Abstract
The role of polymorphonuclear neutrophil granulocytes (PMN) in defense against the intracellular parasite Leishmania is poorly understood. In the present study, the interaction of human PMN with Leishmania major promastigotes was investigated in vitro. In the presence of fresh human serum, about 50% of PMN phagocytosed the parasites within 10 min and the parasite uptake led to PMN activation, resulting in the killing of most ingested parasites. Heat inactivation of the serum markedly reduced the rate of early parasite phagocytosis, suggesting a role of complement components in the early uptake of Leishmania. However, over 50% of PMN were able to ingest parasites in the presence of heat-inactivated serum if the coincubation was extended to 3 h. After 3 h, 10% of the PMN were found to internalize Leishmania even under serum-free conditions. These findings indicate that PMN possess mechanisms for both opsonin/complement-dependent and -independent uptake of Leishmania. Both pathways of uptake could be partially blocked by anti-CR3 antibody. Mannan-binding lectin was found not to be involved in this process. When phagocytosed in the absence of opsonin, the majority of Leishmania parasites survived intracellularly in PMN for at least 1 day. These data suggest a dual role of PMN in the early response to L. major infection. On the one hand, PMN can rapidly eliminate the intracellular parasites, and on the other hand, Leishmania can survive intracellularly in PMN. These data, together with the finding that intact parasites were seen in PMN isolated from the skin of infected mice, suggest that PMN can serve as host cells for the intracellular survival of Leishmania within the first hours or days after infection.
Polymorphonuclear leukocytes (PMN) are the primary effector cells in infection-induced acute inflammatory reactions. PMN are rapidly recruited from the bloodstream in large numbers to the site of infection via transmigration through the vascular endothelium. They play a pivotal role in the phagocytosis of microorganisms that finally leads to the elimination of many pathogenic microorganisms (46). Investigations over the past two decades revealed two basic mechanisms of recognition of microorganisms by phagocytic cells: opsonin dependent and opsonin independent (27). Opsonins are serum components which act by binding both to the surface of the microorganisms and to specific receptors on the phagocyte surface. The best-known opsonins are the C3bi fragment of the C3 component of complement, which binds to complement receptor type 3 (CR3), and immunoglobulins, which bind via their Fc domain to the Fc receptor (FcR) on the phagocytes. Recently, other opsonic serum components, termed collectins, were described; these include the mannan-binding lectin (MBL) (17). In addition to opsonin-mediated mechanisms of uptake, phagocytosis of microorganisms can be mediated by direct recognition of structures on the surface of microorganisms via specific receptors. Recognition of such structures by the phagocytic receptors often leads to uptake of the microorganisms, a process called nonopsonic phagocytosis (27).
Leishmaniasis is initiated by the bite of an infected sand fly and the deposition of the promastigote form of the parasites in the skin of the vertebrate hosts. The principal host cells of these obligate intracellular parasites are mononuclear phagocytes. The elimination of the intracellular parasites depends on the activation of the antimicrobial effector mechanisms in the infected macrophages (for a review, see reference 34). Although macrophages are the major host cells for Leishmania, we and others have demonstrated that neutrophil granulocytes prevail within the first hours after infection in the skin and thus represent the first leukocyte population migrating to the site of infection (25, 37). Although the role of PMN in defense against many extracellular bacteria is well established, their role in the early response to intracellular pathogens such as Leishmania is poorly understood. On the one hand, PMN were reported to exert antileishmanial activity by phagocytosing and killing of Leishmania promastigotes (9, 28), thus playing a role in the early control of these parasites (20). On the other hand, an early wave of PMN was found to be associated with the development of a disease-promoting Th2 response in mice susceptible to L. major (38).
In the present study, the interactions between highly purified human PMN and L. major promastigotes were investigated. We showed that PMN possess mechanisms for both the opsonin-dependent and the opsonin-independent phagocytosis of Leishmania. Opsonin-dependent uptake leads to PMN activation and killing of the intracellular parasites, while Leishmania parasites escape intracellular killing in PMN after opsonin-independent uptake in vitro. The in vitro findings that show the survival of L. major in PMN were confirmed in vivo by the detection of intact intracellular parasites in PMN isolated from the skin of mice infected with L. major.
MATERIALS AND METHODS
Preparation of human peripheral blood neutrophils.
Heparinized blood collected by venipuncture from healthy adult volunteers was layered on Histopaque 1119 (Sigma Diagnostics, Deisenhofen, Germany) and centrifuged for 20 min at 800 × g. The interphase, consisting mainly of lymphocytes and monocytes, was discarded. The granulocyte-rich layer of Histopaque 1119 was collected and washed twice in RPMI 1640 medium (Seromed-Biochrom, Berlin, Germany), and the cells were further fractionated on a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient consisting of layers with densities of 1,105 g/ml (85%), 1,100 g/ml (80%), 1,093 g/ml (75%), 1,087 g/ml (70%), and 1,081 g/ml (65%). After centrifugation for 20 min at 800 × g, the interface between the 80 and 85% Percoll layers was collected and washed twice in RPMI 1640. All procedures were carried out at room temperature. The purity of granulocytes achieved by this isolation technique was always above 99% as determined microscopically after May-Gruenwald-Giemsa staining of cytocentrifuge (Shandon, Pittsburgh, Pa.) slides. The viability of cells was >98% as assessed by trypan blue dye exclusion.
Coincubation of PMN with L.major promastigotes in vitro.
The origin and propagation of the cloned virulent line of L. major (MHOM/IL/81/FEBNI) have been described elsewhere (33). Stationary-phase promastigotes were collected from in vitro cultures in biphasic NNN blood agar medium. A total of 106 PMN were coincubated, at 37°C under a humidified atmosphere containing 5% CO2, with L. major promastigotes at a parasite-to-PMN ratio of 5:1 in 1 ml of complete medium (RPMI 1640 medium [Gibco Laboratories, Eggenstein, Germany] supplemented with 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 10 mM HEPES, 100 μg of penicillin per ml, and 160 μg of gentamicin per ml [all Seromed-Biochrom]). In designated experiments, this medium was supplemented with 20% fresh or heat-inactivated autologous human serum or with 20% heat-inactivated fetal calf serum (FCS) (Seromed-Biochrom). Heat inactivation was carried out at 56°C for 30 min. The number of infected cells was determined by microscopic evaluation of ≥200 PMN after May-Gruenwald-Giemsa staining of cytocentrifuge preparations.
Coincubation of PMN with L.major promastigotes in the presence of sera deficient in MBL and with purified MBL.
