Abstract
Experimental severe malaria (ESM; also known as experimental cerebral malaria) is an acute lethal syndrome caused by infection with Plasmodium berghei ANKA and associated with coma and other neurological manifestations in mice. Various inbred strains of mice exhibit differences in susceptibility to the development of ESM. For example, C57BL/6 mice are highly susceptible and DBA/2 mice are relatively resistant. We report here the results of a genomewide scan for host genomic regions that control resistance to ESM in DBA/2 mice using an F2 intercross population of susceptible and resistant strains. A region of mid-chromosome 18 was found to be a major determinant of resistance to ESM.
Infection of some mouse strains with Plasmodium berghei ANKA leads to the development of an acute syndrome associated with coma and other neurological manifestations, usually resulting in death in 48 h after onset (7, 17). This syndrome is commonly known as experimental cerebral malaria or experimental severe malaria (ESM).
A number of studies using antibodies, recombinant cytokines, and knockout mice have shown that ESM is an immunopathological process mediated by proinflammatory cytokines and other immune system components. Depletion of CD4+ or CD8+ T cells (9), tumor necrosis factor (18), gamma interferon (19), or intercellular adhesion molecule 1 (3) prevents the disease.
Histologically, a breakdown of the blood-brain barrier and intravascular sequestration of macrophages, polymorphonuclear neutrophils, and platelets are the most prominent events (2, 16, 20, 23). Because alterations of the microcirculation are not restricted to brain vessels (1, 3, 20), some authors prefer to call this syndrome ESM rather than experimental cerebral malaria (16, 20).
Various inbred strains of mice exhibit differences in susceptibility to the development of ESM. For example, the C57BL/6 (B6) strain is highly susceptible (10, 14); almost all infected mice die from ESM 6 to 12 days after infection, when their parasitemia (percentage of parasitized erythrocytes) is below 20%. In contrast, DBA/2 (D2) is relatively resistant (15); the majority of infected mice survive the period of ESM and die much later (>20 days after infection) without neurological implications. This late phase of death is thought to be due to severe anemia and an overwhelming parasitemia (usually over 60%) (7). Little is known about the genetic basis of the different susceptibilities to ESM.
To detect the host genomic region responsible for resistance to ESM, an F2 intercross of the susceptible (B6) and resistant (D2) mouse strains was produced and a genomewide scan using polymorphic microsatellite markers was carried out. In this analysis, we identified a region of mid-chromosome 18 that shows significant linkage to ESM resistance.
MATERIALS AND METHODS
Mice.
C57BL/6NCrj, DBA/2NCrj, and (C57BL/6NCrj × DBA/2NCrj) F1 mice were purchased from Charles River, Japan (Kanagawa, Japan). F1 males and females were bred to generate F2 mice and were housed in our facilities. All animal experiments were approved by the committee for guidelines and regulation of animal experiments in our institute.
Parasites and infection.
P. berghei ANKA was a kind gift from M. Torii (Ehime University, Matsuyama, Japan). Mice 8 weeks of age were infected with P. berghei ANKA by intraperitoneal inoculation with 105 parasitized red blood cells obtained from male F1 mice. Giemsa-stained blood smears were examined to monitor parasitemia twice a day from day 4.
Determination of phenotype.
The phenotypes for the ESM susceptibility trait were determined based on survival until day 14. Mice surviving to day 14 were defined as ESM resistant; those dying before day 14 were defined as ESM susceptible.
Genotyping.
Genomic DNA was prepared from mice prior to infection. Fluorescently labeled primers for a PCR were either obtained from Research Genetics (Huntsville, Ala.) or synthesized in accordance with sequences obtained through the Whitehead Institute/Massachusetts Institute of Technology mouse genome database (www.genome.wi.mit.edu/cgi-bin/mouse/index). PCR was performed with 96-well plates and a GeneAmp PCR system 9700 (Perkin-Elmer, Norwalk, Conn.). PCR products were analyzed by an ABI Prism 3700 GeneScan system (Perkin-Elmer).
Ninety-three informative microsatellite markers were used, and 87% of the genome was within 10 cM of a marker. All F2 mice (82 males and 101 females) were genotyped.
Determination of statistical significance.
