Abstract
This study was designed to investigate the molecular mechanisms of Staphylococcus aureus adherence to human airway epithelium. Using a humanized bronchial xenograft model in the nude mouse and primary cultures of human airway epithelial cells (HAEC), we showed that S. aureus adhered mainly to undifferentiated HAEC whereas weak adherence (11- to 20-fold lower) to differentiated HAEC was observed (P < 0.01). A fibronectin (FN)-binding protein (FnBP)-deficient strain of S. aureus had a fivefold-lower adherence level to undifferentiated HAEC than did the parental strain (P < 0.005), suggesting that S. aureus FN-binding capacity is involved in the adherence to HAEC. We also showed that 97% of 32 S. aureus clinical strains, isolated from the airway secretions of cystic fibrosis patients (n = 18) and patients with nosocomial pneumonia (n = 14), possessed the two fnb genes. The strains from pneumonia patients had a significantly (P < 0.05) higher FN-binding capacity than did the strains from CF patients. This result was confirmed by the expression of FnBPs, investigated by Western ligand affinity blotting. Our results suggest a major role of FnBPs in the colonization of the airways by S. aureus and point to the importance of the adhesin regulatory pathways in the staphylococcal infectious process.
Staphylococcus aureus is a major human pathogen involved in nosocomial respiratory tract infections and is one of the first pathogens to colonize the respiratory tract of cystic fibrosis (CF) patients. Due to an increasing number of infections caused by methicillin-resistant S. aureus (MRSA) strains, therapy has become problematic (43). Moreover, the recent emergence of MRSA strains with intermediate sensitivity to vancomycin (4, 42) has increased the need for a better understanding of the staphylococcal pathogenesis. Microbial adherence to cells and matrix components is considered to promote colonization and infection (2) and, as such, could represent an attractive target for the development of new antimicrobial therapeutic and preventive approaches.
A well-characterized family of staphylococcal surface adhesins, called MSCRAMMs (for “microbial surface components recognizing adhesive matrix molecules”) are known to mediate adherence of the bacteria to host extracellular matrix components, such as collagen, fibrinogen, and fibronectin (FN). S. aureus FN-binding activity is mediated by two closely related FN-binding proteins (FnBPs) (9, 10) encoded by two adjacent genes, fnbA and fnbB (16). The structural genes coding for FnBPs are partially redundant, since both of them need to be inactivated to suppress bacterial interaction with FN (13). Numerous in vitro and in vivo studies have underlined the role of FN and FnBPs in the staphylococcal infectious process. In a rat model of endocarditis, Kuypers and Proctor (19) showed that a low-FN-binding mutant of S. aureus had a markedly reduced adherence to traumatized heart valves. Schennings et al. (35) induced protection against S. aureus-mediated endocarditis by immunizing animals against the FN-binding domains of FnBPs. Rozalska and Wadström (32) showed that bacteria are more rapidly eliminated by S. aureus-infected mice when they are opsonized with antibodies directed against FnBPA. FnBPs also mediate adherence of S. aureus to endothelial cells, epithelial cells, and fibroblasts and the subsequent internalization of the bacteria (28, 38), thus contributing to the invasion of deeper tissues. These studies point to the importance of bacterial adherence to cells and extracellular matrix molecules as an essential first step in the pathogenesis of infectious diseases and suggest that FnBPs could represent potential targets for the development of vaccines against S. aureus.
In contrast, S. aureus adherence to airway epithelium has been poorly documented. Studies of chronically infected lung tissues from CF patients have shown that S. aureus does not adhere to pseudostratified CF airway epithelium (24, 41). We have previously reported that S. aureus is able to adhere to CF airway epithelium in remodeled or injured areas, at lobar as well as at segmental levels (24). Moreover, the adherence of CF isolates to bronchial epithelial cell lines has been described to be significantly higher than that of non-CF isolates, and CF and non-CF S. aureus strains bound equally to CF and non-CF cells (36). S. aureus interacts with the carbohydrate sequence GalNAcβ1-4Gal (18), found in the asialylated ganglioside asialo-GM1, one of the transmembrane glycolipid receptor present and overexpressed at the surface of CF cells (33) and poorly differentiated respiratory epithelial cells (6, 7). However, S. aureus surface proteins that promote adherence to remodeled human airway epithelium, as well as the corresponding cellular receptors, have yet to be identified.
The present study was designed to investigate the role of several S. aureus surface proteins in the adherence to human airway epithelium. For that purpose, we used reference strains as well as protein A (SpA)-, FnBPs-, and clumping factor (ClfA)-deficient strains, and 18 CF clinical strains and 14 non-CF strains obtained from hospitalized patients suffering from nosocomial pneumonia. Adherence of these strains was investigated by using FN-coated wells, in vitro in contact with airway epithelial cells and in vivo in a humanized bronchial model of S. aureus infection, and was correlated with the presence or absence of S. aureus fnb genes and the expression of FnBPs, as determined by PCR and Western ligand affinity blotting, respectively.
MATERIALS AND METHODS
Bacterial strains.
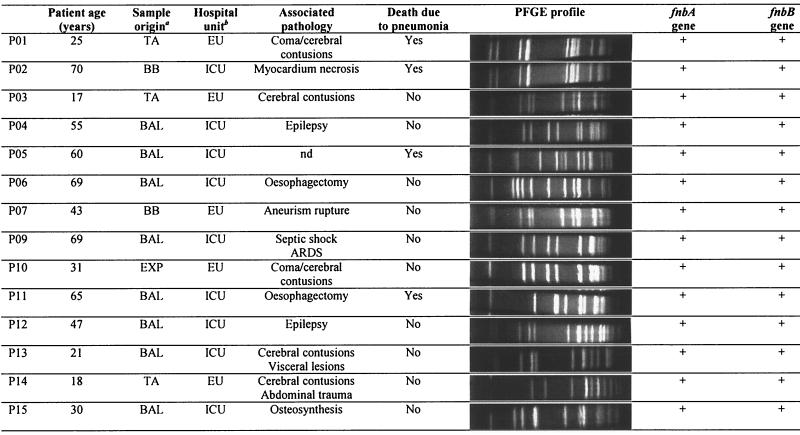
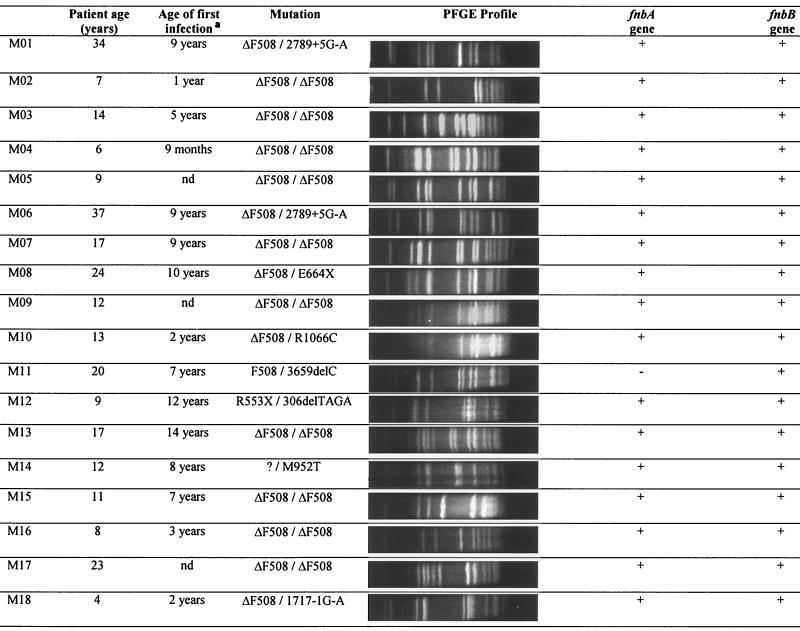
The S. aureus strains and their sources are listed in Table 1. 8325-4, DU5875 (SpA−), DU5883 (FnBP−), DU5883(pFNBA4) (FnBPA+), and DU5880 (ClfA−) strains were generous gifts from T. J. Foster, Department of Microbiology, Trinity College, Dublin, Ireland. Clinical strains isolated from the airways of intensive-care unit (ICU) patients with nosocomial pneumonia (P strains) and CF patients (M strains) are also listed (see Tables 2 and 3). P strains (n = 14) were isolated from the respiratory tracts of 14 ICU patients (mean age, 44.2 ± 20.4 years; range, 17 to 70 years) with nosocomial pneumonia. The pneumonia, following cerebral contusions, myocardial necrosis, or esophagectomy, was the cause of death for four patients. For six patients, S. aureus was the only pathogen isolated (strains P01, P02, P03, P07, P10, and P12). All the P strains were susceptible to methicillin. M strains (n = 18) of S. aureus were isolated from the airway secretions of 16 CF patients (mean age, 15.4 ± 9.2 years; range, 4 to 37 years) during acute infectious episodes. The M01 and M06 strains and the M05 and M09 strains were isolated from the airways of the same CF patients in different acute infectious episodes. One strain, M15, was methicillin resistant. Ten CF patients were homozygous for the ΔF508 mutation. Six CF patients were coinfected with S. aureus (strains M01/M06, M02, M07, M10, M11, and M13) and Pseudomonas aeruginosa. In one patient, S. aureus (strain M05) was isolated as a unique pathogen.
