Abstract
Akt promotes cell survival by phosphorylating and inhibiting components of the intrinsic cell death machinery. Akt translocates into the nucleus upon exposure of cells to survival factors, but little is known about its functions in the nucleus. Here, we show that acinus, a nuclear factor required for apoptotic chromatin condensation, is a direct target of Akt. We demonstrate that Akt phosphorylation of acinus on serine 422 and 573 results in its resistance to caspase cleavage in the nucleus and the inhibition of acinus-dependent chromatin condensation. Abolishing acinus phosphorylation by Akt through mutagenesis accelerates its proteolytic degradation and chromatin condensation. Acinus S422, 573D, a mutant mimicking phosphorylation, resists against apoptotic cleavage and prevents chromatin condensation. Knocking down of acinus substantially decreases chromatin condensation, and depletion of Akt provokes the apoptotic cleavage of acinus. Thus, Akt inhibits chromatin condensation during apoptosis by phosphorylating acinus in the nucleus, revealing a specific mechanism by which nuclear Akt promotes cell survival.
Keywords: acinus, Akt, chromatin condensation, phosphorylation
Introduction
Nerve growth factor (NGF) regulates survival of several types of neurons by provoking a variety of signaling cascades including the PI3K/Akt pathway, which plays an essential role in this process. PI3K/Akt signaling blocks cell death by both impinging on the cytoplasmic apoptotic machinery and by mediating the expression of genes involved in cell death and survival (Yuan and Yankner, 2000; Brunet et al, 2001). For instance, Akt phosphorylates the proapoptotic Bcl-2 family member BAD, thereby inhibiting BAD proapoptotic functions (Datta et al, 1997; del Peso et al, 1997). In addition to its effects on the cytoplasmic apoptotic machinery, Akt controls a major class of transcription factors—the Forkhead box transcription factor, by phosphorylating FOXOs and inhibiting their ability to induce the expression of death genes (Biggs et al, 1999; Brunet et al, 1999; Kops et al, 1999). In addition to its function as a suppressor of critical death genes, under some circumstances activation of the PI3K/Akt survival pathway also triggers the expression of survival genes by phosphorylating CREB and IKK (Du and Montminy, 1998; Ozes et al, 1999; Romashkova and Makarov, 1999). PI3K and Akt predominantly locate in the cytoplasm, but they also occur in the nucleus, or translocate to the nucleus upon stimulation (Ahmed et al, 1993; Neri et al, 1994; Meier et al, 1997; Kim, 1998; Lu et al, 1998; Marchisio et al, 1998). For example, after 20–30 min growth factor treatment, Akt translocates to the nucleus. However, whether nuclear translocated Akt also impinges on the apoptotic machinery in the absence of de novo gene expression remains unknown. There is evidence that Akt regulation of apoptosis is dependent on its phosphorylation of key substrates in the nucleus (Ahn et al, 2004; Shiraishi et al, 2004), but the identities of these substrates are unknown.
Acinus, predominantly located in the nucleus, induces apoptotic chromatin condensation after cleavage by caspases (Sahara et al, 1999). Acinus is expressed in three different isoforms, termed Acinus-L, Acinus-S and Acinus-S′. They contain 1341, 583 and 568 amino-acid residues, respectively, with apparent molecular weights at 220, 98 and 94 kDa. Acinus is cleaved by caspases on both its N- and C-termini, producing a p17 active form (amino acids (aa) 987–1093), which triggers chromatin condensation in the absence of caspase-3. Full-length acinus-S is unable to condense chromatin, suggesting that caspase-mediated cleavage is necessary for this activity. Acinus undergoes several proteolytic cleavages during apoptosis, and p17 is one of the active forms to induce chromatin condensation. Acinus contains a region similar to the RNA-recognition motif of Drosophila splicing regulator Sxl, suggesting that acinus is implicated in RNA metabolism. As a matter of fact, recent studies reveal that acinus is a component of functional splicesomes (Rappsilber et al, 2002; Zhou et al, 2002). Moreover, different acinus isoforms are found in the apoptosis- and splicing-associated protein (ASAP) complex, which is comprised of the proteins SAP18, RNPS1 and distinct isoforms of Acinus. Addition of the complex to in vitro splicing assays inhibits RNA processing, and microinjection of ASAP into mammalian cells accelerates the progress of cell death after induction of apoptosis (Schwerk et al, 2003).