A total of 106 PMN were coincubated with L. major promastigotes, at 37°C in a humidified atmosphere containing 5% CO2, at a parasite-to-PMN ratio of 5:1 in 1 ml of complete medium (see above) in the presence of 20% normal human serum or 20% serum from patients with MBL deficiency. The two samples of normal human sera (sera 1 and 2 in Fig. 3) contained 2.3 and 2.8 μg of MBL per ml, respectively. The MBL content of the two MBL-deficient sera was 16 ng/ml (serum 3 in Fig. 3) and 12 ng/ml (serum 4 in Fig. 3). The allotypes of the the two MBL-deficient patients were LYPB/HYPD (serum 3) and LXPA/LYPB (serum 4), determined as described previously (35). Purified MBL (MBL/MASP complex SSI, lot MO-04, stabilized with 5 mg of HAS per ml [Statens Serum Institut, Copenhagen, Denmark]) was added to serum-free complete RPMI 1640 medium at a final concentration of 3.3 μg/ml.
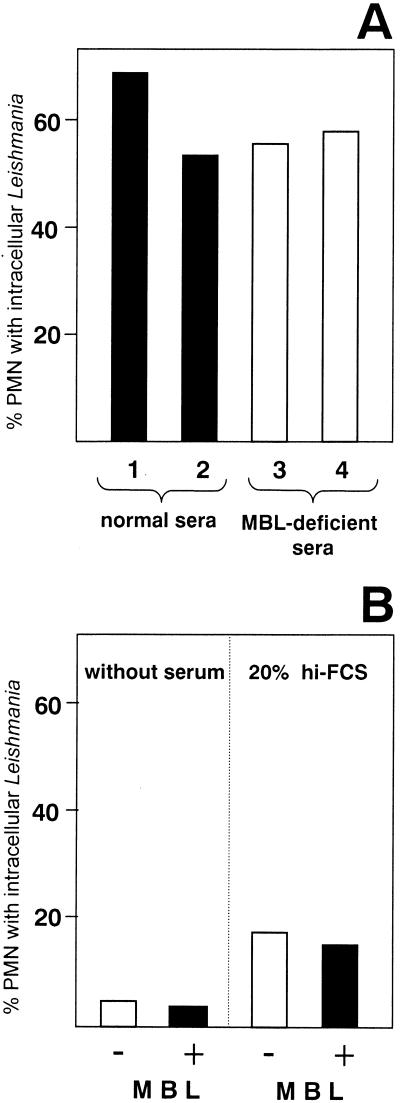
FIG. 3.
Effect of MBL on the uptake of L. major by PMN. (A) PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 10 min at 37°C in medium supplemented with 20% fresh serum from healthy individuals (containing 2.3 μg of MBL per ml ([serum 1] or 2.8 μg of MBL per ml [serum 2]). (B) PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 10 min at 37°C without MBL or in the presence of 3.3 μg of purified MBL in medium without serum supplementation or in medium supplemented with 20% heat-inactivated FCS (hi-FCS). Data are from one representative experiment of two performed.
Separation of PMN from nonphagocytosed Leishmania promastigotes with a fluorescence-activated cell sorter (FACS).
PMN were coincubated with L. major promastigotes, for 3 h at 37°C in a humidified atmosphere containing 5% CO2, at a parasite-to-PMN ratio of 5:1 in 1 ml of complete medium (see above) in the presence of 20% fresh autologous serum or 20% hi-FCS or without serum supplement. The PMN were then washed and resuspended in phosphate-buffered saline (PBS). To separate PMN from nonphagocytosed L. major promastigotes after coincubation, PMN were sorted using the FACS Vantage SE cell sorter system (BD Biosciences, San Jose, Calif.). Leishmania promastigotes were easily identified due to their low forward- and side-scatter signal intensities. The distinct PMN population was then sorted at a flow rate of 1,500 to 2,000 events/s. Sterile PBS was used as sheath fluid, and the collected cells were sorted into 5-ml polystyrene tubes precoated with FCS. After centifugation (300 × g for 10 min at 4°C), PMN were resuspended in medium and either analyzed by flow cytometry or used for cultures.
Determination of the viability of intracellular L.major in PMN.
To estimate the viability of the intracellular parasites, PMN were first separated from nonphagocytosed free Leishmania by flow cytometer sorting (see above). The sorted PMN were then incubated in RPMI 1640 medium complemented with 10% FCS at 37°C in humidified air with 5% CO2 for 24 h to allow the intracellular killing of parasites by PMN. The viability of ingested parasites was subsequently assessed by using a limiting-dilution culture assay for Leishmania as described previously (19). Briefly, serial twofold dilutions of sorted PMN were plated in 96-well flat-bottom microtiter plates containing 50 μl of NNN blood agar and 100 μl of RPMI 1640 medium supplemented with 10% FCS. The plates were then incubated at 27°C in a 5% CO2 humidified atmosphere for 1 week to allow the growth of Leishmania. The wells were assessed microscopically for growth of promastigotes. The number of viable Leishmania organisms in the sorted PMN preparations was calculated by assessing the last PMN dilution resulting in parasite growth and considering the average plating efficiency of 10 promastigotes as determined for L. major previously.
Acridine orange-ethidium bromide staining was applied to visualize the viability of intracellular L. major in PMN as described previously (19, 36). PMN were coincubated with L. major promastigotes for 3 h in culture medium containing 20% heat-inactivated FCS. PMN were separated from nonphagocytosed free Leishmania and cultivated for 24 h at 37°C. The cells were then stained with 2.5 mg of acridine orange (Sigma) per ml and 25 mg of ethidium bromide (Sigma) per ml for 10 min at room temperature, washed twice in PBS, fixed in 1% paraformaldehyde, and visualized under a fluorescence microscope (Axioskop 2; Carl Zeiss, Oberkochen, Germany).
Blocking of CR3.
PMN were preincubated with 10 μg of rat anti-mouse CD11b monoclonal antibody (MAb) (integrin αM chain, Mac-1 chain) per ml without sodium azide (clone M1/70; BD/Pharmingen, Heidelberg, Germany) for 20 min at 37°C. The anti-CR3 MAb, M1/70, is a rat immunoglobulin G2b (IgG2b) specific for mouse and human CR3 and blocks the binding of iC3b-coated targets to CR3 (4). A rat IgG1 isotype control MAb (BD/Pharmingen) was used as a negative control. The cells were washed twice in medium before being coincubated with L. major.
Assessment of PMN activation by flow cytometry analysis.
