Abstract
We have investigated the adherence of Burkholderia pseudomallei, cultured under a number of different conditions, to six human epithelial cell lines. While several complex medium compositions had relatively little effect on adherence, growth at 30°C was found to significantly increase adherence to all cell lines relative to that of cultures grown at 37°C (P < 0.001).
Melioidosis is a disease of humans and animals, the etiologic agent of which is Burkholderia pseudomallei. The major region of endemicity encompasses Southeast Asia and northern Australia, though this is considered to be expanding (8, 10, 11, 24, 25). B. pseudomallei is a gram-negative saprophytic bacterium which can frequently be found inhabiting the soil and water of regions of endemicity (9, 35). While infections acquired from the environmental reservoir via percutaneous inoculation have been documented and this is considered the major mode of infection, the roles of inhalation and ingestion are less clear (7-9, 17). Person-to-person or animal-to-person transmission of melioidosis is considered to be rare (7-9). Disease presentation is diverse, including chronic and acute forms of localized and disseminated infection, with the lungs, liver, and spleen being the most commonly affected organs (25). Melioidosis is associated with a high fatality rate and is a major cause of death from community-acquired bacteremic pneumonia in regions of endemicity (7, 23).
Adherence of pathogens to host surfaces is a prerequisite step in the pathogenesis of almost all infectious diseases. Bacterial adherence requires the specific interaction of bacterial molecules, termed adhesins, with host cell membrane molecules or extracellular matrix proteins (2, 16). A modification of this typical interaction is employed by the attaching and effacing enteropathogens which insert the bacterial protein Tir into host cell membranes for use as the receptor for the bacterial adhesin intimin (21). Disruption of the adhesin-receptor interaction in many paradigm systems, by mutagenesis or competitive inhibition, resulting in a nonadherent phenotype, is typically associated with a significant reduction in virulence of the pathogen (16, 26, 36). Conversely, the elucidation of specific roles of individual adhesins is complicated by redundancy attributable to the presence of multiple adhesins, as is the case with many species, for example Bordetella pertussis (29).
A significant effort has been put into preliminary characterization of B. pseudomallei interactions with eukaryotic cells, in particular the ability of B. pseudomallei to survive within mammalian phagocytic cells. It has been shown that B. pseudomallei has the ability to survive within polymorphonuclear leukocytes (12, 19, 27), macrophages (13, 14, 19, 27), and nonphagocytic cell lines (14, 19). Similar interactions with Acanthamoeba have also been described (18). Recently, B. pseudomallei has been reported to cause mammalian cell fusion (22), an apparently novel phenotype among those displayed by bacterial pathogens. Relative to the interest in intracellular survival, the preceding steps in pathogenesis, including adherence to host surfaces, have received scant attention. There has been one report of adherence of B. pseudomallei to human pharyngeal cells, from which it was concluded that B. pseudomallei displayed low attachment ability (1). Despite this, intrabronchial inoculation of mice with B. pseudomallei resulted in significant bacterial loads in the lungs, indicating that B. pseudomallei has the ability to adhere to the respiratory tract in vivo (1).
Given the importance of adherence to host surfaces in microbial pathogenesis, we decided to investigate the adherence of B. pseudomallei. It was our intention to clarify this situation by developing an in vitro model of adherence using the standard methodology of allowing bacteria to adhere to epithelial cell lines in culture, as opposed to primary cells shaking in suspension (1).
Identification of the B. pseudomallei adherent phenotype.