TABLE 1.
Reference and isogenic mutant strains of S. aureus used in this study
| Strain | Relevant genotype | Relevant phenotypes or properties | Source or reference |
|---|---|---|---|
| Cowan III | Human pleural liquid isolate | ATCC 12600 | |
| 8325-4 | fnbA+fnbB+ | FnBPA+ FnBPB+, NCTC 8325 cured of prophages, plasmid free | 26 |
| DU5875 | spa::Tcr | Spa−, isogenic mutant of 8325-4 | 27 |
| DU5883 | fnbA::TcrfnbB::Emr | FnBPA− FnBPB−, isogenic mutant of 8325-4 | 13 |
| DU5883(pFNBA4) | fnbA::TcrfnbB::Emr (pFNBA4::fnbA+ Cmr) | Mutant strain of 8325-4 defective in FnBPs, complemented with multicopy plasmid expressing fnbA+ | 13 |
| DU5880 | clfA1::Tn 917 (Emr) | Mutant strain of 8325-4 defective in fibrinogen-binding protein ClfA | 21 |
TABLE 2.
Characteristics of S. aureus clinical strains isolated from the airways of patients with nosocomial pneumonia
TA, tracheal aspiration; BB, bronchial brush; BAL, broncholalveolar lavage; EXP, expectoration. ICU, intensive-care unit; EU, emergency unit; ARDS, acute respiratory distress syndrome; nd, not determined.
TABLE 3.
Characteristics of S. aureus clinical strains isolated from the expectorations of CF patients
nd, not determined.
Bacterial isolates were stored at −20°C in Trypticase soy broth (Institut Pasteur) containing 20% (vol/vol) glycerol. Bacteria were cultured overnight in Trypticase soy broth at 37°C to obtain stationary-phase bacteria or were 50-fold diluted and grown for a further 3 h at 37°C for logarithmic-phase bacteria. They were then harvested by centrifugation and resuspended in RPMI 1640 culture medium (Seromed; Biochrom KG, Berlin, Germany) containing 20 mM HEPES; Sigma Chemical Co., St Louis, Mo. The bacterial suspension was adjusted to a final concentration of 108 CFU/ml. Bacterial self-aggregation was limited by extruding ex tempo the bacterial suspension through a fine needle.
Human airway tissues.
Fresh human airway tissues, collected from non-CF nasal polyps or nasal mucosa (turbinates), were immediately transferred to the laboratory in RPMI 1640 medium culture supplemented with 20 mM HEPES and antibiotics (200 U of penicillin per ml and 200 μg of streptomycin per ml [Sigma]).
In vitro S.aureus interaction with HAEC primary cultures.
Fresh airway tissues were cut into small explants (2 mm2), seeded onto a type I collagen matrix in 24-well plates in a defined culture medium, i.e., RPMI 1640 supplemented with insulin (1 μg/ml; Sigma Chemical Co.), apo-transferrin (1 μg/ml; Serva, Heidelberg, Germany), epidermal growth factor (10 ng/ml; Serva), hydrocortisone (0.5 μg/ml; Sigma), retinoic acid (10 ng/ml; Sigma), penicillin (100 U/ml), and streptomycin (100 μg/ml), and grown at 37°C under 5% CO2 (5). After 3 to 4 days of culture, explants were surrounded by an outgrowth resulting from both cell migration and proliferation. Well-differentiated (ciliated) as well as nondifferentiated (flattened) human airway epithelial cells (HAEC) could be identified in the center and at the periphery respectively, of the explant-outgrowth cell culture.
On day 3 or 4, antibiotics were removed from primary HAEC cultures by repetitive rinsing with RPMI 1640 culture medium. A 200-μl aliquot of bacterial suspension was added to each HAEC culture and incubated for 1 h at 37°C. To remove nonadherent bacteria, cultures were rinsed three times with 0.5 ml of 0.1 M phosphate-buffered saline (PBS) (pH 7.2) and prepared for scanning electron microscopy.
In vivo S.aureus interaction with HAEC in the humanized tracheal xenograft model.
Humanized tracheal xenografts, two per mouse, were prepared as previously described (8). Briefly, tracheas of male Wistar rats (obtained from Charles River France, Saint-Aubin-lès-Elbeuf and weighing 220 to 250 g) were frozen at −80°C and thawed three times to remove the rat surface epithelium. Rat tracheas were aseptically tied at their distal end to sterile polyethylene tubing. The tracheas were then stored at −80°C until used for inoculation with dissociated HAEC.
Dissociated HAEC from nasal polyps were obtained by enzymatic dissociation (5). The cell viability, as assessed using the trypan blue exclusion procedure, was higher than 90%. Cells were resuspended in the defined culture medium at a density of 107 cells/ml, and 0.75 × 106 cells were inoculated into each rat trachea. Tracheas were then subcutaneously implanted in the flanks of female recipient nude mice (see Fig. 2A) anesthetized by an intraperitoneal injection of pentobarbital (40 mg/kg). The mice (8 weeks old) were housed under pathogen-free conditions and used for experimentation. Xenografts were flushed weekly to clear the lumen of cell debris.