The cell surface expression of CD62L and CD66b was measured as markers for PMN activation. CD62L is known to be downregulated on PMN upon activation (5), while CD66b is expressed at a higher level on activated PMN (17). PMN were stained with fluorescein isothiocyanate (FITC)-conjugated MAb to human CD66b (clone 80H3; Immunotech, Hamburg, Germany) or phycoerythrin (PE)-conjugated MAb to CD62L (clone Dreg-56; BD/Pharmingen) and analyzed with a FACS-Calibur instrument using CellQuest software (Becton Dickinson, San Diego, Calif.). FITC- and PE-conjugated isotype control antibodies were purchased from BD/Pharmingen (mouse IgG1). The PMN isolation technique applied in our studies allowed us to work with non-preactivated PMN, as shown by the high CD62L expression of the cells used in the experiments (see Fig. 4A). This was achieved by using a method for the isolation of PMN which did not include a hypotonic step for lysing erythrocytes. As reported previously, membrane fragments of lysed erythrocytes stimulate PMN (10).
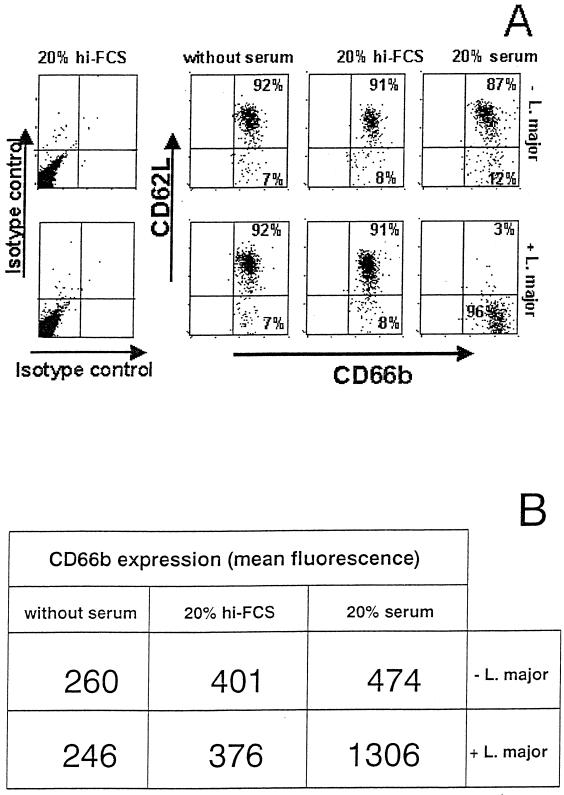
FIG. 4.
Effect of L. major on the cell surface expression of CD62L and CD66b on PMN. (A) PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 90 min at 37°C in RPMI 1640 medium supplemented with either 20% fresh autologous serum or 20% heat-inactivated FCS (hi-FCS) or without serum supplementation. PMN were then stained with FITC-conjugated MAb to CD66b and PE-labeled MAb to CD62L and analyzed by flow cytometry (A). (B) Mean fluorescence values of CD66b staining.
Determination of oxidative radical production by PMN.
Intracellular production of reactive oxygen radicals was assayed by using the fluorogenic substrate dihydrorhodamine 123 (Medac, Hamburg, Germany), which is fluorescent on interaction with reactive oxygen species (H2O2 and HO·) (32). Briefly, 106 PMN were incubated with 20 μl of substrate in 100 μl. The cells were then fixed in 1 ml of FACS lysing solution (Medac), washed twice in PBS, and stored on ice in the dark until used for flow cytometry analysis. Based on experiments with phorbol myristate acetate-stimulated PMN, a fluorescence intensity (FL-1) of higher than 103 on the arbitrary scale was interpreted as a positive intracellular oxidative burst (data not shown).
Determination of IL-8 production in PMN supernatants by ELISA.
Interleukin-8 (IL-8) in the culture supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) (DuoSet ELISA Development System; R&D Systems, Wiesbaden, Germany) as specified by the manufacturer.
Subcutaneous air pouch technique.
Female BALB/c mice were obtained from Charles River Breeding Laboratories (Sulzfeld, Germany) and used at 8 to 12 weeks of age. Air pouches were raised on the dorsum by subcutaneous injection of 2 ml of sterile air. Then 107 L. major promastigotes in 1 ml of PBS were injected into the air pouch. At 24 h after infection, the mice were killed and the exudate cells were washed out of the air pouch with ice-cold PBS. A total of 105 exudate cells were centrifuged on microscope slides at 500 rpm for 5 min using a cytocentrifuge (Shandon). The slides were air dried, fixed with methanol, and stained with Giemsa.
RESULTS
PMN possess mechanisms for both serum-dependent and serum-independent phagocytosis of Leishmania promastigotes.
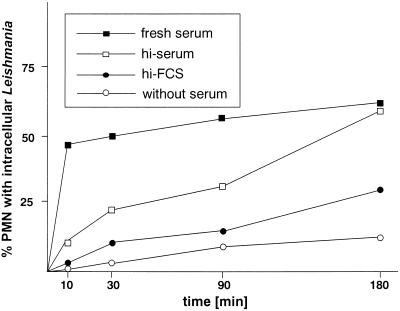
PMN were coincubated with L. major promastigotes in culture medium supplemented with fresh or heat-inactivated autologous human serum, with heat-inactivated FCS, or without serum supplement. Uptake of Leishmania could be observed in all cultures; however, the kinetics of phagocytosis was markedly different under the various conditions. In the presence of fresh serum, the uptake of L. major was very rapid; 47.2% ± 13.3% (n = 5) of PMN were parasitized after 10 min. The ratio of cells with intracellular parasites increased slowly during prolonged incubation and reached a level of 70.5% ± 10.2% (n = 5) after 3 h (Fig. 1).
FIG. 1.
Kinetics of phagocytosis of L. major promastigotes by PMN in vitro. PMN were incubated with L. major promastigotes at 37°C at a PMN-to-parasite ratio of 1:5 in RPMI 1640 medium supplemented with either 20% fresh autologous serum, 20% heat-inactivated autologous serum (hi-serum), or 20% heat-inactivated FCS (hi-FCS) or without serum supplementation. The percentage of PMN harboring at least one intracellular parasite was assessed by microscopic evaluation of Giemsa-stained cytocentrifuge preparations at the given time points. The data shown are from one experiment representative of four experiments performed.
Heat inactivation of the serum resulted in a significantly slower uptake of L. major promastigotes by PMN. After a 10-min coincubation, not more than 11.3% ± 8% of the cells contained Leishmania. However, over 50% of cells could phagocytose parasites if the coincubation was extended to 3 h (Fig. 1). In the presence of heat-inactivated FCS, 22.8% ± 10.3% of PMN phagocytosed Leishmania during a coincubation period of 3 h (Fig. 1).