To calculate the linkage of individual markers to ESM, 2 × 2 contingency tables (χ2 test of independence) were applied (21), where one category was the phenotype data (ESM susceptible or ESM resistant) and the other was the genotype data at each marker. Additive, dominant, and recessive models were tested. For the additive model, P values of less than 3.4 × 10−3 and 1.0 × 10−4 were considered to indicate suggestive and significant linkages, respectively. For the dominant and recessive models, the suggestivity level was 2.4 × 10−3 and the significance level was 7.2 × 10−5 (12).
RESULTS
Experimental infection and phenotype determination.
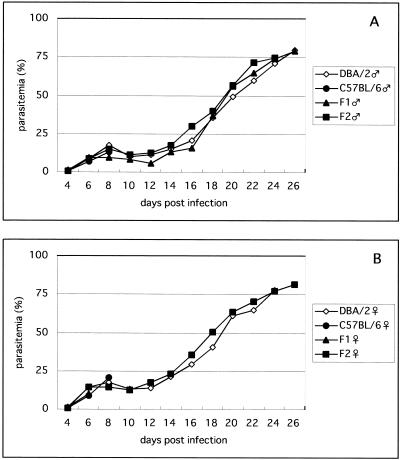
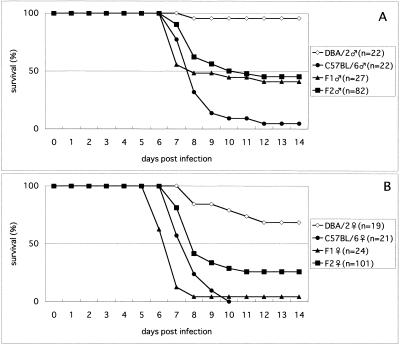
The time courses of parasitemia are shown in Fig. 1. No major difference in parasitemia between strains or between sexes was observed. Most deaths from ESM occurred on days 7 and 8, when the parasitemia was about 15% (Fig. 1 and 2). The phenotypes for the ESM susceptibility trait of all of the mice tested are summarized in Table 1. Almost all of the B6 mice were ESM susceptible, with 95% of the males and 100% of the females dying between days 6 and 12 (Fig. 2). Many mice exhibited symptoms such as ataxia and coma. In contrast, almost all (95%) of the male D2 mice and a majority (68%) of the female D2 mice were ESM resistant and survived the 6- to 12-day period of ESM with only minor symptoms and died after day 20. This late phase of death was not accompanied by the symptoms described above and was presumably due to severe anemia and overwhelming parasitemia. Most of the animals that died in this late phase showed very high parasitemia (more than 60%) before death, as expected (Fig. 1). Of the male F1 mice, 59% were ESM susceptible and 41% were resistant. On the other hand, 96% of the female F1 mice were ESM susceptible, like the B6 females. The phenotypes of the F2 mice were also determined and subjected to linkage analysis.
FIG. 1.
Courses of parasitemia in D2, B6, F1, and F2 mice infected with P. berghei ANKA. Parasitemias from Giemsa-stained blood films were monitored from day 4. The average value of surviving mice in each group is presented. A, male mice; B, female mice.
FIG. 2.
Survival of D2, B6, F1, and F2 mice infected with P. berghei ANKA. A, male mice; B, female mice.
TABLE 1.
Summary of the ESM susceptibility trait in parental strains and F1 and F2 hybrids
| Mice | No. (%) ESM susceptiblea | No. ESM resistantb | Total no. |
|---|---|---|---|
| Males | |||
| B6 | 21 (95) | 1 | 22 |
| D2 | 1 (5) | 21 | 22 |
| F1 | 16 (59) | 11 | 27 |
| F2 | 45 (55) | 37 | 82 |
| Females | |||
| B6 | 21 (100) | 0 | 21 |
| D2 | 6 (32) | 13 | 19 |
| F1 | 27 (96) | 1 | 28 |
| F2 | 75 (74) | 26 | 101 |
Mice dead by day 14 postinfection.
Mice that survived beyond day 14 postinfection.
Linkage analysis.