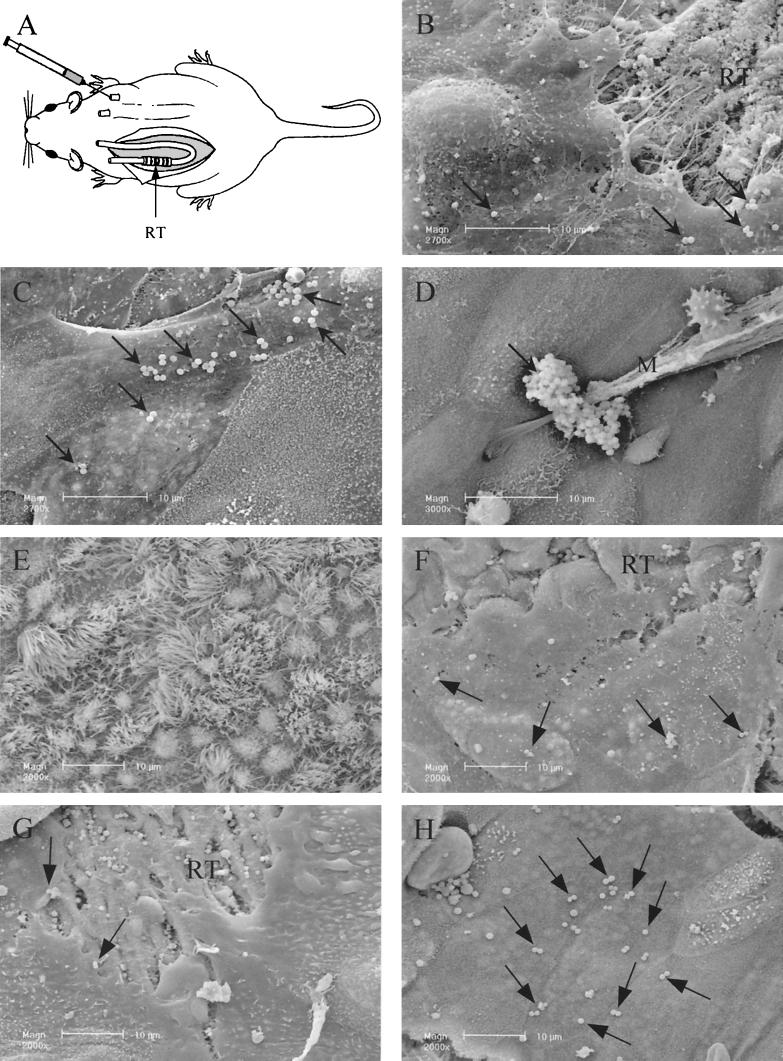
FIG. 2.
S. aureus adherence to HAEC in the humanized tracheal xenograft model. (A) Schematic drawing of the humanized xenograft model in the nude mouse. The rat tracheas (RT), two per mouse, are seeded with HAEC and subcutaneously implanted in the flanks of a nude mouse. The tracheas are connected to polyethylene tubing and can be externally filled with culture medium or bacterial suspension. (B to E) Adherence of Cowan III during the regeneration and maturation process of the human airway epithelium after 3 days (B), 5 days (C), 15 days (D), and 30 days (E) of xenograft implantation in the nude mouse. (F to H) Adherence of strain 8325-4 (F) and FnBPs-deficient (G) and FnBPA-overexpressing (H) strains of S. aureus to HAEC after 3 days of xenograft implantation in the nude mouse. RT, rat trachea; M, mucus.
After various times of xenograft implantation (3 to 30 days), the mice were anesthetized by intraperitoneal injection of pentobarbital (40 mg/kg). Xenografts were repetitively flushed with RPMI 1640 culture medium to remove antibiotics and then filled with the bacterial suspension. After 1 h of incubation, the xenografts were rinsed with 1.5 ml of RPMI 1640 culture medium to remove nonadherent bacteria. To remove tracheal xenografts, the mice were sacrificed by injection of an overdose of pentobarbital. Each tracheal xenograft was then prepared for scanning electron microscopy (SEM).
Observation by SEM.
Samples were fixed with 2.5% glutaraldehyde in 0.1 M PBS for 2 h at 4°C, rinsed three times in PBS, postfixed with 2% osmium tetroxide, dehydrated through graded ethanol concentrations, critical-point- dried with CO2, affixed to stubs, coated with 15-nm-diameter gold-palladium, and observed by using a Philips XL30 scanning electron microscope operating at 10 kV.
Quantification of bacterial adherence to HAEC.
Quantification of staphylococcal adherence to HAEC was performed using a computer-assisted scanning electron microscope, as previously described (11). The software has been adapted for S. aureus quantification. Briefly, optical series were obtained by confocal microscopy with a Z-step of 0.4 μm on S. aureus aggregates labeled as previously reported (24) and by three-dimensional reconstructions of these aggregates. A filling rate of S. aureus aggregates was determined, allowing the quantification of a number of S. aureus cells within an aggregate observed by SEM. A total of 35 fields screening the periphery of the outgrowth (nondifferentiated epithelial cells) and 35 fields of the explant-neighboring region of the outgrowth (containing ciliated differentiated and nonciliated differentiated epithelial cells) were analyzed at a constant magnification of 2,700 per explant. Each value represents the mean of several different explants, as explained in the section on statistical analysis. Bacteria, isolated and in aggregates, were numbered and reported as the total number of adherent bacteria per square micrometer of airway epithelial surface. For isogenic mutants and clinical strains, adherence data were presented as a percentage of the strain 8325-4 adherence value.
Adherence of S.aureus to fibronectin-coated microtiter wells.
We coated 96-well microtiter plates with 100 μl of human cellular FN (Sigma) or PBS supplemented with 1% bovine serum albumin (BSA) (Sigma) to estimate nonspecific binding. A dose-dependent FN-binding assay was performed with coating levels ranging from 0.5 to 50 μg/ml for reference strains and for three clinical strains of S. aureus in stationary phase. Identical experiments were performed for reference strains in the logarithmic growth phase [8325-4, DU5875, DU5883, and DU5883(pFNBA4)].
Further experiments were performed with 50 μg of FN per ml for all the S. aureus strains. The purity and integrity of FN were assessed by loading and running at 150 V on sodium dodecyl sulfate-8% polyacrylamide gels. After overnight incubation with FN at 4°C under constant agitation, the wells were washed three times with PBS-1% BSA for 20 min. Then 100-μl volumes of the different bacterial suspensions (109 CFU/ml in PBS) were deposited in duplicate for 30 min at 37°C under mild agitation and washed three times with PBS to remove nonadherent bacteria. Bacteria were fixed with 2.5% glutaraldehyde in 0.1 M PBS for 2 h at 4°C, stained with 0.1% crystal violet for 30 min at room temperature, and rinsed in water. The stain was then extracted from bacteria by using 0.2% Triton X-100 (Sigma) at room temperature for 30 min and read with a spectrophotometer (MR5000; Dynatec, Guernsey, Channel Islands) at 570 nm. A blank value corresponding to BSA-coated wells was automatically subtracted, and each assay point was performed in duplicate. Values of FN binding of the DU5883 strain (defective in FnBPs) were taken as reference values. These experiments were repeated at least twice; since similar results were obtained in independent experiments, only one representative experiment is shown in the graphs.
Pulsed-field gel electrophoresis (PFGE) analysis of S.aureus strains.
