, P., Chen, J., Brun, R. P., Spiegelman, B. M. & Evans, R. M. (1995) Cell 83, 803-812. [DOI] [PubMed] [Google Scholar]
- 16.Fang, X., Kaduce, T. L., Weintraub, N. L., Harmon, S., Teesch, L. M., Morisseau, C., Thompson, D. A., Hammock, B. D. & Spector, A. A. (2001) J. Biol. Chem. 276, 14867-14874. [DOI] [PubMed] [Google Scholar]
- 17.Frangos, J. A., Eskin, S. G., McIntire, L. V. & Ives, C. L. (1985) Science 227, 1477-1479. [DOI] [PubMed] [Google Scholar]
- 18.Gill, S. S., Ota, K. & Hammock, B. D. (1983) Anal. Biochem. 131, 273-282. [DOI] [PubMed] [Google Scholar]
- 19.Morisseau, C., Goodrow, M. H., Newman, J. W., Wheelock, C. E., Dowdy, D. L. & Hammock, B. D. (2002) Biochem. Pharmacol. 63, 1599-1608. [DOI] [PubMed] [Google Scholar]
- 20.Newman, J. W., Watanabe, T. & Hammock, B. D. (2002) J. Lipid Res. 43, 1563-1578. [DOI] [PubMed] [Google Scholar]
- 21.Yamada, T., Morisseau, C., Maxwell, J. E., Argiriadi, M. A., Christianson, D. W. & Hammock, B. D. (2000) J. Biol. Chem. 275, 23082-23088. [DOI] [PubMed] [Google Scholar]
- 22.Bernlohr, D. A., Simpson, M. A., Hertzel, A. V. & Banaszak, L. J. (1997) Annu. Rev. Nutr. 17, 277-303. [DOI] [PubMed] [Google Scholar]
- 23.Pearce, M. J., McIntyre, T. M., Prescott, S. M., Zimmerman, G. A. & Whatley, R. E. (1996) Biochem. Biophys. Res. Commun. 218, 500-504. [DOI] [PubMed] [Google Scholar]
- 24.Iiyama, K., Hajra, L., Iiyama, M., Li, H., DiChiara, M., Medoff, B. D. & Cybulsky, M. I. (1999) Circ. Res. 85, 199-207. [DOI] [PubMed] [Google Scholar]
- 25.Hajra, L., Evans, A. I., Chen, M., Hyduk, S. J., Collins, T. & Cybulsky, M. I. (2000) Proc. Natl. Acad. Sci. USA 97, 9052-9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taba, Y., Sasaguri, T., Miyagi, M., Abumiya, T., Miwa, Y., Ikeda, T. & Mitsumata, M. (2000) Circ. Res. 86, 967-973. [DOI] [PubMed] [Google Scholar]
- 27.Krey, G., Braissant, O., L'Horset, F., Kalkhoven, E., Perroud, M., Parker, M. G. & Wahli, W. (1997) Mol. Endocrinol. 11, 779-791. [DOI] [PubMed] [Google Scholar]
- 28.Karara, A., Wei, S., Spady, D., Swift, L., Capdevila, J. H. & Falck, J. R. (1992) Biochem. Biophys. Res. Commun. 182, 1320-1325. [DOI] [PubMed] [Google Scholar]
- 29.VanRollins, M., Kaduce, T. L., Knapp, H. R. & Spector, A. A. (1993) J. Lipid Res. 34, 1931-1942. [PubMed] [Google Scholar]