Three patients had diverse clinical manifestations, all relating to the same disease (Figures 1–3). A representative skin biopsy is seen in Figure 4. What is the diagnosis, and what therapeutic options should be considered?
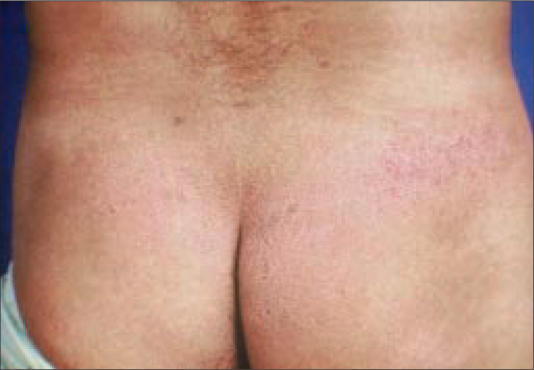
Figure 1.
Scaly, red pruritic patches on the buttocks.
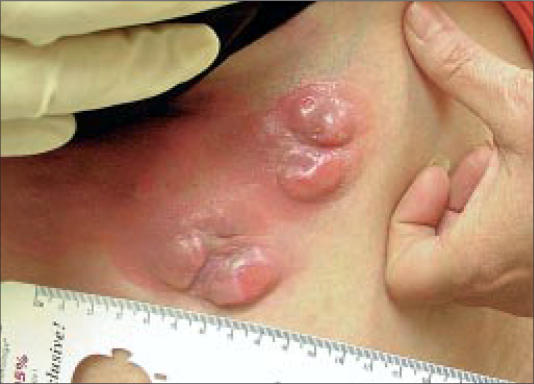
Figure 3.
Generalized exfoliative erythroderma.
Figure 4.
Routine histopathology slide from the patient shown in Figure 1 (hematoxylin-eosin stain, ×40).
DIAGNOSIS: Mycosis fungoides.
DISCUSSION
Approximately two thirds of cutaneous lymphomas are of T-cell origin. Cutaneous T-cell lymphoma (CTCL) comprises a heterogeneous group of malignancies, the most common form of which is mycosis fungoides (MF) (Table 1). MF is a rare, low-grade lymphoma with an estimated incidence of 0.36 cases per 100,000 people (1). Approximately 1000 new cases are found yearly. The incidence in African Americans is 1.6 that in Caucasians. Men are affected twice as often as women (1).
Table 1.
T-cell lymphomas involving the skin
| Cutaneous T-cell lymphomas |
| • Mycosis fungoides |
| • Sézary syndrome (leukemic variant of mycosis fungoides) |
| • CD30 (Ki-1)+ large-cell lymphomas (includes pleomorphic, anaplastic, and immunoblastic variants) |
| • CD30− large-cell lymphomas (includes pleomorphic, anaplastic, and immunoblastic variants) |
| Human T-lymphotropic virus type 1–related T-cell disorders |
| Peripheral T-cell lymphoma (includes subcutaneous panniculitis-like lymphoma) |
| Natural killer cell lymphoma (CD56+) |
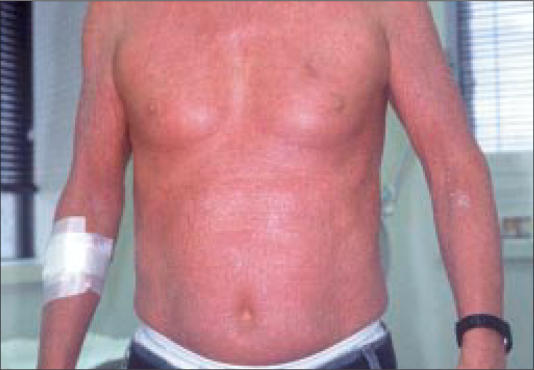
Clinically, early lesions of MF present as scaly, red patches or plaques in double-covered areas such as the buttocks (Figure 1), groin, or breasts. In ethnic patients, hyper- and hypopigmentation from the lesions may be striking and of great concern to affected individuals. More advanced cutaneous lesions consist of tumors (Figure 2) and a generalized exfoliative erythroderma (Figure 3). When erythrodermic patients have ≥10%–20% circulating atypical lymphocytes, they may be classified as having Sézary syndrome.