Genomic DNA extraction and SmaI restriction were performed as described by Matushek et al. (20). DNA fragments were separated by pulsed-field gel electrophoresis PFGE in a Gene Navigator system (Pharmacia) for 18 h at 200 V with the switching times ramped from 5 to 30 s. The SmaI genomic digestion of S. aureus NCTC 8325 was used as a molecular size marker (40). The similarity of macrorestriction patterns was determined by visual comparison. Patterns differing from more than three fragments were considered to be distinct clones, and those differing by 1-3 fragments were considered as subclonal variants (40).
S.aureus DNA extraction.
Three colonies were suspended in 50 μl of lysostaphin (Sigma; 100 μg/ml in H2O) and incubated at 37°C for 10 min. Then 50 μl of proteinase K solution (100 μg/ml) (Merck, Darmstadt, Germany) and 150 μl of 0.1 M Tris-HCl (pH 7.5) were added, and incubation proceeded for a further 10 min at 37°C. The samples were then heated for 5 min at 100°C. The DNA concentration was measured by spectrophotometry (absorbance at 260 nm) and adjusted to 0.135 μg/μl.
fnbA and fnbB PCR.
Four primers specific for fnbA and four primers specific for fnbB were designed using the gene sequences published by Signds et al. (37) for fnbA (GenBank accession number J04151) and by Jönsson et al. (16) for fnbB (GenBank accession number X62992). The primers were synthesized by MWG-Biotech (Courtaboeuf, France).
For the detection of fnbA, primer 1 (5"-AAATGATATTGAAGATAAGGTTGATG-3") and primer 2 (5"-GTCTAGATTCTTCAAAGTCAATTGGA-3") were used to amplify a 985-bp fragment of the A domain of fnbA. Primer 3 (5"-GATACAAACCCAGGTGGTGG-3") and primer 4 (5"-TGTGCTTGACCATGCTCTTC-3") were used to amplify a highly conserved region of 191 bp in the fibronectin-binding domain D3 of fnbA.
For the detection of fnbB, primer 5 (5"-AATCTATTTATTGATCCTACAACAG-3") and primer 6 (5"-CGTATATTCTTTCGTATCTTCAATT-3") were used to amplify a 1,007-bp fragment of the A domain of fnbB. Primer 7 (5"-GGAGCGGCCTCAGTATTCTT-3") and primer 8 (5"-AGTTGATGTCGCGCTGTATG-3") were used to amplify a 201-bp fragment including part of the signal peptide sequence and A domain of fnbB.
PCR was performed in a DNA thermal cycler (GeneAmp PCR System 9700; PE Applied Biosystems). The following cycling parameters were used: 94°C for 30 s, 52°C (primers 1, 2, 5, and 6) or 55°C (primers 3, 4, 7, and 8) for 30 s, and 72°C for 30 s, for 30 cycles. The identity of the PCR products (obtained with primers 1, 2, 5, and 6) was confirmed by digestion with XbaI and NheI for the fnbA and fnbB genes, respectively.
Extraction and expression of FnBPs.
Cell extracts were prepared on both stationary- and logarithmic-phase cultures. After pelleting, the cells were resuspended in PBS-1.1 M sucrose supplemented with 50 μg of lysostaphin (Sigma) per ml and incubated for 10 min at 37°C (39). After centrifugation (10,000 × g for 30 min), the protein extracts were ultrafiltered (10-kDa cutoff) to remove sucrose.
Equivalent amounts of total extracted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 8% acrylamide resolving gels. After being transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), FnBPs were probed with 30 μg of human FN solution per ml for 2 h at 4°C and then incubated with a mouse monoclonal anti-FN antibody (Chemicon, Temecula, Calif.). Bound antibody was detected with goat anti-mouse antibody conjugated with horseradish peroxidase (Jackson Immunoresearch). Detection was enhanced by chemiluminescence (Pierce, Rockford, Ill.).
Statistical analysis.
Adherence of Cowan III and 8325-4 strains to undifferentiated and differentiated respiratory epithelial cells was quantified using six different cultures from the same patient. Adherence of Cowan III, 8325-4, isogenic mutants of 8325-4, and clinical strains P14, P15, M01, and M16 to undifferentiated HAEC was quantified using nine cell cultures from three different patients (three cultures per patient). Data are expressed as mean ± standard error. A nonparametric Kruskal-Wallis test was used to analyze the interpatient variability. Values of adherence were compared by the nonparametric Mann-Whitney test. A P value of <0.05 was considered significant.
RESULTS
PFGE analysis of S.aureus strains.
PFGE confirmed the clonality of mutant strains derived from the parental strain 8325-4 (data not shown). PFGE profiles of the P and M clinical strains of S. aureus are shown in Tables 2 and Table 3, respectively. PFGE profiles of P strains showed 11 different clones among the 15 strains. P01 and P02, P03 and P07, and P09 and P10 strains, although they had been isolated from different patients, exhibited the same PFGE profiles and could be closely related. Fifteen different clones and three pairs of subclonal variants (M01 and M05, M02 and M16, and M08 and M09) were identified in the 18 strains isolated from the airways of CF patients. Interestingly, the M01 and M06 pairs and the M05 and M09 pairs of strains, which had been isolated from the airways of the same patient, exhibited more than three different bands in their PFGE patterns and did not appear to be clonally related.
Quantification of in vitro S.aureus adherence to HAEC.
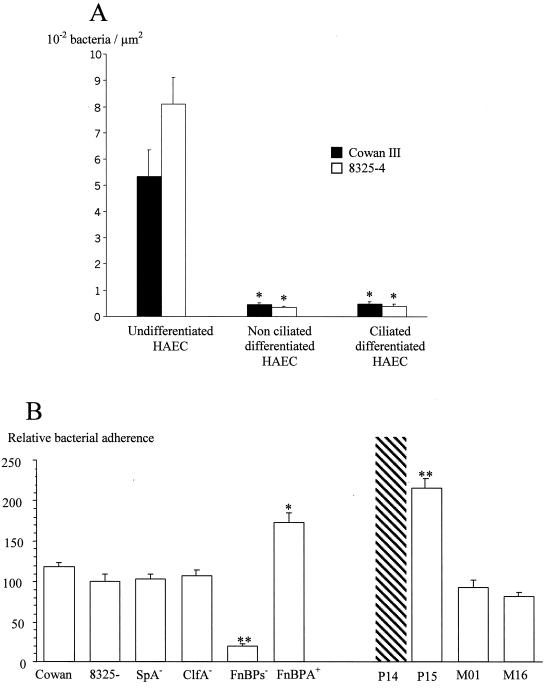
Adherence to differentiated and undifferentiated airway epithelium was investigated using the primary explant outgrowth culture model (Fig. 1A). For both Cowan III and 8325-4 reference strains, bacterial adherence to nonciliated (0.49 × 10−2 ± 0.08 × 10−2 and 0.39 × 10−2 ± 0.09 × 10−2 bacterium/μm2, respectively) and to ciliated (0.45 × 10−2 ± 0.07 ×10−2 and 0.33 × 10−2 ± 0.06 × 10−2 bacterium/μm2, respectively) differentiated HAEC was weak. In contrast, adherence to undifferentiated HAEC was significantly increased, 11-fold for the Cowan III strain (5.35 × 10−2 ± 1.02 bacteria/μm2; P < 0.01) and more than 20-fold for the 8325-4 strain (8.07 × 10−2 ± 1.04 bacteria/μm2; P < 0.01), compared to adherence to differentiated ciliated HAEC. No significant difference between the Cowan III and 8325-4 strains was observed.