In order to see if B. pseudomallei was capable of adhering to epithelial cells, a number of variables were tested. This was to consider the possibility that any potential adherent phenotype could be regulated by growth conditions or possibly be specific for different types of cells. We decided to test adherence to six epithelial cell lines, each derived from different tissues. The cell lines A549, NCI-H292, HEp-2, KB, Chang, and ME-180 were derived from alveolar, bronchial, laryngeal, oral, conjunctival, and cervical tissues, respectively. B. pseudomallei strain 08 (6) cultures were grown in Luria-Bertani (LB) medium containing high salt, low salt, and high salt-low iron concentrations at temperatures of 30 and 37°C. All assays were conducted in triplicate with the nonadherent Escherichia coli strain DH5α as a negative control (32). The statistical significance of the differences between the means was assessed using a one-way analysis of variance performed with the Bonferroni post hoc test. The results from these experiments (Fig. 1A) show that B. pseudomallei grown at 30°C adheres to each cell line significantly more than B. pseudomallei grown at 37°C or DH5α (P < 0.001). While the levels of adherence to each individual cell line show considerable variation, there is no outstanding lack of adherence to any particular cell line compared to the other cell lines. This is perhaps indicative of the broad tropism of B. pseudomallei. While this might be the case, experimental evidence from many groups suggesting the ability of B. pseudomallei to survive within macrophages (13, 14, 19, 27) indicates that organ tropism may be complicated by dissemination within such cells (34). Bacteria grown in low-salt and high-salt-low-iron media adhered to a similar degree to that of those grown in high-salt media for each tested cell line (results not shown). Three other strains of B. pseudomallei (RBH, THP375, and 03) (6) grown at 30 and 37°C in standard LB medium were also tested for adherence to ME-180 cells. While there was some variation in the degree of adherence displayed by each strain, in each case the 30 and 37°C phenotypes paralleled that seen with strain 08 (results not shown).
FIG. 1.
Adherence of B. pseudomallei, grown under different conditions, to various cell lines. (A) Adherence of B. pseudomallei strain 08 grown at 30 and 37°C and E. coli DH5α grown at 37°C to ME-180, KB, Chang, A549, NCI-H292, and HEp-2 cells. (B) Adherence of B. pseudomallei 08 grown to early logarithmic and stationary phases at 30 and 37°C to ME-180 cells. Percent adherence was determined by dividing the number of adherent bacteria by the inoculum and multiplying by 100.
To examine whether this apparent increase in the adherence of B. pseudomallei grown at 30°C relative to adherence with growth at 37°C was an artifact, E. coli DH5α grown at these temperatures was tested for adherence to ME-180 cells. Adherence of cultures grown at 37 and 30°C was found to be 1.33% ± 0.09% and 1.04% ± 0.08%, respectively. Similarly, strain E264 of the closely related but relatively avirulent species Burkholderia thailandensis (3, 4) was grown at 37 and 30°C and the degrees of adherence to ME-180 cells were 4.76% ± 0.33% and 4.61% ± 1.21%, respectively. This is potentially another important difference between these species, in addition to the previously demonstrated difference in virulence (3, 4). These results clearly demonstrate that the adherence phenotypes displayed by B. pseudomallei grown at 30 and 37°C are not an artifact of the methods employed in this investigation. The apparent temperature regulation of adherence could be the reason why a previous study found B. pseudomallei to adhere to pharyngeal cells only at low levels (1).
All of the preceding experiments were conducted with stationary-phase cultures, and even though cultures grown at 30°C were of an optical density equivalent to that of those grown at 37°C at the time of assay, it was decided that any potential effects of the growth phase should be identified at each temperature. The results shown in Fig. 1B clearly demonstrate that bacteria from stationary-phase 30°C cultures adhered significantly more than logarithmic-phase 30°C cultures and both logarithmic- and stationary-phase 37°C cultures (P < 0.001). A modest increase in adherence was observed in the logarithmic-phase 37°C cultures in comparison to the stationary-phase 37°C cultures. Taken together, with no information regarding the molecular basis of the demonstrated phenotypes, adherence would appear to be regulated in a complex manner.
Characterization of temperature effects on B. pseudomallei adherence.
The fact that B. pseudomallei cells grown at 30°C adhered significantly more to epithelial cells than those grown at 37°C led us to consider the optimal growth temperature for bacterial adherence to ME-180 cells. Cultures were therefore grown at 5°C intervals from 22 to 37°C. As can be seen from the results shown in Fig. 2A, the optimal temperature for adherence appeared to be 27°C.
FIG. 2.
The effect of temperature on B. pseudomallei adherence to ME-180 cells. (A) Adherence of B. pseudomallei strain 08 cultures grown at 22, 27, 32, and 37°C. (B) Adherence of B. pseudomallei strain 08 cultures to ME-180 cell monolayers following incubation times ranging from 15 min to 8 h. (C) Adherence of B. pseudomallei strain 08 to ME-180 cells following a temperature shift. B. pseudomallei strain 08 was grown at 30°C to stationary phase before being incubated at 37°C in LB medium for defined lengths of time prior to assaying adherence to ME-180 cells. Percent adherence was determined by dividing the number of adherent bacteria by the inoculum and multiplying by 100.