Figure 2.
Violaceous tumors on the left anterior abdomen.
Patients typically have nondescript ‘rashes’ for years prior to diagnosis and are misdiagnosed as having common inflammatory dermatoses like eczema, lupus erythematosus, tinea, pityriasis rubra pilaris, or psoriasis. In the early stages, pathology of involved areas likewise may be indeterminate (see Diagnostic procedures).
Pathogenesis
The cause of MF is unknown; however, many consider chronic antigenic stimulation (2) and defective apoptosis (3) important in the pathogenesis. MF is a malignancy of skin-homing memory T lymphocytes (4). Usually the malignant lymphocytes are CD4+CLA+; however, CD8+ variants do exist (5). MF usually arises from one malignant clone; hence, diagnosis can be aided by obtaining molecular biology studies of the T-cell receptor. Initially, the cells are epidermotropic (migrate to skin); however, with time the malignant T cells lose their affinity for skin and may disseminate internally (6).
Diagnostic procedures
To rule out the possibility of MF, a biopsy should be taken of any chronic rash that has been refractory to traditional topical therapy. For the most accurate results, biopsies should be performed when patients have been off therapy for at least 2 weeks. More information will be found if biopsies are done on the thickest lesions found in sun-protected areas like the buttocks. The histopathologic diagnosis of MF is difficult and requires an experienced dermatopathologist (7, 8). When MF is strongly suspected, repeat biopsies may be required at 6-month intervals for definitive diagnosis. On histopathology, small sheets of lymphocytes abutting the dermal-epidermal junction and collections of atypical lymphocytes in the epidermis (Pautrier's microabscesses, Figure 4) are helpful in the diagnosis of MF.
Adjunctive tests may be required if the diagnosis cannot be determined from routine slides stained with hematoxylin-eosin. For patients with lesions highly suspicious for MF but with equivocal or nondiagnostic histopathology, immunophenotyping will frequently provide more information (9). The most common phenotype is CD2+CD3+CD4+CD5+CD7–CLA+CD45RO+. Additionally, immunophenotyping may detect CD8+ variants, which express CD7 but lack CD2 and may have a more aggressive clinical course (5). For cases with equivocal histopathology and immunohistochemistry, polymerase chain reaction (PCR)-based studies have aided in the diagnosis of MF by detecting, with a sensitivity of 1%, the presence of a dominant clonal T-cell receptor gene rearrangement (10).
Staging and prognosis
After the diagnosis of MF is established, staging evaluations are required for determining appropriate therapy. Additionally, a patient's prognosis can be predicted by the stage of disease. The baseline examination required is dictated by clinical examination; however, most patients will require complete blood count (CBC), screening chemistry tests, flow cytometry of the peripheral blood (to detect malignant circulating T cells), chest x-rays, and computed tomography of the abdomen and pelvis. Bone marrow biopsies are required for patients with abnormal CBC results or detectable circulating atypical cells (i.e., Sézary cells). The subsequent staging system is summarized in Table 2 (11). While some physicians do not perform staging evaluations for early disease (stage IA), early stage patients with a detectable T-cell clone may have a worse prognosis and a higher rate of treatment failure (12).
Table 2.
Staging and prognosis of mycosis fungoides∗
| Stage | Skin disease | Adenopathy | Lymph node pathology | Visceral involvement | Survival at 10 years |
| IA | Patch/plaque (<10% BSA) | − | − | – | 100% |
| IB | Patch/plaque (>10% BSA) | − | − | − | 100% |
| IIA | Patches or plaques | + | − | − | 64% |
| IIB | Tumors | ± | − | − | 64% |
| III | Generalized erythroderma | ± | − | − | 45% |
| IVA | Patch/plaque, tumors, or erythroderma | ± | + | − | Poor |
| IVB | Patch/plaque, tumors, or erythroderma | ± | + | + | Poor |
∗From reference 11. BSA indicates body surface area.
Recently, the value of this staging system has come into question. Data from a cohort of 450 patients with MF and Sézary syndrome revealed that those with clinically positive lymph nodes with negative histology had the same prognosis as those with extensive patch disease (13). In addition, patients with extensive plaques had a worse prognosis than those with extensive patches. Finally, erythroderma and tumor stage patients had similar prognoses. If these findings are indeed confirmed, revisions in current staging and management are likely.