To examine whether PMN possess mechanisms for the serum-independent direct recognition and uptake of Leishmania, phagocytosis experiments were carried out with medium without serum supplementation. Under serum-free conditions, 10.8% ± 5.7% of PMN were able to phagocytose Leishmania promastigotes during the 3-h incubation (Fig. 1). These experiments demonstrate that the presence of heat-labile serum factors in the culture medium facilitates the uptake of L. major promastigotes by granulocytes. However, PMN are also able to phagocytose L. major under serum-free conditions, indicating that PMN possess mechanisms for the opsonin-independent uptake of Leishmania as well.
The complement receptor CR3 plays a major role in both serum-dependent and complement-independent uptake of L.major by PMN.
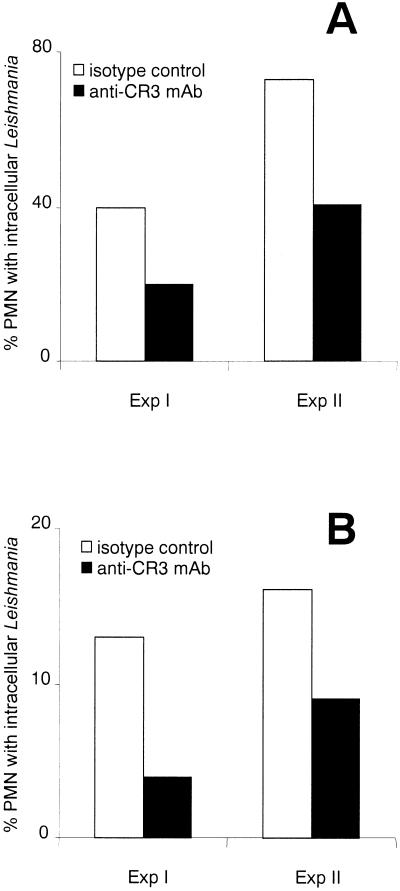
As shown above, heat treatment of serum reduced the initial uptake rate of Leishmania to a minimum level (Fig. 1). Heat treatment of serum at 56°C for 30 min results in the inactivation of heat-labile serum factors, including components of the complement system. Complement components are therefore likely to contribute to the rapid uptake of Leishmania by PMN. It has been reported that binding of complement-opsonized Leishmania promastigotes to the complement receptor CR3 contributes to the binding and uptake of the parasites by macrophages (21, 22). Therefore, we investigated whether CR3 was involved in the serum-dependent rapid uptake of Leishmania by PMN. Blocking of CR3 in vitro with anti-CD11b MAb significantly reduced the phagocytosis of L. major promastigotes by PMN compared to the phagocytosis by PMN treated with isotype control antibody (Fig. 2A).
FIG. 2.
Effect of anti-CR3 MAb treatment on the uptake of L. major by PMN. PMN were preincubated with anti-CR3 MAb for 20 min at 37°C, and control cultures were incubated with isotype control MAb. PMN were subsequently washed and coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 2 h at 37°C in RPMI 1640 medium containing 20% fresh autologous serum (A) or 20% heat-inactivated FCS (B). The uptake of parasites was assessed in two experiments (Exp I and Exp II) by microscopic evaluation of Giemsa-stained cytocentrifuge preparations. Exp I and Exp II were performed with cells and serum from two different donors.
In addition to mediating the uptake of complement-opsonized parasites, CR3 plays a major role in the opsonin-independent binding of Leishmania to macrophages (23, 42). Neutrophil granulocytes have also been shown to possess mechanisms for the CR3-mediated nonopsonic uptake of bacteria (1, 39). Therefore, the effect of anti-CR3 MAb on the complement-independent uptake of L. major by PMN was analyzed. In vitro blocking of CR3 resulted in a markedly reduced uptake of L. major by PMN in medium containing heat-inactivated FCS (Fig. 2B).
Opsonization through MBL does not contribute to the serum-dependent uptake of Leishmania by PMN.
In the experiments above, treatment of PMN with anti-CR3 antibody resulted in only a partial blocking of uptake of Leishmania. These data suggest that in addition to complement components binding to CR3, other heat-labile serum factors are involved in the uptake of Leishmania by PMN. A candidate heat-labile molecule is MBL, which mediates the lectin pathway of complement activation (37, 38). MBL was reported to bind to the surface of Leishmania promastigotes (15). The possible role of MBL was tested in two sets of experiments. First, the uptake of Leishmania by PMN was investigated in the presence of fresh serum obtained from healthy volunteers (MBL content, 2.3 and 2.8 μg/ml) as well as from patients with MBL deficiency (MBL content, 16 and 12 ng/ml). The significantly lower MBL concentration in the MBL-deficient sera did not affect the rate of Leishmania uptake; about 60% of PMN could phagocytose Leishmania in the presence of both normal and MBL-deficient serum (Fig. 3A).To exclude the possibility that the low MBL level (16 and 12 ng/ml) in the MBL-deficient sera still could mediate MBL-dependent uptake, a second set of experiments was carried out. Serum-free medium was supplemented with purified MBL, and the uptake of L. major by PMN was analyzed. Supplementation of the culture medium with purified MBL did not result in increased uptake of Leishmania by PMN (Fig. 3B). These data indicate that MBL-mediated opsonization has little if any effect on the phagocytosis of Leishmania by PMN.
Only opsonin-dependent uptake of Leishmania activates PMN.
Having observed that PMN ingest L. major promastigotes, we investigated whether parasitized PMN are able to activate antimicrobial effector mechanisms and to eliminate the intracellular pathogens. First, the cell surface expression of the activation markers CD62L and CD66b in PMN was analyzed. The expression of CD62L is a widely used marker for PMN activation since it results in the rapid shedding of surface CD62L (5). Also, the upregulation of CD66b was measured as an additional activation marker on PMN (18).
Coincubation of PMN with L. major in the presence of fresh serum led to PMN activation as demonstrated by the loss of CD62L expression (Fig. 4A)and increased expression of CD66b (Fig. 4B). In contrast, similar coincubation in the presence of heat-inactivated FCS or in serum-free medium did not result in the activation of PMN; the cells retained their high expression of CD62L, and the expression of CD66b was not enhanced (Fig. 4).