A genomewide screen was carried out with 93 markers and DNA from 183 F2 mice. Results of chromosomes that showed at least suggestive linkage are shown in Table 2. When combined data of both sexes were analyzed, two markers in the middle region of chromosome 18 (D18Mit123 and D18Mit202) showed the strongest linkage to ESM. P values for both markers (4.1 × 10−8 and 5.7 × 10−6) were less than the significance level (P < 1.0 × 10−4; recessive model). We thus concluded that a major determinant that strongly affects susceptibility to ESM is located in this chromosomal segment. Markers on chromosomes 5, 8, and 14 (D5Mit10, D5Mit136, D8Mit249, D14Mit206, and D14Mit54) showed suggestive linkages in the analysis of combined data. The effects of marker loci on chromosomes 18, 5, and 14 were similar to those expected by the parental phenotypes. On the other hand, a marker locus on chromosome 8 had the opposite effect on susceptibility to ESM.
TABLE 2.
Analysis of ESM linkage with chromosomes 18, 5, 8, 11, 14, and 19a
| Chromosome and marker | Position (cM)b | Both sexes
|
Males
|
Females
|
Modele | Susceptibility | |||
|---|---|---|---|---|---|---|---|---|---|
| χ2c | Pd | χ2 | P | χ2 | P | ||||
| 18 | |||||||||
| D18Mit34 | 12 | 13.6 | 2.3 × 10−4 | 6.8 | (−)g | 8.6 | (−) | R | B6>D2f |
| D18Mit202 | 22 | 20.6 | 5.7 × 10−6h | 14.4 | 1.5 × 10−4 | 9.6 | 1.9 × 10−3 | ||
| D18Mit123 | 31 | 30.1 | 4.1 × 10−8 | 21.5 | 3.5 × 10−6 | 9.6 | 1.9 × 10−3 | ||
| D18Mit33 | 44 | 13.0 | 3.2 × 10−4 | 5.2 | (−) | 7.5 | (−) | ||
| D18Mit4 | 57 | 5.3 | (−) | 1.8 | (−) | 3.0 | (−) | ||
| 5 | |||||||||
| D5Mit294 | 8 | 1.4 | (−) | 0.4 | (−) | 1.2 | (−) | R | B6>D2 |
| D5Mit81 | 28 | 8.8 | (−) | 7.6 | (−) | 8.2 | (−) | ||
| D5Mit356 | 41 | 3.3 | (−) | 2.4 | (−) | 3.4 | (−) | ||
| D5Mit10 | 54 | 12.0 | 5.4 × 10−4 | 4.1 | (−) | 11.4 | 3.0 × 10−3 | ||
| D5Mit136 | 65 | 10.1 | 1.5 × 10−3 | 1.1 | (−) | 10.5 | 2.7 × 10−4 | ||
| D5Mit221 | 78 | 1.4 | (−) | 0.0 | (−) | 2.9 | (−) | ||
| 8 | |||||||||
| D8Mit223 | 11 | 0.4 | (−) | 1.1 | (−) | 0.1 | (−) | D | D2>B6 |
| D8Mit191 | 21 | 0.9 | (−) | 2.4 | (−) | 0.1 | (−) | ||
| D8Mit249 | 37 | 11.0 | 8.9 × 10−4 | 3.2 | (−) | 7.4 | (−) | ||
| D8Mit47 | 57 | 2.4 | (−) | 0.6 | (−) | 0.9 | (−) | ||
| D8Mit56 | 73 | 0.2 | (−) | 0.1 | (−) | 0.1 | (−) | ||
| 11 | |||||||||
| D11Mit229 | 14 | 3.9 | (−) | 5.6 | (−) | 0.4 | (−) | A | B6>D2 |
| D11Mit86 | 28 | 1.4 | (−) | 3.9 | (−) | 0.0 | (−) | ||
| D11Mit219 | 43 | 4.5 | (−) | 13.4 | 2.5 × 10−4 | 0.4 | (−) | ||
| D11Mit99 | 59.5 | 1.2 | (−) | 7.8 | (−) | 1.0 | (−) | ||
| D11Mit180 | 66 | 0.8 | (−) | 3.5 | (−) | 0.2 | (−) | ||
| 14 | |||||||||
| D14Mit206 | 2.5 | 8.6 | 3.3 × 10−3 | 1.2 | (−) | 7.7 | (−) | A | B6>D2 |
| D14Mit54 | 12.5 | 9.5 | 2.0 × 10−3 | 5.3 | (−) | 3.5 | (−) | ||
| D14Mit203 | 28.3 | 1.4 | (−) | 1.5 | (−) | 0.1 | (−) | ||
| D14Mit195 | 44.3 | 0.1 | (−) | 0.7 | (−) | 0.1 | (−) | ||
| D14Mit165 | 52 | 1.3 | (−) | 0.2 | (−) | 1.5 | (−) | ||
| 19 | |||||||||
| D19Mit79 | 6 | 1.2 | (−) | 2.1 | (−) | 0.0 | (−) | D | B6>D2 |
| D19Mit46 | 33 | 0.4 | (−) | 0.4 | (−) | 0.1 | (−) | ||
| D19Mit91 | 47 | 0.7 | (−) | 2.1 | (−) | 0.2 | (−) | ||
| D19Mit35 | 53 | 4.6 | (−) | 9.6 | 1.9 × 10−3 | 0.0 | (−) | ||
Results of chromosomes that showed at least suggestive linkages.