The temperature regulation of adherence is interesting when the following is considered. Bacteria grown at 37°C do not show appreciable adherence to host cells, whereas bacteria grown at 30°C do show significant degrees of adherence, and these bacteria are adhering during incubation at 37°C. We therefore investigated the effect of incubation with ME-180 cells at 37°C by performing a time course experiment, in order to see if the incubation at 37°C progressively decreased the levels of adherence. This involved quantifying adherence following incubation for up to 8 h during the assay itself. The results for the 30 and 37°C cultures indicate that levels of adherence progressively increased with incubation time (Fig. 2B), presumably due to the longer period, which allowed attachment and increases in bacterial cell numbers. Given this result, adherence assays were then performed using cultures grown to stationary phase at 30°C, followed by incubation at 37°C for defined lengths of time, before use in a standard 2-h incubation adherence assay. The results shown in Fig. 2C clearly illustrate that, following growth at 30°C, an incubation of 15 min at 37°C prior to conducting the assay is enough to cause a significant reduction in adherence (P < 0.005). These results are not due to a decrease in bacterial viability following the temperature shift from 30 to 37°C (results not shown). The mechanism for the reduction of adherence manifested following a temperature shift in LB medium from 30 to 37°C could involve a requirement for de novo protein synthesis for the maintenance of adhesins, assuming adhesins are not synthesized at 37°C in LB medium. Indeed, it has been demonstrated that Haemophilus influenzae adherence requires de novo protein synthesis (31). To test this hypothesis, we incubated strain 08 cultures grown overnight at 30°C with tetracycline (10 μg ml−1) at 30°C and without tetracycline at 37°C for 2 h prior to assaying adherence. The results for these experiments were 10.01% ± 1.07% for the tetracycline culture and 11.93% ± 2.97% for the 30-to-37°C temperature-shifted culture, which were both significantly different from the result for the 30°C control culture without tetracycline (50.00% ± 3.92%, P < 0.001). This indicates that de novo protein synthesis is required for B. pseudomallei adherence. In addition, the similarity of results for protein synthesis inhibition at 30°C and the temperature shift to 37°C could indicate that adhesin molecules are not synthesized at 37°C.
The temperature shift experiments described above present an interesting point: bacteria grown at 30°C and incubated at 37°C in LB medium without epithelial cells show a significant reduction in adherence, whereas those incubated at 37°C with epithelial cells in cell culture medium during the assay maintain high levels of adherence. To address this, another temperature shift experiment was devised to uncouple any potential influence of the cell culture medium from the epithelial cells on adherence. Cultures used in the assay were grown at 30°C to stationary phase, pelleted by centrifugation, resuspended in cell culture medium (McCoy's 5a medium), and incubated at either 30 or 37°C for 4 h prior to adherence assays. Respectively, these results were 91.65% ± 21.19% and 31.96% ± 2.94%. Comparison with the results shown in Fig. 2C indicates that incubation of 30°C-grown stationary-phase bacteria at 37°C in cell culture medium allows for a substantial maintenance of adherence relative to incubation in LB medium (P < 0.005). Incubation of 30°C-grown stationary-phase bacteria in cell culture medium at 30°C also appeared to increase the levels of detectable adherence to ME-180 cells when compared to that of bacteria from 30°C stationary-phase cultures grown in LB medium. Hence, the regulation of adherence appears to involve signals in addition to growth temperature. In Pseudomonas aeruginosa, the type III secreted protein, ExoS, has been shown to be induced in response to growth in cell culture medium (33), and it is perhaps likely that B. pseudomallei adhesins are similarly induced.