The long-term survival of patients with stage IA disease (diagnosis made without PCR) is similar to that of age-matched controls (14). Over 90% of patients with disease affecting ≤10% body surface area (BSA) and 75% of patients with disease affecting ≥10% BSA do not have progressive disease after treatment (14, 15). Younger patients have a favorable overall survival within any given stage (16). Large-cell transformation within 2 years of diagnosis is associated with a worse prognosis (17). Additionally, serum concentration of soluble IL-2 receptor correlates with tumor burden and prognosis (18).
Treatment
In the only randomized, controlled trial of active treatments for CTCL, early aggressive treatment with chemotherapy did not show a survival benefit when compared with conservative sequential therapies (19). In general, patients with early stage MF (stages IA, IB, and IIA) can be adequately treated with skindirected therapy. In contrast, later-stage patients require systemic therapy with biologic response modifiers, photopheresis, fusion toxins, chemotherapy, or bone marrow transplantation (Table 3).
Table 3.
Treatment of mycosis fungoides and Sézary syndrome
| Therapy | Reference number |
| Skin-directed therapy (early stage disease: stages IA–IIA) | |
| Topical steroids | 20 |
| Nitrogen mustard | 21 |
| Topical carmustine | 22 |
| Bexarotene gel | 23 |
| Phototherapy | |
| •PUVA | 24 |
| •Broadband ultraviolet B | |
| •Narrowband ultraviolet B (wavelength 311 nm) | 25 |
| Radiation (spot or total-body electron beam) | 26 |
| Systemic therapy (refractory early stage disease or stage IIB and above) | |
| Biologic response modifiers | |
| •Retinoids/rexinoids | 27 |
| •Interferon ± PUVA | 28, 29 |
| •Photopheresis | 30 |
| Combined modality treatment | 31 |
| Chemotherapy (single agent and multiagent) | 32–34 |
| Fusion toxin (denileukin diftitox) | 35 |
| Bone marrow transplantation | 36, 37 |
| Experimental: cytokine therapy | |
PUVA indicates psoralen + ultraviolet A.
The 3 patients shown in Figures 1 through 3 were completely staged and initiated on therapy. The patient in Figure 1 had stage IA disease and has been treated with topical steroids, psoralen plus ultraviolet A, and bexarotene gel. The patient in Figure 2 had stage IIB disease and has been treated with denileukin diftitox, bexarotene capsules, interferon alfa-2a, and, most currently, combination chemotherapy. The patient in Figure 3 had Sézary syndrome and responded well to bexarotene capsules; however, he developed Hodgkin's disease with extensive liver involvement that was refractory to chemotherapy.
A CTCL clinic is held at Baylor every other Thursday in the Collins Building and is staffed by Drs. Jennifer Cather (dermatologist) and Estil Vance (oncologist and infectious disease specialist); both have extensive experience in the diagnosis and treatment of CTCL. Patients are evaluated, and treatment options are proposed to both the patient and referring doctor. Longterm follow-up is needed for patients with MF and Sézary syndrome to ensure stability of their disease and to provide surveillance for possible secondary malignancies, especially other lymphomas and lung cancer (38). All investigative procedures and therapies described in this article are available to patients through the clinic.