From 2000 Chromosome Committee reports (www.informatics.jax.org/ccr/searches/ index.cgi?year=2000).
Calculated by using a 2 × 2 contingency table, where one category is the phenotype data (ESM susceptible or ESM resistant) and the other is the genotype data at each marker.
Calculated in association with χ2 value and χ2 distribution with a single degree of freedom.
Calculations were done with three models; the protective effect of each microsatellite locus works in a dominant (D), recessive (R), or additive (A) manner.
B6>D2 means that mice possessing a B6-derived allele(s) were more susceptible to ESM than those with a D2-derived allele(s).
(−), no indication of linkage.
Underlined values represent significant linkage.
Subsequently, the data on both sexes were analyzed separately. For male mice, D18Mit123 and D18Mit202 showed significant linkage and D11Mit219 and D19Mit35 exhibited suggestive linkage. For female mice, D18Mit123, D18Mit202, D5Mit10, and D5Mit136 exhibited suggestive linkage. The tendency to show linkage of markers on chromosomes 11 and 19 was observed only in male mice. The other markers exhibited the same trend in both sexes.
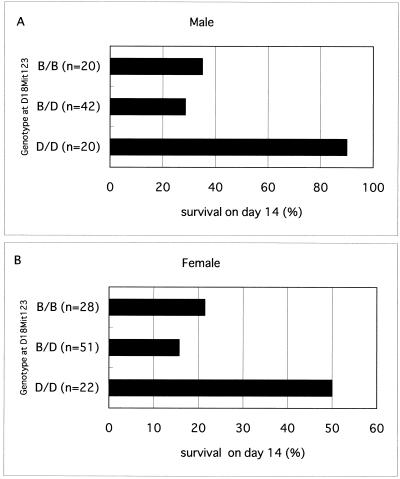
Effects of genotype at D18Mit123 on ESM.
The relationship of the genotype at D18Mit123 to survival rate on day 14 is shown in Fig. 3. The protective effect of this marker locus seemed to be inherited in a recessive manner because heterozygotes for the D18Mit123 alleles were not protected.
FIG. 3.
Relationship of the genotype at D18Mit123 to survival on day 14. B/B, homozygotes for B6-derived allele; B/D, heterozygotes; D/D, homozygotes for D2-derived allele.
DISCUSSION
Inheritance of ESM susceptibility did not follow simple Mendelian patterns and was influenced by the sexual background (female mice were more susceptible than males). The phenotype of ESM susceptibility was incompletely penetrant. This means that a particular genotype does not guarantee the expression of the phenotype in 100% of the F2 animals that carry it. It may indicate involvement of environmental factors in addition to genetic factors.
In the analysis of a locus that segregates in a simple Mendelian manner, the total number of markers required for a first mapping of the locus to a subchromosomal interval is calculated to be 60 when 52 backcross animals are used (22). On the other hand, the greater the genetic complexity, the larger the number of animals that have to be bred and the number of markers that have to be analyzed. We considered the sample size and marker density used in this study (183 animals and 93 markers) to be sufficient to detect the existence of loci that may have a large effect on the phenotypes. However, since loci with moderate effects span a wide range of detectability and cannot necessarily be detected at high power with crosses of reasonable size (13), there might be other potential loci not detected in this genome scan. In addition, 13% of the genomic regions were more than 10 cM from any marker in this study. This also suggests the possibility of the existence of undetected potential loci. Some markers on chromosomes 1, 3, 4, 9, 14, and 17 showed segregation distortion. Although the calculation of chi-square values was not dependent on the theoretical segregation ratio in this study, there is a possibility that our results were affected by the distorted segregation through increased or decreased detection powers.