In the present study, we show that Akt phosphorylates acinus at both S422 and 573 residues, and prevents its proteolytic cleavage in vitro and in vivo. However, the protective effect is predominantly associated with S422, but not 573 phosphorylation. Cells transfected with acinus (S422, 573A) mutant, unable to be phosphorylated by Akt, reveal robust apoptotic degradation of acinus and chromatin condensation, which are prevented by acinus (S422, 573D), a mutant mimicking phosphorylation. These findings point to acinus as a key substrate for Akt and demonstrate a novel mechanism by which nuclear Akt controls chromatin condensation and apoptosis.
Results
Acinus is a physiological nuclear substrate of Akt
In exploring the sequence of acinus, we noticed that aa 398–404, RERTRSE, 417–423, RSRSRSR and 568–574, RSRSRST correspond to a motif that is identified as a consensus Akt phosphorylation element present in numerous Akt substrates. We prepared GST-recombinant proteins from three fragments of acinus, with each containing a putative phosphorylation domain (Figure 1A). We examined their ability to be phosphorylated by Akt through in vitro kinase assays. Fragment B (aa 404–567), C (aa 460–583) and positive control GSKβ were robustly phosphorylated by active Akt. By contrast, fragment A or GST alone was not phosphorylated (Figure 1B, left panel). Mutation with S422A or S573A in acinus abolishes the phosphorylation of fragment B and C by active Akt, suggesting that both residues can be phosphorylated by Akt in vitro (Figure 1C, lower panel). To further characterize Akt-mediated acinus phosphorylation, we generated rabbit polyclonal anti-phospho-S422 specific antibody. In vitro phosphorylation with full-length wild-type acinus and mutants demonstrate that this antibody selectively recognizes phosphorylated serine 422 in acinus (Figure 1D). To explore whether acinus can be phosphorylated by Akt in intact cells, we transfected HEK 293 cells with GST-tagged acinus wild-type and mutant constructs. EGF treatment triggers potent S422 phosphorylation in wild-type acinus and (S573A) mutant, which is markedly diminished by LY294002. As expected, no phosphorylation is detected in acinus (S422A) or acinus (S422AS573A) mutant, although Akt in all samples is activated upon EGF treatment, suggesting that S422 residue is phosphorylated in vivo (Figure 1E, left second and third panels). Subcellular fractionation reveals that Akt also occurs in the nucleus (Figure 1E, right panels).
Figure 1.
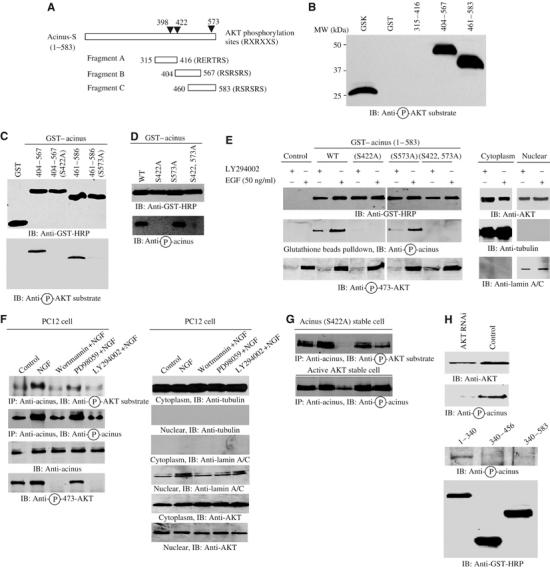
Acinus is a physiological nuclear substrate of Akt. (A) Diagram of acinus-S. Acinus-S possesses three putative Akt phosphorylation motifs (RXRXXS/T) as indicated (▾). The three fragments with each containing a putative phosphorylation motif are indicated with residue numbers. (B) In vitro Akt kinase assay. Purified recombinant GST-fusion proteins were incubated with active Akt. Both fragments B and C were robustly phosphorylated, while fragment A was not. (C) S422 and 573 residues in acinus-S are phosphorylated by Akt. Wild-type fragments, but not S422A and S573A mutants, were phosphorylated (lower panel). Equal amount of GST proteins was employed (upper panel). (D) Phospho-S422 antibody selectively recognizes phosphorylated acinus. While S422 site was markedly phosphorylated in wild-type and S573A acinus, no S422 phosphorylation was detected in S422A and S422, 573A mutants (lower panel). (E) Acinus-S can be phosphorylated in intact cells. HEK 293 cells were transfected with GST–acinus wild-type and mutants. One group was treated with LY294002, and the other group was stimulated by EGF for 20 min. While S422 was markedly phosphorylated