References
- 1.Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999;89:1240–1244. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood GS. Lymphocyte activation in cutaneous T-cell lymphoma. J Invest Dermatol. 1995;105(1 Suppl):105S–109S. doi: 10.1111/1523-1747.ep12316249. [DOI] [PubMed] [Google Scholar]
- 3.Dereure O, Levi E, Vonderheid EC, Kadin ME. Infrequent Fas mutations but no Bax or p53 mutations in early mycosis fungoides: a possible mechanism for the accumulation of malignant T lymphocytes in the skin. J Invest Dermatol. 2002;118:949–956. doi: 10.1046/j.1523-1747.2002.01794.x. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 5.Agnarsson BA, Vonderheid EC, Kadin ME. Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol. 1990;22:569–577. doi: 10.1016/0190-9622(90)70074-r. [DOI] [PubMed] [Google Scholar]
- 6.Rappaport H, Thomas LB. Mycosis fungoides: the pathology of extracutaneous involvement. Cancer. 1974;34:1198–1229. doi: 10.1002/1097-0142(197410)34:4<1198::aid-cncr2820340431>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Olerud JE, Kulin PA, Chew DE, Carlsen RA, Hammer SP, Weir TW, Patterson SD, Bolen JW, Kadin ME, Barker E, et al. Cutaneous T-cell lymphoma: evaluation of pretreatment skin biopsy specimens by a panel of pathologists. Arch Dermatol. 1992;128:501–507. doi: 10.1001/archderm.128.4.501. [DOI] [PubMed] [Google Scholar]
- 8.Smoller BR, Bishop K, Glusac E, Kim YH, Hendrickson M. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423–1430. doi: 10.1097/00000478-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Smoller BR, Bishop K, Glusac E, Kim YH, Bhargava V, Warnke RA. Reassessment of lymphocyte immunophenotyping in the diagnosis of patch and plaque stage lesions of mycosis fungoides. Applied Immunohistochemistry. 1995;3:32–36. [Google Scholar]
- 10.Wood GS. Analysis of clonality in cutaneous T cell lymphoma and associated diseases. Ann N Y Acad Sci. 2001;941:26–30. [PubMed] [Google Scholar]
- 11.Bunn PA, Jr, Lamberg SI. Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep. 1979;63:725–728. [PubMed] [Google Scholar]
- 12.Fraser-Andrews EA, Woolford AJ, Russell-Jones R, Seed PT, Whittaker S. Detection of a peripheral blood T cell clone is an independent prognostic marker in mycosis fungoides. J Invest Dermatol. 2000;114:117–121. doi: 10.1046/j.1523-1747.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 13.Kashani-Sabet M, McMillan A, Zackheim HS. A modified staging classification for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2001;45:700–706. doi: 10.1067/mjd.2001.117722. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Jensen RA, Watanabe GL, Varghese A, Hoppe RT. Clinical stage IA (limited patch and plaque) mycosis fungoides: a long-term outcome analysis. Arch Dermatol. 1996;132:1309–1313. [PubMed] [Google Scholar]
- 15.Kim YH, Chow S, Varghese A, Hoppe RT. Clinical characteristics and longterm outcome of patients with generalized patch and/or plaque (T2) mycosis fungoides. Arch Dermatol. 1999;135:26–32. doi: 10.1001/archderm.135.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Crowley JJ, Nikko A, Varghese A, Hoppe RT, Kim YH. Mycosis fungoides in young patients: clinical characteristics and outcome. J Am Acad Dermatol. 1998;38(5 Pt 1):696–701. doi: 10.1016/s0190-9622(98)70198-7. [DOI] [PubMed] [Google Scholar]
- 17.Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/Sézary syndrome: clinical characteristics and prognosis. Blood. 1998;92:1150–1159. [PubMed] [Google Scholar]
- 18.Wasik MA, Vonderheid EC, Bigler RD, Marti R, Lessin SR, Polansky M, Kadin ME. Increased serum concentration of soluble interleukein-2 receptor in cutaneous T-cell lymphoma. Arch Dermatol. 1996;132:42–47. [PubMed] [Google Scholar]
- 19.Kaye FJ, Bunn PA, Jr, Steinberg SM, Stocker JL, Ihde DC, Fischmann AB, Glatstein EJ, Schechter GP, Phelps RM, Foss FM, et al. A randomized trial comparing combination electron-beam radiation and chemotherapy with topical therapy in the initial treatment of mycosis fungoides. N Engl J Med. 1989;321:1784–1790. doi: 10.1056/NEJM198912283212603. [DOI] [PubMed] [Google Scholar]