The relevance to pathogenesis of a highly adherent phenotype only when bacteria are grown at temperatures below 37°C certainly merits discussion. It is especially noteworthy that B. pseudomallei is an environmental bacterium, and infection is almost exclusively acquired from soil and water rather than from infected animals or humans. Taking into account the temperature regulation of adherence indicated by these experiments, we propose that this may be important for initial adherence to the host following an encounter from the environmental reservoir. A similar temperature regulation of adherence is shown by Bordetella bronchiseptica (28). In this case, the relative increase in adherence of bacteria grown at the lower temperature is less than that seen with B. pseudomallei, due mainly to the fact that B. bronchiseptica displays relatively high levels of adherence when grown at 37°C (28). Importantly, this temperature regulation of adherence in B. bronchiseptica is reflected by colonization levels in vivo, suggesting an important role in overcoming initial clearance mechanisms (5). The precise temperatures of typical environmental reservoirs of B. pseudomallei are unknown, and as such the potential relevance of such regulation is speculative. A major paradigm of virulence gene regulation is the ToxR regulon of Vibrio cholerae, and this system is influenced by a number of environmental stimuli, including temperature (30). Included in the ToxR regulon are the toxin coregulated pilus and accessory colonization factors (30). During in vitro growth of classical biotype strains in LB medium, the growth temperature for optimal expression of the regulon is 30°C (30), though this is not considered to be relevant to natural infections. Hence, it remains a formal possibility that the temperature regulation of adherence described herein is simply an artifact of in vitro culture of B. pseudomallei.
Qualitative investigation of B. pseudomallei adherence using microscopy.
In order to confirm both that B. pseudomallei strain 08 was indeed adhering to epithelial cells and that the levels were increased in cultures grown at 30°C, monolayers were Giemsa stained (pH 6.7) and examined by light microscopy. These experiments were conducted using ME-180 cells grown on coverslips. Light microscopy indeed confirmed that bacteria were adhering to cells and that they were far more prevalent in cultures grown at 30°C (Fig. 3). Typically, bacteria grown at 30°C could often be seen clustered together in microcolonies, adhering to one or a few adjacent epithelial cells, while many neighboring epithelial cells in the monolayer were left relatively free of bacteria. Observation of the adherence of bacteria grown at 37°C showed that while the number of bacterium-cell contacts was considerably lower, adherent microcolonies were essentially absent compared with that seen in bacteria grown at 30°C. At the incubation time used (2 h), there was no consistent and obvious cytopathic effect common to cells with adherent bacteria when compared to uninfected controls.
FIG. 3.