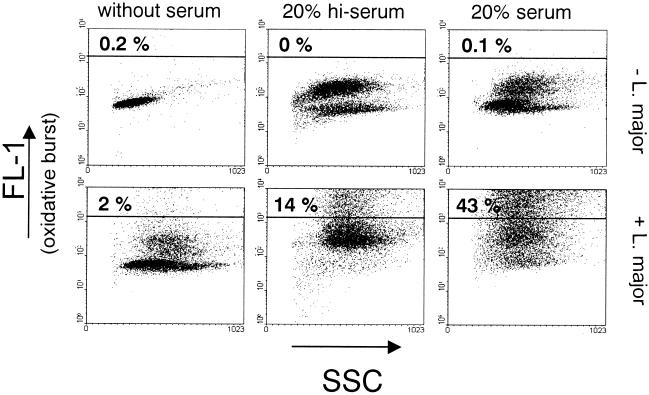
To determine whether L. major parasites were able to induce the oxidative burst in PMN, the production of reactive oxygen radicals in PMN was measured after 10 min of coincubation with L. major by using an intracellular staining technique. In the presence of fresh serum, over 40% of the cells responded to the parasites with a strong oxidative burst (Fig. 5),compared to only about 10 to 15% of the cells after incubation in medium supplemented with heat-inactivated serum. None of the PMN showed a strong oxidative response when the cells were coincubated with L. major in medium without serum supplement (Fig. 5).
FIG. 5.
Effect of L. major on induction of the oxidative burst by PMN. PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 10 min at 37°C in RPMI 1640 medium supplemented with either 20% fresh autologous serum or 20% heat-inactivated serum (hi-serum) or without serum supplementation. The oxidative burst in PMN was assessed by using the fluorogenic substrate dihydrorhodamine 123 and measured by flow cytometry (FL-1). The percentage of burst positive cells is shown in the upper left corner.
Induction of a strong oxidative burst in PMN results in the elimination of intracellular L.major, while the parasites survive in inactivated PMN.
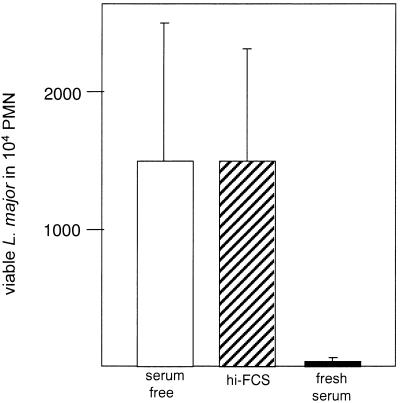
Next, the effect of PMN activation and the induction of the oxidative burst in PMN on the viability of intracellular parasites was investigated. After coincubation of PMN with L. major promastigotes under various culture conditions, the nonphagocytosed free parasites were separated from the PMN by fluorescence-activated cell sorting. Sorted PMN were subsequently cultivated for 24 h at 37°C to allow the intracellular killing of parasites by PMN. The number of PMN-associated viable parasites was then determined by using a limiting-dilution in vitro culture assay. The number of viable parasites was very small in the PMN samples which had taken up Leishmania in the presence of fresh serum (Fig. 6).A large number of viable parasites was detected in PMN that phagocytosed the parasites in medium supplemented with heat-inactivated FCS or in medium without serum supplement (Fig. 6).
FIG. 6.
Viability of intracellular Leishmania in PMN. PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 3 h at 37°C in RPMI 1640 medium supplemented with either 20% fresh autologous serum or 20% heat-inactivated FCS (hi-FCS) or without serum supplementation. PMN were then separated from nonphagocytosed L. major promastigotes by fluorescence-activated cell sorting and subsequently incubated for 24 h at 37°C in complete RPMI 1640 medium containing 10% FCS. The viability of L. major in PMN was then assessed by a limiting-dilution Leishmania culture assay. The y axis shows the mean and standard deviation of the three parallel dilutions of PMN.
Although the uptake rate of L. major under serum-free conditions and in medium containing heat-inactivated FCS was significantly lower than in the presence of fresh serum (Fig. 1), the viability of the intracellular parasites was much higher after they were internalized in the absence of fresh serum. These data clearly show that the majority of parasites which had been internalized in the presence of fresh serum were killed rapidly by PMN. On the other hand, intracellular Leishmania survived in PMN if the uptake had taken place in heat-inactivated serum or in medium without serum supplementation.
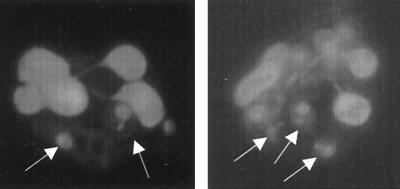
Acridine orange-ethidium bromide staining was applied to confirm the viability of intracellular L. major in PMN. Previously, this method was shown to detect both viable (green-stained) and killed (red-stained) intracellular Leishmania parasites in viable host cells (19, 36). PMN were coincubated with L. major promastigotes for 3 h in culture medium containing 20% heat-inactivated FCS, separated from nonphagocytosed free Leishmania, and cultivated for 24 h at 37°C. The cells were then stained with acridine orange and ethidium bromide. Figure 7shows that intracellular L. major parasites in PMN are viable (green staining) and morphologically intact, since the nuclei and kinetoplasts are clearly seen. These results again show that opsonin-independent uptake of L. major by PMN does not lead to intracellular killing of the parasites.
FIG. 7.
Staining of viable intracellular L. major in PMN with ethidium bromide and acridine orange. PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 3 h at 37°C in RPMI 1640 medium supplemented with 20% heat-inactivated FCS, separated from nonphagocytosed L. major promastigotes, and subsequently incubated for 24 h at 37°C. The viability of L. major in PMN was then assessed by staining with acridine orange to visualize viable cells or parasites (green staining) and ethidium bromide, which stains dead cells. Viable L. major parasites (arrows) are seen in nonapoptotic viable PMN. The morphological appearance of the nuclei and kinetoplasts is typical for intact parasites.
Leishmania can escape intracellular killing after being internalized by PMN in vivo.
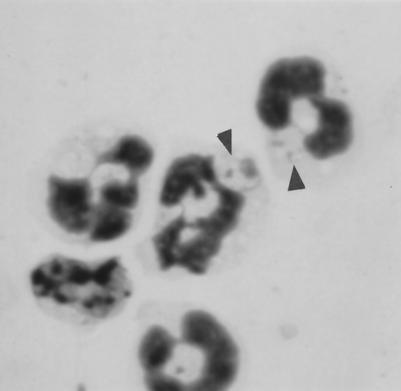
The finding that L. major can survive intracellularly in PMN in vitro suggested that this may also occur in vivo in the early phase of infection. To test this hypothesis, L. major promastigotes were injected into subcutaneous air pouches of BALB/c mice. Previous studies demonstrated that L. major induces the migration of PMN into the infected skin within the first few hours after experimental infection of mice (25, 37). Inflammatory exudate cells were collected from the air pouch 24 h after L. major infection, and Giemsa-stained cytospin slides were assessed for the presence of infected PMN. Intracellular Leishmania parasites were seen in PMN, and these parasites were morphologically intact (Fig. 8),demonstrating clearly that at least in some PMN the in vivo uptake of Leishmania does not lead to rapid cell activation and intracellular killing of L. major.