In spite of the limitations of this study stated above, the effects of genotypes at the D18mit123 marker locus were striking. The chances for survival of male and female animals genotyped D2/D2 at this marker locus were 2.94 and 2.82 times greater than those of mice with the other two genotypes, respectively. Adjacent to this marker (position 31 cM; Chromosome Committee report), there are several interesting candidate genes. These include those for the colony-stimulating factor 1 receptor (Csf1r, position 30 cM), the platelet-derived growth factor receptor, the beta peptide (Pdgfrb, position 30 cM), the CD14 antigen (Cd14, position 31cM), and the Ia-associated invariant chain (Ii, position 32 cM).
Markers on chromosomes 5, 8, 11, 14, and 19 showed suggestive linkages to ESM. Because one suggestive linkage can occur at random at a genome scan (12), some of them may be detected false positively. However, the one on chromosome 8 is interesting. Two independent reports demonstrated the existence of a locus in the middle of this chromosome that controls the peak parasitemia of mice infected with P. chabaudi, another rodent malaria parasite (4; A. Fortin, A. Belouchi, M. F. Tam, L. Cardon, E. Skamene, M. M. Stevenson, and P. Gros, Letter, Nat. Genet. 17:382–383, 1997). Because parasitemia was suppressed transiently during the period of ESM (Fig. 1), a host response that suppresses parasitemia and is influenced by the locus on chromosome 8 may be involved in the development of ESM.
During the period of ESM (days 6 to 12), parasitemias were consistently low and rarely exceeded 30%. Sometimes we had an opportunity to examine parasitemias of mice immediately after their death. Even in such cases, we could not observe high parasitemia (data not shown). Therefore, overwhelming parasitemia did not seem to be the cause of deaths that occurred in this period in not only parental strains but also F2 progenies.
Unexpectedly, we observed marked sex differences in ESM susceptibility. There is a possibility that sex hormones or substances that are regulated by them affect the development of ESM. For example, testosterone is known to influence the outcome of P. chabaudi infection in several strains of mice (25). Some markers exhibited suggestive linkages only in male mice. This may indicate the existence of genes near the markers that show sex-influenced expression.
In humans, infection with P. falciparum can result in severe complications, leading eventually to death, with symptoms ranging from respiratory distress to coma (24). The pathogenesis of coma is poorly understood and might be different from that of mouse ESM. The major difference is that there is no sequestration of parasitized erythrocytes in the microvessels of the mouse brain. Monocytes and lymphocytes are the principal cell populations sequestered in the venules of rodents (11). However, some similarities like elevated serum tumor necrosis factor levels (5, 8) and thrombocytopenia in human (24) and mouse ESM (6) were also reported. The present study will aid further analysis of the actual genes involved in the development of ESM and may lead to better understanding of the pathogenesis of severe malaria in humans.
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Japanese Ministry of Education, Science, Sports and Culture (11470067) and the Japanese Ministry of Health and Welfare (H12-shinko-17).
We thank Nirbhay Kumar of Johns Hopkins University and Walter Stahl of Tokai University for critical reading of the manuscript.