- 20.Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides: experience in 79 patients. Arch Dermatol. 1998;134:949–954. doi: 10.1001/archderm.134.8.949. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe RT, Abel EA, Deneau DG, Price NM. Mycosis fungoides: management with topical nitrogen mustard. J Clin Oncol. 1987;5:1796–1803. doi: 10.1200/JCO.1987.5.11.1796. [DOI] [PubMed] [Google Scholar]
- 22.Zackheim HS, Epstein EH, Jr, Crain WR. Topical carmustine (BCNU) for cutaneous T cell lymphoma: a 15-year experience in 143 patients. J Am Acad Dermatol. 1990;22:802–810. doi: 10.1016/0190-9622(90)70112-u. [DOI] [PubMed] [Google Scholar]
- 23.Breneman D, Duvic M, Kuzel T, Yocum R, Truglia J, Stevens VJ. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol. 2002;138:325–332. doi: 10.1001/archderm.138.3.325. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann JJ, Roenigk HH, Jr, Hurria A, Kuzel TM, Samuelson E, Rademaker AW, Rosen ST. Treatment of mycosis fungoides with photochemotherapy (PUVA): long-term follow-up. J Am Acad Dermatol. 1995;33:234–242. doi: 10.1016/0190-9622(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 25.Clark C, Dawe RS, Evans AT, Lowe G, Ferguson J. Narrowband TL-01 phototherapy for patch-stage mycosis fungoides. Arch Dermatol. 2000;136:748–752. doi: 10.1001/archderm.136.6.748. [DOI] [PubMed] [Google Scholar]
- 26.Jones GW, Hoppe RT, Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1057–1076. [PubMed] [Google Scholar]
- 27.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 28.Bunn PA, Jr, Ihde DC, Foon KA. The role of recombinant interferon alfa- 2a in the therapy of cutaneous T-cell lymphomas. Cancer. 1986;57(8 Suppl):1689–1695. doi: 10.1002/1097-0142(19860415)57:8+<1689::aid-cncr2820571311>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Roenigk HH, Jr, Kuzel TM, Skoutelis AP, Springer E, Yu G, Caro W, Gilyon K, Variakojis D, Kaul K, Bunn PA, Jr, et al. Photochemotherapy alone or combined with interferon alpha-2a in the treatment of cutaneous T cell lymphoma. J Invest Dermatol. 1990;95(6 Suppl):198S–205S. doi: 10.1111/1523-1747.ep12875523. [DOI] [PubMed] [Google Scholar]
- 30.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, Vonderheid E, Knobler R, Wolff K, Plewig G, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy: preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 31.Duvic M, Lemak NA, Redman JR, Eifel PJ, Tucker SL, Cabanillas FF, Kurzrock R. Combined modality therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 1996;34:1022–1029. doi: 10.1016/s0190-9622(96)90282-0. [DOI] [PubMed] [Google Scholar]
- 32.Zackheim HS, Kashani-Sabet M, Hwang ST. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. J Am Acad Dermatol. 1996;34:626–631. doi: 10.1016/s0190-9622(96)80062-4. [DOI] [PubMed] [Google Scholar]
- 33.Kurzrock R, Pilat S, Duvic M. Pentostatin therapy of T-cell lymphomas with cutaneous manifestations. J Clin Oncol. 1999;17:3117–3121. doi: 10.1200/JCO.1999.17.10.3117. [DOI] [PubMed] [Google Scholar]
- 34.Bunn PA, Jr, Hoffman SJ, Norris D, Golitz L, Aeling JL. Systemic therapy of cutaneous T-cell lymphomas (mycosis fungoides and the Sézary syndrome) Ann Intern Med. 1994;121:592–602. doi: 10.7326/0003-4819-121-8-199410150-00007. [DOI] [PubMed] [Google Scholar]
- 35.Duvic M, Cather JC. Immunotoxin DAB389-interleukin-2 (ONTAK) in the management of cutaneous T cell lymphoma. Current Practice of Medicine. 1999;2(5):167–170. [Google Scholar]
- 36.Burt RK, Guitart J, Traynor A, Link C, Rosen S, Pandolfino T, Kuzel TM. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides: evidence of a graft-versus-tumor effect. Bone Marrow Transplant. 2000;25:111–113. doi: 10.1038/sj.bmt.1702099. [DOI] [PubMed] [Google Scholar]
- 37.Molina A, Nademanee A, Arber DA, Forman SJ. Remission of refractory Sézary syndrome after bone marrow transplantation from a matched unrelated donor. Biol Blood Marrow Transplant. 1999;5:400–404. doi: 10.1016/s1083-8791(99)70017-0. [DOI] [PubMed] [Google Scholar]
- 38.Vakeva L, Pukkala E, Ranki A. Increased risk of secondary cancers in patients with primary cutaneous T cell lymphoma. J Invest Dermatol. 2000;115:62–65. doi: 10.1046/j.1523-1747.2000.00011.x. [DOI] [PubMed] [Google Scholar]