FIG. 8.
Uptake and survival of L. major in PMN in vivo. L. major promastigotes were injected into subcutaneous air pouches in BALB/c mice. At 24 h after infection, the exudate cells were collected from the air pouches. Cytocentrifuge slides of exudate cells were stained with Giemsa. Intracellular Leishmania organisms (arrows) are seen in PMN.
Leishmania induces IL-8 secretion in PMN.
The early inflammatory reaction in infected tissue plays a major role in the development of the specific immune response to Leishmania (for a review, see reference 34). One of the proinflammatory cytokines involved in inflammatory reactions is the chemokine IL-8, of which PMN are a major source (8). We analyzed whether coincubation of human PMN with L. major under various culture conditions resulted in IL-8 release. Coincubation of PMN with L. major for 24 h in medium with fresh serum led to secretion of high levels of IL-8 (>3,400 pg/ml) (Table 1). The IL-8 release did not occur immediately after activation of PMN by Leishmania, since the IL-8 content in the supernatant after 2 h was low (Table 1). L. major promastigotes also induced the release of significant amounts of IL-8 in the presence of heat-inactivated FCS (Table 1). However, no significant levels of IL-8 were induced by Leishmania in medium without serum supplementation (Table 1).
TABLE 1.
Secretion of IL-8 by PMN upon coincubation with L. majora
| Coincubation time (h) | Expt | IL-8 content (pg/ml) in PMN culture supernatants in medium containing:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No serum
|
20% hi-FCS
|
20% fresh autologous serum
|
|||||||
| −L. major | +L. major | −L. major | +L. major | −L. major | +L. major | ||||
| 2 | I | 2 | 3 | 72 | 11 | 85 | 211 | ||
| II | 2 | 17 | 2 | 52 | 2 | 97 | |||
| 24 | III | 18 | 283 | 47 | 1,892 | 25 | 3,994 | ||
| IV | 41 | 349 | 65 | 2,433 | 85 | 3,438 | |||
PMN were coincubated with L. major promastigotes at a PMN-to-parasite ratio of 1:5 for 2 and 24 h at 37°C in RPMI 1640 medium supplemented with either 20% fresh autologous serum or 20% heat-inactivated FCS. The IL-8 content of the culture supernatants was determined by ELISA.
DISCUSSION
In the present study, the serum-dependent and serum-independent in vitro uptake of Leishmania promastigotes by PMN was described and characterized. Although the uptake of Leishmania by macrophages has been characterized in detail, no such comprehensive study with PMN has been carried out so far. The importance of the interactions between PMN and Leishmania is highlighted by the fact that within the first few hours after infection, PMN, not macrophages, are recruited to the site of subcutaneous Leishmania infection (25, 37).
Our results show that serum-dependent phagocytosis of L. major leads to PMN activation, resulting in the rapid killing of intracellular parasites. Phagocytosis of L. major in the absence of fresh serum, however, did not lead to PMN activation, and the majority of intracellular parasites survived the first day after being phagocytosed by PMN. The possible biological relevance of these results was underlined by the finding that intact parasites were observed intracellularly in PMN isolated from the skin of mice 24 h after infection with L. major. We have also demonstrated that L. major induced the production of IL-8 by PMN.
In medium containing fresh serum, the majority of PMN ingested L. major within minutes; this was followed by the intracellular killing of the parasites. Heat inactivation of the serum resulted in abolition of the rapid early uptake and of subsequent killing of Leishmania by PMN. Therefore, the rapid uptake which leads to the intracellular killing of L. major promastigotes by PMN depends on the presence of heat-labile serum factors. Heat treatment of serum at 56°C for 30 min inactivates components of the complement system. Complement-mediated opsonophagocytosis by neutrophils is known to involve the complement receptors CR3 and CR1. The functional phagocytic receptor is CR3, while CR1 appears mostly as a coreceptor, facilitating the function of CR3 (46). In our experiments, the serum-dependent rapid uptake of L. major by PMN could be partially blocked by treatment with anti-CR3 antibody, showing a role of CR3-mediated mechanisms in the uptake of Leishmania by PMN. Since CR3 is stored in the secondary and tertiary granules of PMN, its surface expression is rapidly upregulated on activation of the cell (6). In the presence of serum, L. major was previously shown to activate the alternative pathway of complement, leading to C3b deposition on the surface of L. major promastigotes and CR3-mediated uptake of L. major by macrophages (24, 30). Our results show the involvement of CR3 in the uptake of L. major by PMN. Therefore, PMN and macrophages are likely to use the same or similar CR3-mediated mechanisms for the uptake of opsonized Leishmania parasites.
Treatment of PMN with anti-CR3 antibody resulted in only a partial blocking of serum-dependent Leishmania uptake. Therefore, in addition to CR3, there are certainly other surface molecules involved in the binding of Leishmania parasites to PMN. Antibodies bound to the surface of microorganisms can mediate opsonophagocytosis by PMN mediated by the Fcγ receptors FcγRIIA (CD32) and FcγRIIIB (CD16) (46). Natural antibodies recognizing Leishmania have been found in fresh sera of most vertebrates (31). The binding of natural antibodies may therefore directly opsonize L. major promastigotes and lead to FcR-mediated phagocytosis of the parasites by PMN in the presence of heat-inactivated serum. Moreover, binding of natural antibodies may activate the classical complement pathway and deposit C3 on the parasite surface, resulting in parasite opsonization.
In addition to components of the complement system, Leishmania promastigotes were shown to interact with and bind to other serum proteins to promote uptake by host macrophages. One heat-labile serum factor which plays an important role in the activation of complement and in the uptake of microorganisms by phagocytes is MBL (43). MBL can play an opsonic role and was shown to enhance the uptake of mannose-rich pathogens by PMN (17). MBL binds to mannose-terminating oligosaccharides present in Leishmania lipophosphoglycan and acts as an activator of the complement cascade. Binding of MBL to the surface of Leishmania promastigotes provides an additional mechanism for the formation of C3b, which participates in the attachment to macrophages (15). However, our results using MBL-deficient sera and purified MBL did not support a role for MBL opsonization in the serum-dependent uptake of L. major by PMN. Therefore, in addition to complement factors binding to CR3, heat-labile serum factors other than MBL are involved in the serum-mediated uptake of Leishmania by PMN.