Editor: J. M. Mansfield
REFERENCES
- 1.Carvalho, L. J., H. L. Lenzi, M. Pelajo-Machado, D. N. Oliveira, C. T. Daniel-Ribeiro, and M. F. Ferreira-da-Cruz. 2000. Plasmodium berghei: cerebral malaria in CBA mice is not clearly related to plasma TNF levels or intensity of histopathological changes. Exp. Parasitol. 95:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Clark, I. A., J. D. MacMicking, K. M. Gray, K. A. Rockett, and W. B. Cowden. 1992. Malaria mimicry with tumor necrosis factor. Contrasts between species of murine malaria and Plasmodium falciparum. Am. J. Pathol. 140:325–336. [PMC free article] [PubMed] [Google Scholar]
- 3.Favre, N., C. Da Laperousaz, B. Ryffel, N. A. Weiss, B. A. Imhof, W. Rudin, R. Lucas, and P. F. Piguet. 1999. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1:961–968. [DOI] [PubMed] [Google Scholar]
- 4.Foote, S. J., R. A. Burt, T. M. Baldwin, A. Presente, A. W. Roberts, Y. L. Laural, A. M. Lew, and V. M. Marshall. 1997. Mouse loci for malaria-induced mortality and the control of parasitaemia. Nat. Genet. 17:380–381. [DOI] [PubMed] [Google Scholar]
- 5.Grau, G. E., L. F. Fajardo, P. F. Piguet, B. Allet, P. H. Lambert, and P. Vassalli. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210–1212. [DOI] [PubMed] [Google Scholar]
- 6.Grau, G. E., P. F. Piguet, D. Gretener, C. Vesin, and P. H. Lambert. 1988. Immunopathology of thrombocytopenia in experimental malaria. Immunology 65:501–506. [PMC free article] [PubMed] [Google Scholar]
- 7.Grau, G. E., P. F. Piguet, P. Vassalli, and P. H. Lambert. 1989. Tumor necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol. Rev. 112:49–70. [DOI] [PubMed] [Google Scholar]
- 8.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P. H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586–1591. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen, C., T. van de Wiel, E. Mommers, R. Sauerwein, and W. Eling. 1997. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 114:7–12. [DOI] [PubMed] [Google Scholar]
- 10.Jennings, V. M., J. K. Actor, A. A. Lal, and R. L. Hunter. 1997. Cytokine profile suggesting that murine cerebral malaria is an encephalitis. Infect. Immun. 65:4883–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landau, I., and P. Gautret. 1998. Animal models: rodents, p.401–417. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 12.Lander, E., and L. Kruglyak. 1995. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat. Genet. 11:241–247. [DOI] [PubMed] [Google Scholar]
- 13.Manly, K. F., and J. M. Olson. 1999. Overview of QTL mapping software and introduction to map manager QT. Mamm. Genome 10:327–334. [DOI] [PubMed] [Google Scholar]
- 14.Moumaris, M., C. Sestier, F. Miltgen, A. Halbreich, M. Gentilini, and D. Sabolovic. 1995. Effect of fatty acid treatment in cerebral malaria-susceptible and nonsusceptible strains of mice. J. Parasitol. 81:997–999. [PubMed] [Google Scholar]
- 15.Neill, A. L., and N. H. Hunt. 1992. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology 105:165–175. [DOI] [PubMed] [Google Scholar]
- 16.Piguet, P. F., C. Da Laperrousaz, C. Vesin, F. Tacchini-Cottier, G. Senaldi, and G. E. Grau. 2000. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infect. Immun. 68:3822–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rest, J. R. 1982. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans. R. Soc. Trop. Med. Hyg. 76:410–415. [DOI] [PubMed] [Google Scholar]
- 18.Rudin, W., H. P. Eugster, G. Bordmann, J. Bonato, M. Muller, M. Yamage, and B. Ryffel. 1997. Resistance to cerebral malaria in tumor necrosis factor-alpha/beta-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am. J. Pathol. 150:257–266. [PMC free article] [PubMed] [Google Scholar]
- 19.Rudin, W., N. Favre, G. Bordmann, and B. Ryffel. 1997. Interferon-gamma is essential for the development of cerebral malaria. Eur. J. Immunol. 27:810–815. [DOI] [PubMed] [Google Scholar]
- 20.Senaldi, G., C. Vesin, R. Chang, G. E. Grau, and P. F. Piguet. 1994. Role of polymorphonuclear neutrophil leukocytes and their integrin CD11a (LFA-1) in the pathogenesis of severe murine malaria. Infect. Immun. 62:1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silveira, P. A., A. G. Baxter, W. E. Cain, and I. R. van Driel. 1999. A major linkage region on distal chromosome 4 confers susceptibility to mouse autoimmune gastritis. J. Immunol. 162:5106–5111. [PubMed] [Google Scholar]
- 22.Silver, L. M. 1995. Mouse genetics, p.195–263. Oxford University Press, New York, N.Y.
- 23.Thumwood, C. M., N. H. Hunt, I. A. Clark, and W. B. Cowden. 1988. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology 96:579–589. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1–65. [PubMed] [Google Scholar]
- 25.Wunderlich, F., H. Mossmann, M. Helwig, and G. Schillinger. 1988. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect. Immun. 56:2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]