FIG. 1.
S. aureus adherence to HAEC in primary culture. (A) Adherence of strains Cowan III and 8325-4 to undifferentiated, nonciliated differentiated, and ciliated HAEC airway epithelial cells. ∗, P < 0.01 (mean bacterial adherence to nonciliated and ciliated differentiated HAEC versus undifferentiated HAEC). (B) Adherence of Cowan III, 8325-4, isogenic mutants of 8325-4, and clinical strains (P14, P15, M01, and M16) of S. aureus to undifferentiated HAEC. Values of adherence are expressed relative to the 100% adherence value of strain 8325-4 to HAEC. The adherence of P14 was not quantifiable (>0.5 bacteria/μm2). ∗, P < 0.01; ∗∗, P < 0.005 (mean adherence to HAEC of mutant and clinical strains versus strain 8325-4).
The contribution of some staphylococcal adhesins involved in the adherence to undifferentiated HAEC was further investigated in vitro by using isogenic mutants of 8325-4 (Fig. 1B). The adherence of strain 8325-4 was set at 100%, and values of the adherence of other strains were expressed relative to that of 8325-4. Adherence of SpA− and ClfA− isogenic mutant strains to undifferentiated HAEC (102.6% ± 6.9% and 107.4% ± 6.7%, respectively) was not significantly different from that of strain 8325-4 (Fig. 1B). In contrast, adherence of the FnBP− mutant was significantly reduced (19.9% ± 3%; P < 0.005), suggesting that FnBPs are the major adhesins responsible for S. aureus adherence to undifferentiated HAEC. Further evidence for the role of FnBPs in S. aureus adherence to HAEC was provided by the 8325-4 FnBP-deficient strain complemented with a multicopy plasmid encoding FnBPA (FnBPA+ strain). Adherence of the FnBPA+ strain was significantly higher (172.9% ± 12.2%) than that of the FnBP-deficient strain (P < 0.01) and the 8325-4 parental strain (P < 0.005) (Fig. 1B).
In vivo S.aureus adherence to HAEC.
Using a humanized tracheal xenograft model in the nude mouse (Fig. 2A), we investigated the adherence of the Cowan III strain to HAEC in relation to the degree of differentiation and maturation of the airway epithelium (Fig. 2B to E). In 3-day-old tracheas, a strong interaction between Cowan III and poorly differentiated (and migrating) HAEC was observed (Fig. 2B, arrows), as we previously showed in vitro. In 1-week-old tracheas, bacteria were found to adhere only to flattened cells (Fig. 2C, arrows). As the differentiation of the epithelium increased, S. aureus adherence to HAEC decreased. S. aureus was trapped by the mucus (Fig. 2D), and no bacteria were observed at the surface of ciliated HAEC (Fig. 2E) in 15- and 30-day-old tracheas.
To confirm in vivo the implication of the FnBPs in the adherence of S. aureus to undifferentiated HAEC, we further investigated the adherence of 8325-4, DU5883 FnBP−, and DU5883(pFNBA4) FnBPA+ strains of S. aureus to poorly differentiated HAEC in 3-day-old xenografts. Adherence of the 8325-4 strain to HAEC (Fig. 2F) was similar to that of the Cowan III strain (Fig. 2B). Bacteria were observed only at the surface of undifferentiated HAEC, and bacterial adherence decreased as the differentiation of the human airway epithelium increased (data not shown). Adherence of the FnBP− strain was weak; very few bacteria were observed at the surface of undifferentiated HAEC (Fig. 2G) whereas numerous bacteria still adhered to the denuded rat trachea. In contrast, numerous FnBPA+ bacteria adhered to undifferentiated HAEC (Fig. 2H).
FN-binding capacity of P and M clinical strains.
Because in vitro and in vivo studies highlight the major role of staphylococcal FnBPs in adherence to host tissue, we further investigated the FN-binding capacity of reference strains, isogenic mutants, and 32 clinical strains isolated during 14 nosocomial pneumonia episodes and 18 acute infectious episodes in CF.
Reference strains and three clinical strains were investigated for their abilities to bind to FN in microplates coated with 0.5, 5, 25, or 50 μg of human cellular FN per ml. All these strains exhibited a dose-dependent increase of their FN-binding activity, which reached a plateau value at 50 μg/ml (data not shown). Complementary FN-binding assays with 8325-4, DU5875 (SpA−), DU5883 (FnBP−), and DU5883(pFNBA4) (FnBPA+) strains were also performed to compare FN binding in the logarithmic phase to that in the stationary phase, and we did not observe any significant difference according to the growth phase (data not shown). Therefore, further experiments with clinical strains were conducted using stationary-phase cultures and a coating of 50 μg of FN per ml, representing the plateau value.
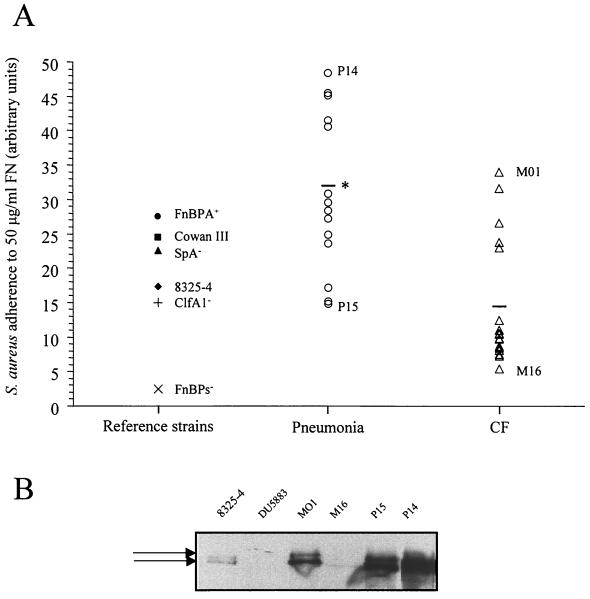
Interestingly, the P and M strains were characterized by different FN-binding capacities. Adherence of S. aureus clinical strains isolated from the airway secretions of patients suffering from nosocomial pneumonia or CF is shown in Fig. 3. The FN-binding capacity was significantly higher for P strains than for M strains (31.95% ± 3.02% and 14.36% ± 2.13%, respectively; P < 0.05). Among clinical strains, two P and two M strains were selected for their FN-binding capacity. These include the P14 (binding to 50 μg of FN per ml, 48.38%) and P15 (binding, 14.8%) strains for the pneumonia group and the M01 (binding, 33.9%) and M16 (binding, 5.4%) strains for the CF group, which have the highest and the lowest values of adherence on FN, respectively.
FIG. 3.
S. aureus adherence to FN and FnBP expression. (A) Adherence of reference and clinical strains was tested in microplates coated with 50 μg of FN per ml. Values of adherence are expressed in comparison to the FnBP-deficient strain. ∗, P < 0.05 (mean adherence of the 14 pneumonia strains versus the 18 CF strains). (B) Levels of expression of FnBPs (arrows) in strain 8325-4 strain, its isogenic mutant lacking fnbA and fnbB, two CF strains (M01 and M16), and two P strains (P15 and P14) were examined by Western ligand affinity blotting.
Identification of fnbA and fnbB in clinical strains of S.aureus.