We showed that PMN are able to phagocytose L. major promastigotes even under serum-free conditions. Although complement-mediated phagocytosis of bacteria by PMN in serum is considered important, studies have shown the nonopsonic uptake of bacteria by PMN in the absence of antibody and active complement (11, 13, 27). Direct binding to the mannose receptor was reported to be involved in the serum-independent uptake of Leishmania by macrophages (44, 45). Since PMN do not express the mannose receptor, the mechanism of Leishmania binding to PMN under serum-free conditions remains to be elucidated. In our studies, anti-CR3 MAb treatment inhibited not only the serum-dependent but also the serum-independent uptake of L. major by PMN, suggesting a role of CR3 in the nonopsonic recognition and uptake of L. major by PMN. Such a role of CR3 was shown to be involved in the opsonin-independent uptake of group B streptococci by PMN (1). The CR3-mediated nonopsonic recognition of L. major by PMN is possibly a result of the binding of Leishmania lipophosphoglycan and gp63 to CR3, as described previously (39).
In the present study, the expression of activation markers and the intracellular production of reactive oxygen intermediates were analyzed in the context of the intracellular survival of Leishmania in PMN. Activation of the PMN oxidative burst was found to be associated with the elimination of intracellular parasites. This finding is in line with earlier reports that reactive oxygen intermediates are major mediators of intracellular killing of Leishmania in granulocytes (9, 26). In the presence of fresh autologous serum, canine PMN were also reported to phagocytose and efficiently kill L. infantum promastigotes, and reactive oxygen radicals were suggested to be responsible for the killing (7). The survival of parasites after being taken up by PMN in medium containing heat-inactivated serum and in medium without serum supplement is consistent with the low level of activation and with the lack of oxidative burst induction in these cells.
Coincubation of PMN with L. major was found to result in the secretion of IL-8. Although IL-8 is secreted by a variety of cells including T lymphocytes, epithelial cells, keratinocytes, fibroblasts, and endothelial cells, neutrophils are the most abundant source of this chemokine. On the other hand, neutrophils are also the primary cellular target of IL-8; thus, IL-8 production can act as an amplifying loop of neutrophil granulocytes (14). In addition to the chemotactic activity, IL-8 activates other cellular functions of PMN, such as phagocytosis (2). The Leishmania-induced production of IL-8 by PMN is therefore likely to accelerate the recruitment of neutrophils to the site of infection and to facilitate the uptake of the parasites. Moreover, the chemotactic activity of IL-8 on T lymphocytes may play a role in the development of a T-cell-mediated immune response to the parasite. Whether the functional murine homologues of IL-8 (MIP-2 and KC) (12) are involved in the recently described granulocyte-dependent promotion of a Th2-dominated response in mice (38) remains to be elucidated.
Since mononuclear phagocytes are a major source of several cytokines, it was important to exclude the possibility that monocyte contamination could account for the release of IL-8 attributed to PMN. The high purity (>99%) argues against this possibility. Additional evidence for the purity of the PMN population was the absence of IL-6 mRNA (data not shown), which is readily detectable in monocyte but not in PMN preparations (3).
The results presented here show that serum-dependent phagocytosis of L. major leads to the rapid killing of intracellular parasites while the majority of intracellular parasites survived in PMN when the uptake of L. major occurred in the absence of heat-labile serum factors. These data suggest a possible dual role of PMN regarding the interaction with L. major parasites. On the one hand, by killing the intracellular parasites, PMN can play a role in the early defense against this pathogen. On the other hand, PMN may provide an intracellular milieu for the survival of the parasites within the first hours to days of infection. The survival of intracellular pathogens such as Leishmana in the host organism depends on phagocytic cells which recognize and internalize these microorganisms. They thus escape antimicrobial agents of immune and nonimmune origin in the surrounding milieu. The data presented here indicate that in addition to macrophages, neutrophil granulocytes can function as cells providing such a protective intracellular milieu for Leishmania. Indeed, morphologically intact Leishmania organisms were seen in PMN isolated from infected skin 24 h after infection. The parasites seen intracellularly in PMN isolated from the skin of mice after L. major infection did not show any morphological sign of damage. These data clearly indicate that although serum factors are expected to opsonize Leishmania promastigotes in vivo, opsonin-independent uptake leading to the “silent entry” of parasites into PMN occurs in the skin soon after infection. Complement activation by Leishmania membrane components was reported to lead to local complement depletion necessary for the pathology of Leishmania infection (16, 29). Therefore, a local exhaustion of complement after Leishmania infection can provide a milieu for the complement-independent uptake of the parasites by PMN, leading to a prolonged survival of the parasites.
Myeloid cells have long been thought to serve as “safe targets” for the survival of Leishmania (21). Since L. major can survive in PMN after nonopsonic phagocytosis, these cells can serve as safe targets within the first hours to days after infection. This is in line with the view that the rapid uptake of Leishmania promastigotes by leukocytes early after infection is the parasite's earliest survival strategy (10).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 367/B10 and So220/5-1).
We thank Ger van Zandbergen for critically reading the manuscript.