in wild-type and S573A acinus upon EGF treatment, no S422 phosphorylation was detected in S422A and S422, 573A mutants (middle left panel). Equal amount of GST proteins was precipitated (top left panel). Akt phosphorylation was also monitored (bottom left panel). Cytosolic and nuclear markers and Akt distribution in the assays (right panels). (F) Endogenous acinus-S can be phosphorylated by Akt in PC12 cells. PC12 cells were pretreated with wortmannin (20 nM) or LY294002 (10 μM) or MEK1 inhibitor PD98059 (10 μM) for 30 min, before NGF was introduced. NGF elicited robust phosphorylation on acinus-S, but PI3K inhibitors markedly blocked it. By contrast, MEK1 inhibitor had no effect (top and second panels). Equal amount of acinus-S was pulled down (third panel). Akt activation was selectively inhibited by PI3K inhibitor, but not MEK1 inhibitor (fourth panel). Cytosolic and nuclear markers and Akt distribution in the assays (right panels). (G) Acinus phosphorylation on acinus (S422A) mutant. Acinus S422A stable cells were induced and treated as described above. PI3K inhibitors substantially blocked NGF-provoked acinus (S573) phosphorylation (upper panel). The S422 phosphorylation was also assessed in NLS-Akt-CA stably transfected PC12 cells. S422 phosphorylation was evident even in control cells, but it was partially blocked by wortmannin (lower panel). (H) Knocking down of Akt blocked acinus S422 phosphorylation (top panel). C-terminus of acinus prevents endogenous acinus phosphorylation by Akt. GST–acinus fragments were transfected into HEK 293 cells, and treated with EGF. Endogenous acinus was analyzed with anti-phospho-422 antibody (middle panel). The expression of transfected GST–acinus fragments (bottom panel).
To investigate further whether acinus can be selectively phosphorylated by Akt in PC12 cells, we pretreated PC12 cells with PI3K inhibitors, wortmannin and LY294002, and MEK1 inhibitor PD98059, respectively, and followed by NGF stimulation. Endogenous acinus was immunoprecipitated, and the precipitated proteins were analyzed with anti-phospho-Akt substrate and anti-phospho-S422 antibodies. NGF treatment elicits robust acinus phosphorylation, which is substantially blocked by PI3K inhibitors but not MEK1 inhibitor, suggesting that PI3K/Akt pathway but not MAPK cascade accounts for its phosphorylation (Figure 1F, left panels). Subcellular fractionation demonstrates that Akt distributes in both the cytosolic and nuclear fractions (Figure 1F, right panels). We observed the similar phosphorylation pattern for Acinus (S422A) mutant in its stable cell line, indicating that S573 site is also phosphorylated by Akt in intact cells (Figure 1G, top panel). Acinus S422 phosphorylation in constitutively active (CA) Myc-nuclear localization signal (NLS)-Akt (T308DS473D) stably transfected PC12 cells is unable to be inhibited by LY294002 pretreatment, although it is partially inhibited by wortmannin, suggesting that endogenous Akt can be inhibited by PI3K inhibitors, but the transfected active Akt is still able to phosphorylate acinus (Figure 1G, bottom panel). Knocking down of Akt by its shRNAi adenovirus abolishes NGF-mediated S422 phosphorylation in acinus (Figure 1H, top panel). Moreover, overexpression of GST–acinus (340–456) and (340–583) fragments but not N-terminal (1–340) segment abolishes endogenous acinus S422 phosphorylation by EGF (Figure 1H, middle and bottom panels), suggesting that the phosphorylated regions act as dominant-negative effectors by sequestering endogenous Akt and preventing it from phosphorylating acinus. Collectively, these data support that acinus acts as a physiological Akt substrate in the nucleus.