PCR detection of fnbA and fnbB was performed on the six reference and isogenic mutant strains of S. aureus and on the 32 clinical strains. Cowan III, 8325-4, SpA-deficient, and ClfA-deficient strains were positive for both genes, giving the expected size of PCR products for both pairs of primers for the fnbA and fnbB genes (data not shown). Using primer pairs 1-2 and 5-6, the PCR detection of fnbA and fnbB was negative in the DU5883 FnBP-deficient strain (data not shown), in contrast to a positive reaction for the isogenic parental strain 8325-4, since both the design of the primers (chosen surrounding the restriction site where interposon conferring resistance was inserted in both genes) and the PCR conditions were adapted for that purpose. The PCR detection was positive for the fnbA gene in the DU5883 strain complemented with a plasmid expressing FnBPA and negative for the fnbB gene (data not shown). PCR products obtained with primers 1 plus 2 and 5 plus 6 were digested with XbaI and NheI for the fnbA and fnbB genes, respectively. Two bands of approximately 480 and 500 bp for fnbA and 485 and 520 bp for fnbB were generated, confirming the identity of the PCR products (data not shown).
All 14 strains isolated from patients with nosocomial pneumonia possessed both the fnbA and fnbB genes (Table 2), and the adherence of P strains to fibronectin was not related to the presence of the fnb genes.
Among the 18 strains isolated from CF patients, M11 was the only strain found to lack the fnbA gene in assays with both primer pairs 1 plus 2 and 3 plus 4 (Table 3), although all the M strains possessed the fnbB gene. The level of adherence of the M11 strain (8.1% [50 μg of FN/ml]) was similar to the adherence of M13 and M18 strains (8.6 and 8.4%, respectively), although these two strains possessed both fnb genes. As reported for the P strains, the adherence of M strains was not only related to the presence of fnb genes. We could detect both fnb genes in strains with either poor adherence (e.g., M16, M17, and M18) or good adherence (e.g., M01 and M02) to FN.
Expression of FnBPs.
Lysostaphin extracts of several clinical strains (P14, P15, M01, and M16) and of reference strains such as 8325-4 and its isogenic mutant lacking both fnbA and fnbB genes in stationary- and logarithmic-phase cultures were subjected to Western ligand affinity blotting.
As previously shown (3, 39), FnBPs appeared as two major bands of approximately 200 kDa. Several other bands corresponding to truncated forms of FnBPs and to protein A could also be observed (data not shown). In stationary-phase cultures, no detectable amount of FnBPs was seen. De novo synthesis of FnBPs took place during early growth (1 h), and the observed quantities of FnBPs decreased dramatically between 2 and 4 h of growth, whatever the clinical or reference strains (data not shown), except for the double fnbA-fnbB mutant, for which absolutely no signal corresponding to FnBPs was detected. When the expression profiles of FnBPs were compared during the early growth phase (1 h), it seemed obvious that total FnBPs (FnBPA plus FnBPB) revealed by FN affinity and the relative amounts of FnBPA and FnBPB proteins varied between strains (Fig. 3B). Total FnBPs were found to be higher in strains P14, P15, and M01 than in strain 8325-4. The levels of FnBPs in strain M16 were very low compared to that in strain 8325-4. Whereas the band with the highest molecular weight, corresponding to FnBPA, was the most intensive band in P1 and P15, this band became less intensive in M01 and was not detectable in M16. Because we were not able to extract FnBPs from cultures in the stationary growth phase but we could identify these FnBPs in the very early growth phase, we verified that during assays of FN affinity in microplates, bacteria were able to multiply. These experiments showed that in fresh medium, in particular in PBS in which bacteria were resuspended in microplates, bacteria were able to divide at least once, even at high inoculum (data not shown).
Adherence of S.aureus clinical strains to HAEC.
Clinical strains remarkable for their FN-binding capacity in microplates, i.e., P14, P15, M01, and M16, were further studied for their adherence to HAEC in vitro and in vivo. In in vitro HAEC cultures, adherence of strain P14 could not be quantified (>0.5 bacteria/μm2), since too many bacteria had adhered to HAEC (Fig. 1B). Adherence of strain P15 (215.6% ± 2.7%) was significantly higher than that of strain 8325-4. In contrast, the levels of adherence of M01 and M16 (93.5% ± 7.9% and 81.2% ± 5.9%, respectively) were not significantly different from that of 8325-4 strain. These in vitro results were confirmed in vivo in the humanized bronchial xenograft model after 3 days of engraftment in the nude mouse (data not shown). P14 and P15 strains strongly adhered to undifferentiated HAEC. Adherence of M01 and M16 strains to undifferentiated HAEC was comparable to that of 8325-4 and Cowan III and lower than that of P14 and P15. For all of these strains, adherence to HAEC decreased during differentiation of the human airway epithelium, and no bacteria was observed at the contact of HAEC in fully mature epithelium (data not shown), as previously observed for the Cowan III strain (Fig. 2).
DISCUSSION
In the present study, we investigated the role of staphylococcal adhesins in the adherence of S. aureus to HAEC. We showed that S. aureus adheres mainly to poorly differentiated HAEC, in vitro as well as in vivo, and that FnBPs are the major staphylococcal adhesins responsible for this adherence.
Very few studies have tried to elucidate the initial mechanisms of colonization, i.e., bacterial adherence, of the airway epithelium by S. aureus. It has been shown that S. aureus specifically interacts with the mucus present in the obstructed airways of CF patients and, at a lower level, with the surface of CF and non-CF nasal epithelial cells grown in primary culture (41). Schwab et al. (36) showed that CF and non-CF S. aureus isolates weakly adhere to ciliated epithelial cells (one to three bacteria per cell). Our results also confirm the weakness of S. aureus adherence to ciliated respiratory epithelial cells. However, few, if any, studies have taken into account the undifferentiated and repairing state of the surface airway epithelium. We previously showed that both intact and remodeled epithelial areas can be equally encountered in the airways of S. aureus-infected CF patients (24). Our in vitro (primary HAEC cultures) and in vivo (humanized bronchial xenografts in the nude mouse) experiments undoubtedly demonstrate that undifferentiated HAEC are the major sites for adherence of reference and clinical S. aureus strains to human airway epithelium.
The use of isogenic mutants of 8325-4 strain for different potential adhesins highlights the major role of FnBPs in S. aureus adherence to HAEC, whereas SpA and ClfA did not play any role in this phenomenon. FN and its cellular receptor, the α5β1-integrin, have been demonstrated to be apically exposed and overexpressed by undifferentiated HAEC engaged in the repair process (14, 31). Several authors have showed that FN acts as a bridging molecule between α5β1-integrins and staphylococcal FnBPs, thus mediating adherence of S. aureus to epithelial cells, endothelial cells, and fibroblasts and their subsequent internalization (1, 38). Moreover, FnBPs on their own are sufficient for adherence and invasion of host cells (39).
Because FnBPs of S. aureus play a key role in bacterial binding to HAEC, we then investigated the FN-binding capacity of 32 clinical strains isolated during nosocomial pneumonia (n = 14) (P strains) and acute bronchial infectious episodes in CF patients (n = 18) (M strains). Few of these strains were clonally related: PFGE showed 26 different clones. The great variability of the FN-binding capacity of the pneumonia and CF isolates could therefore be attributed to the heterogeneity of the strains. In spite of that, the P strains were significantly more adherent than the M strains, to FN as well as to HAEC in vitro and in vivo. Our results are in disagreement with those of Schwab et al. (36), who reported that adherence to bronchial epithelial, nasal, and buccal cells was higher for CF isolates than for non-CF isolates of S. aureus. This discrepancy may be due to differences in the bacterial adherence assay used by the authors, with cells and bacteria in suspension, and to differences concerning S. aureus strains isolated from non-CF patients, but the origin of the strains was not indicated in their study.