Digital images recorded from light microscopy of B. pseudomallei strain 08 adhering to ME-180 cells. All samples were stained with Giemsa (pH 6.7) and examined with a 100× objective. (A and B) B. pseudomallei bacteria cultured at 30°C until stationary phase prior to the adherence assay. A large number of adherent bacteria can be observed in adherent microcolonies. (C and D) B. pseudomallei bacteria cultured at 37°C until stationary phase prior to the adherence assay. Few adherent bacteria can be observed. Bar, 10 μm.
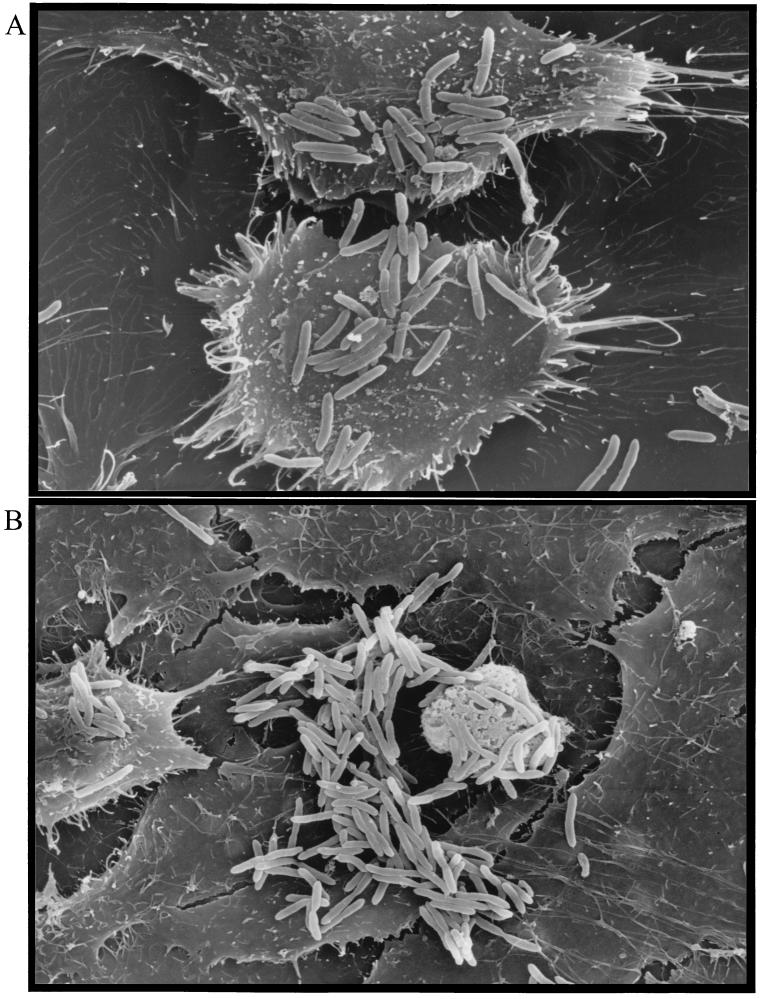
Scanning electron microscopy was utilized to investigate the possible existence of any specific structural features associated with the adherence of B. pseudomallei to epithelial cells. Adherence to all six previously tested cell lines was investigated, and no outstanding structural feature on the surface of the epithelial cells was associated with bacteria-epithelial cell contacts. Likewise, no bacterial structure could be seen mediating attachment to the epithelial cells or to other bacteria. The microcolony formation of adherent bacteria observed was consistent with the results recorded with light microscopy. Epithelial cells associated with adherent microcolonies, in many instances, were associated with many bacteria independent of bacterium-bacterium interactions, and it was apparent that these interactions were intimate (Fig. 4). This illustrates the potential importance of both bacterium-epithelial cell and bacterium-bacterium interactions in the adherence of B. pseudomallei to epithelial cells. It is noteworthy that mechanisms of microcolony formation of other pathogens may involve bacterial as well as host molecules (15, 20).
FIG. 4.
Scanning electron microscopy of B. pseudomallei strain 08 bacteria adhering to epithelial cell lines. (A) Electron micrograph of B. pseudomallei adherence to Chang cells after incubation of bacterial cultures at 30°C until stationary phase, demonstrating bacterium-host and bacterium-bacterium interactions. Magnification, ×3,000. (B) Electron micrograph of B. pseudomallei adherence to A549 cells after incubation of bacterial cultures at 30°C until stationary phase, demonstrating extensive bacterium-host and bacterium-bacterium interactions. Magnification, ×3,600.
Concluding remarks.
This study clearly shows that B. pseudomallei is capable of adhering efficiently to epithelial cell lines. It would appear that the adherent phenotype is regulated in a complex manner by growth temperature, medium composition, and growth phase and that it is dependent on de novo protein synthesis. The development of an animal model which tests preliminary steps in pathogenesis is necessary to test whether this temperature regulation of adherence is important in the pathogenesis of melioidosis. This investigation provides a basis on which to begin the molecular characterization of the bacterial molecules responsible for the initial interactions with host cells as well as the bacterium-bacterium interactions. Since any adhesins are likely to be regulated, experiments involving expression in heterologous hosts may prove challenging. Molecular characterization of the regulatory mechanisms will also be of interest, as will the characterization of the host molecules required for attachment to B. pseudomallei.
Acknowledgments
We are grateful to P. Timms for the gift of the HEp-2 cell line.
N.F.B. is an Australian Postgraduate Award recipient.