Akt phosphorylation prevents in vitro acinus proteolytic cleavage
Acinus-S carries a few putative caspase-3 cleavage sites as described, which might elicit a few fragments (Figure 2A). Truncation analysis suggests that the N-terminus of aa 1–228 and 1–335 display at 55 and 75 kDa, and C-terminal 228–583 and 335–583 express at 45 and 30 kDa, respectively (data not shown). To examine whether Akt phosphorylation could suppress its degradation by caspases, we purified various wild-type and mutant recombinant GST proteins of acinus fragments. After incubation with active Akt, the reaction mixture was introduced into active cell-free apoptotic solution, consisting of HEK 293 cell cytosol supplemented with purified active caspase-3 (Liu et al, 1996, 1997). Immunoblotting analysis reveals that wild-type fragments remain intact, whereas the mutants were substantially cleaved (Figure 2B, lower left panel).
Figure 2.
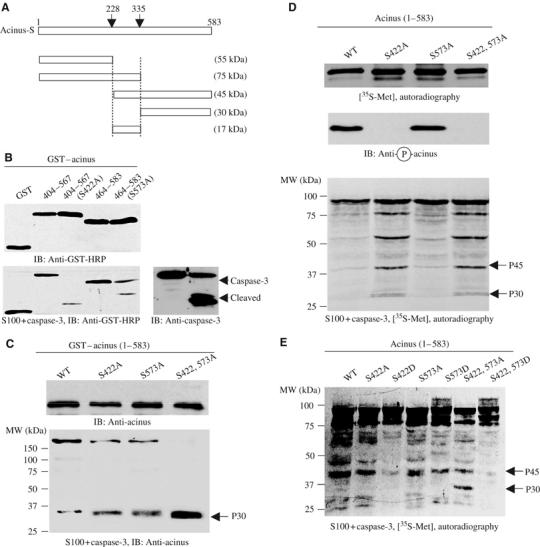
Akt phosphorylation prevents in vitro acinus proteolytic cleavage. (A) Diagram of acinus-S with putative caspase-3 cleavage sites. Residues 228–335 correspond to p17 active form in acinus-L (aa 987–1093). Caspase cleavage sites and the corresponding fragments with molecular weights were labeled. (B) Akt-phosphorylated fragments resist against apoptotic cleavage. Wild-type phosphorylated proteins resisted against apoptotic degradation, but S422A and S573A mutants were robustly cleaved in apoptotic solution (lower left panel). Equal amounts of GST proteins were employed (upper left panel). Caspase-3 was activated in the cell-free apoptotic solution (right panel). (C) Apoptotic cleavage assay with bacterial expressed full-length GST–acinus-S. Purified GST–acinus proteins were analyzed as described above. Immunoblotting was conducted with anti-acinus antibody after in vitro apoptotic degradation assay. Full-length wild type resisted against caspase cleavage, while S422A and S573A mutants were substantially degraded. Interestingly, S422, 573A mutant was almost completely cleaved. A p30 form of acinus was detected in all samples with highest amount in S422, 573A mutant (lower panel). Equal amount of GST proteins was employed (upper panel). (D) Apoptotic analysis with in vitro transcripted and translated acinus-S. Full-length acinus-S wild type and mutants were labeled with 35S-methionine in rabbit reticulocyte, and incubated with active Akt, then the reaction mixture was assayed in cell-free apoptotic solution. Prominent degradation was observed in both S422A and S422A, 573A mutants, but not in wild-type or S573A sample (bottom panel). Equal amount of 35S-labeled proteins was employed (top panel). S422 was robustly phosphorylated in both wild-type and S573A acinus (middle panel). (E) Apoptotic analysis with in vitro transcripted and translated acinus-S in the absence of Akt. Both S422D and S422, 573D mutants, but not wild-type or S573D sample, resisted against apoptotic degradation.