Among the CF isolates, strains M01 and M05 were clonally related and exhibited closely similar FN-binding capacities (33.9% and 31.6% respectively [50 μg of FN/ml]), although they were isolated from the airways of two different patients. The same observations were made for strains M08 and M09, whose PFGE profiles and FN-binding capacities were also very similar (10.4% and 9.7%, respectively). These results led us to conclude that the FN-binding phenotype of S. aureus CF strains can be conserved, independently of the CF genotype and of the evolution of the CF pathology. This is confirmed by the M01 and M06 and the M05 and M09 strain pairs. Although they were isolated from the airways of the same patient, these strains exhibited different PFGE profiles and different FN-binding capacities (33.9% and 12.4%, and 31.6% and 9.7%, respectively). However, in contrast, the clonally related M02 and M16 strains were isolated in different patients and had different FN-binding capacities (26.6% and 5.4%, respectively), suggesting either a lack of ability of PFGE to differentiate these strains or a different regulation of factors promoting bacterial adherence to FN in different environments.
The presence of fnbA and fnbB genes was investigated by amplifying two regions of each fnb gene in the 32 clinical strains of S. aureus. Of 32 clinical strains, 31 were positive for both fnb genes. Only one strain, M11, lacked the fnbA gene, although most of the strains were different clones isolated in different patients. Thus, the presence of at least one of the two fnb genes seems to be an important factor for the virulence of the bacteria. Even if almost 97% of CF and non-CF clinical strains possess one of the two fnb genes, the FN-binding capacity was very different from one clinical strain to another. In a previous study, Montarano et al. (25) showed a strong correlation between a high FN adherence and the presence of the fnb genes in S. aureus and S. epidermidis strains isolated from infected prostheses. In contrast, the studies of Peacock et al. (29) and Rice et al. (30) clearly showed that FN affinity is not related to the number of fnb genes, to fnb locus polymorphism, or to the number of D motifs (FN-binding domains) of FnBPs but, rather is a function of the transcriptional activity of the agr locus and the protease activity. Although the production of FnBPs is negatively regulated, by the global regulator agr, during the late and post-exponential phase of growth, Goerke et al. (12) showed that the agr system of CF isolates was inactive in vivo. This could lead to defective agr-dependent down-regulation of surface proteins synthesis. Moreover, they also reported spontaneous mutations of the agr locus, thus demonstrating that the agr activity seems to be nonessential for the strains infecting CF airways. Mutations in regulatory genes such as agr and sar would then induce differences of the FN-binding properties of mutant strains, as already shown by Wolz et al. (44).
In this study, bacteria were used in either the logarithmic or stationary phase of growth. Surprisingly, we found no difference of the FN-binding capacity in relation to the bacterial growth phase, whereas we could observe a variation of FnBP expression. The fact that the FN-binding capacity did not vary according to the growth phase has been previously described by Holmes et al. (15) using the surface plasmon resonance technique. Although FnBPs were extracted very rapidly at given points of the bacterial growth, the FN-binding capacity in microplates was observed over a period ranging from 30 min to 1 h, a period during which we verified that bacteria were still able to multiply. De novo synthesis of FnBPs by cells at a high inoculum could then occur, since transcription of FnBPs has been reported to be limited to the first cell division by Mohamed et al. (23). Previous reports indicated that the main cause of the decreased adherence of S. aureus to FN during the late and postexponential phases of growth is the increased secretion of serine protease SspA (V8 protease), which is responsible for the degradation of FnBPs at the surface of S. aureus, rather than the decreased transcription of the fnb genes (17, 22). However, the study by Peacock et al. (29) clearly showed that the weak adherence of S. aureus clinical strains to FN (the bacteria were used in stationary phase of growth) was not due to a digestion of FnBPs by proteases. Moreover, the binding of the bacteria to FN is not proportional to the amount of FnBPs present on their surface (34).
P and M clinical strains of S. aureus showed different patterns of FnBP expression, consistent with the results obtained in microplates with entire bacteria. When considering M or P strains, we observed a different expression or FN affinity of FnBPs between strains M01 and M16 and strains P14 and P15, respectively, which is in accordance with results obtained in microplates with entire bacteria. However, Western ligand affinity blotting results showed a similar signal between M01 and P15 strains although they had different FN-binding capacities, suggesting that other factors are essential for entire-cell FN affinity, such as the presence and/or activity of proteases. Furthermore, differences between FN adherence of entire bacteria and profiles of FnBP expression could be attributed to differences in the FN affinity of FnBPs from one strain to another. Indeed, Rice et al. (30) showed that the fnb locus is very polymorphic, especially in MRSA clinical isolates. They pointed out that some MRSA strains may possess an additional D motif, with a reduced ability to bind FN. The expression profiles of FnBPs, as shown in Fig. 3B, could then reflect a difference of FnBP expression combined with a difference of affinity for FN, from one strain to another. All these results suggest that the differences of FN-binding capacities observed in S. aureus clinical strains involve complex relations between different affinities for FN and different regulatory pathways for both the FnBPs and the proteases in charge of their degradation.
In the present study, we have demonstrated that poorly differentiated HAEC are major sites for S. aureus adherence to airway epithelium. This has been achieved using in vitro and in vivo experiments for reference strains but also for clinical strains of S. aureus isolated from the airways of patients with nosocomial pneumonia and with CF. The use of isogenic mutant strains for different potential adhesins highlights the major role of FnBPs in S. aureus adherence to airway epithelium. Nevertheless, the diversity of the FN-binding capacity observed in S. aureus clinical strains may reflect a combination of different affinities for FN and different regulatory pathways for both the FnBPs and the proteases in charge of their degradation.
Acknowledgments
This study was supported by grant 96.34.053.00.470 from DRET/DGA and funded by INSERM and the Association Vaincre la Mucoviscidose. Emmanuel Mongodin was a doctoral fellow of the Ministère de la Défense-DRET/DGA and the Fondation pour la Recherche Médicale (FDT20000710083).
We thank T. J. Foster (Department of Microbiology, Trinity College, Dublin, Ireland) for providing strain 8325-4 and isogenic mutant strains of S. aureus, J. M. Klossek (CHU J. Bernard, Poitiers, France) for providing human nasal tissues, and J. I. Flock (Huddinge, Sweden) and M. McGavin (Toronto, Canada) for promptly and kindly providing antibodies for FnBP detection. We are grateful to D. Ingrand and all the members of his laboratory (Laboratoire de Bactériologie-Virologie-Hygiène, CHU Robert Debré, Reims, France) for useful suggestions and to J. Madoux for technical assistance with PFGE.