Editor:S. H. E. Kaufmann
REFERENCES
- 1.Antal, J. M., J. V. Cunningham, and K. J. Goodrum. 1992. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect. Immun. 60:1114-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazzoni, F., and B. Beutler. 1996. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 334:1717-1725. [DOI] [PubMed] [Google Scholar]
- 4.Beller, D. I., T. A. Springer, and R. D. Schreiber. 1982. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J. Exp. Med. 156:1000-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, M., and S. P. James. 1990. Human neutrophils release the Leu-8 lymph node homing receptor during cell activation. Blood 76:2381-2388. [PubMed] [Google Scholar]
- 6.Berger, M., J. O'Shea, A. S. Cross, T. M. Folks, T. M. Chused, E. J. Brown, and M. M. Frank. 1984. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J. Clin. Investig. 74:1566-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandonisio, O., M. Panunzio, S. M. Faliero, L. Ceci, A. Fasanella, and V. Puccini. 1996. Evaluation of polymorphonuclear cell and monocyte functions in Leishmania infantum-infected dogs. Vet. Immunol. Immunopathol. 53:95-103. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 9.Chang, K. P. 1981. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am. J. Trop. Med. Hyg. 30:322-333. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez, M., and A. Torano. 1999. Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection. J. Exp. Med. 189:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 65:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driscoll, K. E. 1994. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp. Lung Res. 20:473-490. [DOI] [PubMed] [Google Scholar]
- 13.Estabrook, M. M., D. Zhou, and M. A. Apicella. 1998. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect. Immun. 66:1028-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gainet, J., S. Chollet-Martin, M. Brion, J. Hakim, M. A. Gougerot-Pocidalo, and C. Elbim. 1998. Interleukin-8 production by polymorphonuclear neutrophils in patients with rapidly progressive periodontitis: an amplifying loop of polymorphonuclear neutrophil activation. Lab. Investig. 78:755-762. [PubMed] [Google Scholar]
- 15.Green, P. J., T. Feizi, M. S. Stoll, S. Thiel, A. Prescott, and M. J. McConville. 1994. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol. Biochem. Parasitol. 66:319-328. [DOI] [PubMed] [Google Scholar]
- 16.Ilg, T., Y. D. Stierhof, M. J. McConville, and P. Overath. 1995. Purification, partial characterization and immunolocalization of a proteophosphoglycan secreted by Leishmania mexicana amastigotes. Eur. J. Cell Biol. 66:205-215. [PubMed] [Google Scholar]
- 17.Kuhlman, M., K. Joiner, and R. A. Ezekowitz. 1989. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuijpers, T. W., A. T. Tool, C. E. van der Schoot, L. A. Ginsel, J. J. Onderwater, D. Roos, and A. J. Verhoeven. 1991. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 78:1105-1111. [PubMed] [Google Scholar]
- 19.Laskay, T., I. Wittmann, A. Diefenbach, M. Röllinghoff, and W. Solbach. 1997. Control of Leishmania major infection in BALB/c mice by inhibition of early lymphocyte entry into peripheral lymph nodes. J. Immunol. 158:1246-1253. [PubMed] [Google Scholar]
- 20.Lima, G. M., A. L. Vallochi, U. R. Silva, E. M. Bevilacqua, M. M. Kiffer, and I. A. Abrahamsohn. 1998. The role of polymorphonuclear leukocytes in the resistance to cutaneous leishmaniasis. Immunol. Lett. 64:145-151. [DOI] [PubMed] [Google Scholar]
- 21.Mirkovich, A. M., A. Galelli, A. C. Allison, and F. Z. Modabber. 1986. Increased myelopoiesis during Leishmania major infection in mice: generation of “safe targets”, a possible way to evade the effector immune mechanism. Clin. Exp. Immunol. 64:1-7. [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser, D. M., and P. J. Edelson. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135:2785-2789. [PubMed] [Google Scholar]
- 23.Mosser, D. M., T. A. Springer, and M. S. Diamond. 1992. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J. Cell Biol. 116:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser, D. M., H. Vlassara, P. J. Edelson, and A. Cerami. 1987. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J. Exp. Med. 165:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller, K., G. van Zandbergen, B. Hansen, H. Laufs, N. Jahnke, W. Solbach, and T. Laskay. Chemokines, NK cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol., in press. [DOI] [PubMed]
- 26.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofek, I., J. Goldhar, Y. Keisari, and N. Sharon. 1995. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 49:239-276. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, R. D., and R. T. Steigbigel. 1981. Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J. Immunol. 127:1438-1443. [PubMed] [Google Scholar]
- 29.Peters, C., M. Kawakami, M. Kaul, T. Ilg, P. Overath, and T. Aebischer. 1997. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur. J. Immunol. 27:2666-2672. [DOI] [PubMed] [Google Scholar]
- 30.Puentes, S. M., D. L. Sacks, R. P. da Silva, and K. A. Joiner. 1988. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J. Exp. Med. 167:887-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmunis, G. A., and R. Herman. 1970. Characteristics of so-called natural antibodies in various normal sera against culture forms of Leishmania. J. Parasitol. 56:889-896. [PubMed] [Google Scholar]
- 32.Smith, J. A., and M. J. Weidemann. 1993. Further characterization of the neutrophil oxidative burst by flow cytometry. J. Immunol. Methods 162:261-268. [DOI] [PubMed] [Google Scholar]
- 33.Solbach, W., K. Forberg, E. Kammerer, C. Bogdan, and M. Rollinghoff. 1986. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J. Immunol. 137:702-707. [PubMed] [Google Scholar]
- 34.Solbach, W., and T. Laskay. 2000. The host response to Leishmania infection. Adv. Immunol. 74:275-317. [DOI] [PubMed] [Google Scholar]
- 35.Steffensen, R., S. Thiel, K. Varming, C. Jersild, and J. C. Jensenius. 2000. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 241:33-42. [DOI] [PubMed] [Google Scholar]
- 36.Stenger, S., W. Solbach, M. Röllinghoff, and C. Bogdan. 1991. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN) γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL-4. Eur. J. Immunol. 21:1669-1675. [DOI] [PubMed] [Google Scholar]
- 37.Sunderkotter, C., M. Kunz, K. Steinbrink, G. Meinardus-Hager, M. Goebeler, H. Bildau, and C. Sorg. 1993. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J. Immunol. 151:4891-4901. [PubMed] [Google Scholar]
- 38.Tacchini-Cottier, F., C. Zweifel, Y. Belkaid, C. Mukankundiye, M. Vasei, P. Launois, G. Milon, and J. A. Louis. 2000. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 165:2628-2636. [DOI] [PubMed] [Google Scholar]
- 39.Talamas-Rohana, P., S. D. Wright, M. R. Lennartz, and D. G. Russell. 1990. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J. Immunol. 144:4817-4824. [PubMed] [Google Scholar]
- 40.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 41.Turner, M. W. 1998. Mannose-binding lectin (MBL) in health and disease. Immunobiology 199:327-339. [DOI] [PubMed] [Google Scholar]
- 42.Vanek, N. N., S. I. Simon, K. Jacques-Palaz, M. M. Mariscalco, G. M. Dunny, and R. M. Rakita. 1999. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor 3-mediated mechanism. FEMS Immunol. Med Microbiol. 26:49-60. [DOI] [PubMed] [Google Scholar]
- 43.Vorup-Jensen, T., J. C. Jensenius, and S. Thiel. 1998. MASP-2, the C3 convertase generating protease of the MBLectin complement activating pathway. Immunobiology 199:348-357. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, M. E., and R. D. Pearson. 1986. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J. Immunol. 136:4681-4688. [PubMed] [Google Scholar]
- 45.Wilson, M. E., and R. D. Pearson. 1988. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect. Immun. 56:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witko-Sarsat, V., P. Rieu, B. Descamps-Latscha, P. Lesavre, and L. Halbwachs-Mecarelli. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Investig. 80:617-653. [DOI] [PubMed] [Google Scholar]