Editor: V. J. DiRita
REFERENCES
- 1.Ahmed, K., H. D. R. Enciso, H. Masaki, M. Tao, A. Omori, P. Tharavichikul, and T. Nagatake. 1999. Attachment of Burkholderia pseudomallei to pharyngeal epithelial cells: a highly pathogenic bacteria with low attachment ability. Am. J. Trop. Med. Hyg. 60:90-93. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 3.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 4.Brett, P. J., D. DeShazer, and D. E. Woods. 1997. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol. Infect. 118:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockmeier, S. L. 1999. Early colonisation of the upper respiratory tract by temperature modulated Bordetella bronchiseptica. FEMS Microbiol. Lett. 174:225-229. [DOI] [PubMed] [Google Scholar]
- 6.Brown, N. F., and I. R. Beacham. 2000. Cloning and analysis of genomic differences unique to Burkholderia pseudomallei by comparison with B. thailandensis. J. Med. Microbiol. 49:993-1001. [DOI] [PubMed] [Google Scholar]
- 7.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. C. Burrow, D. Lo, S. Selva-Nayagam, N. M. Anstey, S. E. Huffam, P. L. Snelling, P. J. Marks, D. P. Stephens, G. D. Lum, S. P. Jacups, and V. L. Krause. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981-986. [DOI] [PubMed] [Google Scholar]
- 8.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. C. Burrow, S. Selvanayagam, P. L. Snelling, N. M. Anstey, and M. J. Mayo. 2000. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 74:121-127. [DOI] [PubMed] [Google Scholar]
- 9.Dance, D. A. B. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74:159-168. [DOI] [PubMed] [Google Scholar]
- 10.Dance, D. A. B. 2000. Melioidosis as an emerging global problem. Acta Trop. 74:115-119. [DOI] [PubMed] [Google Scholar]
- 11.Dance, D. A. B. 1991. Melioidosis: the tip of the iceberg? Clin. Microbiol. Rev. 4:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harley, V. S., D. A. B. Dance, G. Tovey, and B. S. Drasar. 1994. Interaction of Pseudomonas pseudomallei with macrophages. Biochem. Soc. Trans. 22:88S. [DOI] [PubMed]
- 14.Harley, V. S., D. A. B. Dance, G. Tovey, M. V. McCrossan, and B. S. Drasar. 1998. An ultrastructural study of the phagocytosis of Burkholderia pseudomallei. Microbios 94:35-45. [PubMed] [Google Scholar]
- 15.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 16.Hultgren, S. J., S. Abraham, M. Caparon, P. Falk, J. W. St. Geme III, and S. Normark. 1993. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell 73:887-901. [DOI] [PubMed] [Google Scholar]
- 17.Inglis, T. J. J., S. C. Garrow, M. Henderson, A. Clair, J. Sampson, L. O'Reilly, and B. Cameron. 2000. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg. Infect. Dis. 6:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inglis, T. J. J., P. Rigby, T. A. Robertson, N. S. Dutton, M. Henderson, and B. J. Chang. 2000. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallstrom, H., D. B. Gill, B. Albiger, M. K. Liszewski, J. P. Atkinson, and A.-B. Jonsson. 2001. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell. Microbiol. 3:133-143. [DOI] [PubMed] [Google Scholar]
- 21.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 22.Kespichayawattana, W., R. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leelarasamee, A. 1998. Burkholderia pseudomallei: the unbeatable foe? Southeast Asian J. Trop. Med. Public Health 29:410-415. [PubMed] [Google Scholar]
- 24.Leelarasamee, A. 2000. Melioidosis in Southeast Asia. Acta Trop. 74:129-132. [DOI] [PubMed] [Google Scholar]
- 25.Leelarasamee, A., and S. Bovornkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413-425. [DOI] [PubMed] [Google Scholar]
- 26.Ofek, I., I. Kahane, and N. Sharon. 1996. Toward anti-adhesin therapy for microbial diseases. Trends Microbiol. 4:297-299. [DOI] [PubMed] [Google Scholar]
- 27.Pruksachartvuthi, S., N. Aswapokee, and K. Thankerngpol. 1990. Survival of Pseudomonas pseudomallei in human phagocytes. J. Med. Microbiol. 31:109-114. [DOI] [PubMed] [Google Scholar]
- 28.Register, K. B., and M. R. Ackermann. 1997. A highly adherent phenotype associated with virulent Bvg+-phase swine isolates of Bordetella bronchiseptica grown under modulating conditions. Infect. Immun. 65:5295-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αmβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 30.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 31.St. Geme, J. W., III, and S. Falkow. 1990. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect. Immun. 58:4036-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St. Geme, J. W., III, S. Falkow, and S. J. Barenkamp. 1993. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. USA 90:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallis, A. J., T. L. Yahr, J. T. Barbieri, and D. W. Frank. 1999. Regulationof ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 35.Vuddhakul, V., P. Tharavichitkul, N. Na-Ngam, S. Jitsurong, B. Kunthawa, P. Noimay, P. Noimay, A. Binla, and V. Thamlikitkul. 1999. Epidemiology of Burkholderia pseudomallei in Thailand. Am. J. Trop. Med. Hyg. 60:458-461. [DOI] [PubMed] [Google Scholar]
- 36.Wizemann, T. M., J. E. Adamou, and S. Langermann. 1999. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 5:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]