Editor: E. I. Tuomanen
REFERENCES
- 1.Aly, R., H. R. Shinefield, C. Litz, and H. I. Maibach. 1980. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J. Infect. Dis. 141:463-465. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H. 1981. Bacterial adherence. Adhesin-receptor interactions mediated the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 3.Bisognano C., P. E. Vaudaux, D. P. Lew, E. Y. Ng, and D. C. Hooper. 1997. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 41:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States. JAMA 278:1145-1146. [PubMed]
- 5.Chevillard, M., J. Hinnrasky, J. M. Zahm, M. C. Plotkowski, and E. Puchelle. 1991. Proliferation, differentiation and ciliary beating of human respiratory ciliated cells in different conditions of primary cultures. Cell Tissue Res. 264:49-55. [DOI] [PubMed] [Google Scholar]
- 6.de Bentzmann, S., M. C. Plotkowski, and E. Puchelle. 1996. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am. J. Respir. Crit. Care Med. 154:S155-S162. [DOI] [PubMed]
- 7.de Bentzmann, S., P. Roger, F. Dupuit, O. Bajolet-Laudinat, C. Fuchey, M. C. Plotkowski, and E. Puchelle. 1996. Asialo-GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 64:1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuit, F., D. Gaillard, J. Hinnrasky, E. Mongodin, S. de Bentzmann, E. Copreni, and E. Puchelle. 2000. Differentiated and functional human airway epithelium regeneration in tracheal xenografts. Am. J. Physiol. 278:L165-L176. [DOI] [PubMed]
- 9.Foster, T. J., and D. McDevitt. 1994. Surface-associated proteins of Staphylococcus aureus: their possible role in virulence. FEMS Microbiol. Lett. 118:199-205. [DOI] [PubMed] [Google Scholar]
- 10.Foster, T. J., and M. Höök. 1998. Surface proteins adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 11.Girod de Bentzmann, S., O. Bajolet-Laudinat, M. C. Plotkowski, and N. Bonnet. 1993. Digital stereology to quantify the filling rate of bacterial aggregates of Pseudomonas aeruginosa. J. Microbiol. Methods 17:193-198. [Google Scholar]
- 12.Goerke, C., S. Campana, M. G. Bayer, G. Döring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene, C. 1995. Studies on fibronectin binding proteins of Staphylococcus aureus. Ph.D. thesis. Trinity College, Dublin, Ireland.
- 14.Hérard, A. L., D. Pierrot, J. Hinnrasky, H. Kaplan, D. Sheppard, E. Puchelle, and J. M. Zahm. 1996. Fibronectin and its α5β1-integrin receptor are involved in the wound repair of airway epithelium. Am. J. Physiol. 271:L726-L733. [DOI] [PubMed]
- 15.Holmes, S. D., K. May, V. Johansson, F. Markey, and I. A. Critchley. 1997. Studies on the interaction of Staphylococcus aureus and Staphylococcus epidermidis with fibronectin using surface plasmon resonance (BIAcore). J. Microbiol. Methods 28:77-84. [Google Scholar]
- 16.Jönsson, K., C. Signäs, H. P. Müller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson K., P. Saravia-Otten, K. Tegmark, E. Morfeldt, and S. Arvidson. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus SarA mutants due to up-regulation of extracellular proteases. Infect. Immun. 69:4742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krivan, H. C., D. D. Roberts, and V. Ginsburg. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcβ1-4Gal found in some glycolipids. Proc. Natl. Acad. Sci. USA 85:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers B., and R. P. Proctor. 1989. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of S. aureus. Infect. Immun. 57:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matushek, M. G., M. J. M. Bonten, and M. K. Hayden. 1996. Rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. J. Clin. Microbiol. 34:2598-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDevitt, D., P. François, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 22.McGavin M. J., C. Zahradka, K. Rice, and J. E. Scott. 1997. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect. Immun. 65:2621-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed, N., L. Visai, P. Speziale, and J. M. Ross. 2000. Quantification of Staphylococcus aureus cell surface adhesins using flow cytometry. Microb. Pathog. 29:357-61. [DOI] [PubMed] [Google Scholar]
- 24.Mongodin, E., O. Bajolet, J. Hinnrasky, E. Puchelle, and S. de Bentzmann. 2000. Cell wall-associated protein-A as a tool for immunolocalization of Staphylococcus aureus in infected human airway epithelium. J. Histochem. Cytochem. 48:523-533. [DOI] [PubMed] [Google Scholar]
- 25.Montarano, L., C. R. Arciola, E. Borsetti, S. Collamati, L. Baldassarri, and L. Montarano. 1999. Detection of fibronectin-binding protein genes in staphylococcal strains from peri-prosthesis infections. Microbiologica 22:331-336. [PubMed] [Google Scholar]
- 26.Novick, C. 1967. Properties of a cryptic high frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 27.Patel, A. H., P. Nowlan, E. D. Weavers, and T. J. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 29.Peacock, S. J., N. P. J. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 30.Rice, K., M. Huesca, D. Vaz, and M. J. McGavin. 2001. Variance in fibronectin-binding and fnb locus polymorphisms in Staphylococcus aureus: identification of antigenic variation in a fibronectin-binding protein adhesin of the epidemic CMRSA-1 strain of methicillin-resistant S. aureus. Infect. Immun. 69:3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roger, P., E. Puchelle, O. Bajolet-Laudinat, C. Debordeaux, M. C. Plotkowski, J. M. Cohen, D. Sheppard, and S. de Bentzmann. 1999. Fibronectin and its α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13:1301-1309. [PubMed] [Google Scholar]
- 32.Rozalska, B., and T. Wadström. 1993. Protective opsonic activity of anti-bodies against fibronectin-binding proteins (FnBPs) of Staphylococcus aureus. Scand. J. Immunol. 37:575-580. [DOI] [PubMed] [Google Scholar]
- 33.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialo GM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Investig. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saravia-Otten, P., H. P. Müller, and S. Arvidson. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schennings, T., A. Heimdahl, K. Coster, and J. I. Flock. 1993. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb. Pathog. 15:227-236. [DOI] [PubMed] [Google Scholar]
- 36.Schwab, U. E., A. E. Wold, J. L. Carson, M. Leigh, P. Cheng, P. H. Gilligan, and T. F. Boat. 1993. Increased adherence of Staphylococcus aureus from cystic fibrosis lungs to airway epithelial cells. Am. Rev. Respir. Dis. 148:365-369. [DOI] [PubMed] [Google Scholar]
- 37.Signds, C., G. Raucci, K. Joensson, P. E. Lindgren, G. M. Anantharamiah, M. Hoeoek, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha, B., P. P. François, O. Nüsse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 39.Sinha, B., P. François, Y. A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. H. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulrich, M., S. Herbert, J. Berger, G. Bellon, D. Louis, G. Munker, and G. Döring. 1998. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am. J. Respir. Cell. Mol. Biol. 19:83-91. [DOI] [PubMed] [Google Scholar]
- 42.Vaudaux, P., P. Francois, B. Berger-Bachi, and D. P. Lew. 2001. In vivo emergence of subpopulations expressing teicoplanin or vancomycin resistance phenotypes in a glycopeptide-susceptible, methicillin-resistant strain of Staphylococcus aureus. J. Antimicrob. Chemother. 47:163-170. [DOI] [PubMed] [Google Scholar]
- 43.Voss, A., and B. N. Doebbeling. 1995. The worldwide prevalence of methicillin-resistant S. aureus. Int. J. Antimicrob. Agents 5:101-106. [DOI] [PubMed] [Google Scholar]
- 44.Wolz, C., P. Pölhmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230-243. [DOI] [PubMed] [Google Scholar]