We extend these studies to full-length acinus-S wild-type and mutants. We purified various wild-type and mutant recombinant GST proteins of full-length acinus. After incubation with active Akt, the reaction mixture was introduced into active cell-free apoptotic solution. Compared to S422A or S573A mutants, wild-type acinus-S strongly resists against apoptotic degradation and a large amount of GST–acinus remains intact. Strikingly, acinus-S (S422, 573A) double mutant was almost completely cleaved in active cell-free apoptotic solution. Consistent with these observations, a fragment with molecular weight at 30 kDa appears, with the most abundant p30 form in acinus-S (S422, 573A) double-mutant sample (Figure 2C, lower panel). The 30 kDa fragment does not contain GST tag, suggesting that it arises from the cleaved C-terminus of acinus (data not shown).
We have also investigated acinus cleavage with in vitro transcripted and translated acinus-S in rabbit reticulocyte. 35S-methionine-labeled acinus-S was phosphorylated by active Akt and subjected to apoptotic cleavage in active cell-free apoptosome. After incubation, extensive proteolytic cleavage occurs in both S422A and S422, 573A mutants. By contrast, wild type and S573A reveal faint degradation (Figure 2D, bottom panel). S422 in both wild-type and acinus S573A mutant is robustly phosphorylated. As expected, no S422 phosphorylation is detected in acinus S422A or acinus S422AS573A mutant (Figure 2D, middle panel). To further examine the notion that acinus phosphorylation protects it from cleavage, we mutated S422 and S573 into aspartic acid to mimic their phosphorylation, and performed cleavage assay without Akt treatment. As expected, both S422D and S422, 573D mutants robustly prevent acinus proteolytic degradation. By contrast, S573D mutation only partially antagonizes acinus cleavage (Figure 2E). Therefore, this finding further supports that Akt phosphorylation on S422 is essential for acinus-S to resist against caspase-3-mediated degradation.
Akt phosphorylation prevents acinus proteolytic cleavage in cells
To examine whether Akt phosphorylation on acinus-S protects it from apoptotic degradation in intact cells, we transfected HEK 293 cells with GST-tagged acinus-S wild type and mutants. The transfected cells were treated as described previously (Sahara et al, 1999). The phosphorylation status of acinus was monitored with anti-phospho-S422-specific antibody. S422 phosphorylation occurs in wild-type acinus-S and (S573A) mutant. As expected, no S422 phosphorylation is detected in acinus-S (S422A) or (S422, 573A) mutant. Protein kinase inhibitor staurosporine treatment substantially blocks S422 phosphorylation in wild-type acinus-S and (S573A) mutant (Figure 3A, top panel). Immunoblotting analysis with anti-acinus antibody reveals that p45 and p30 kDa bands, which are faint in wild-type acinus-S-transfected cells, are robustly generated upon apoptotic stimulation with the most abundant in acinus-S (S422, 573) mutant. Nevertheless, they both exist in all acinus-S mutant-transfected cells. Although S422 remains intact in acinus S573A mutant, S422 phosphorylation is substantially decreased in this mutant than in wild type (Figure 3A, top panel). The cleavage pattern of acinus tightly couples to the S422 phosphorylation status, underscoring that S422 phosphorylation is critical for protecting acinus from apoptotic cleavage. Presumably, S573A mutation somehow alters acinus-folding conformation, resulting in decreased S422 phosphorylation by Akt. Moreover, the p17 active form for chromatin condensation is selectively produced in acinus-S (S422A) and (S422, 573A) mutant-transfected cells upon staurosporine treatment (Figure 3A, middle panel), underscoring that S422 phosphorylation by Akt plays an essential role in blocking apoptotic fragment formation from acinus-S.
Figure 3.
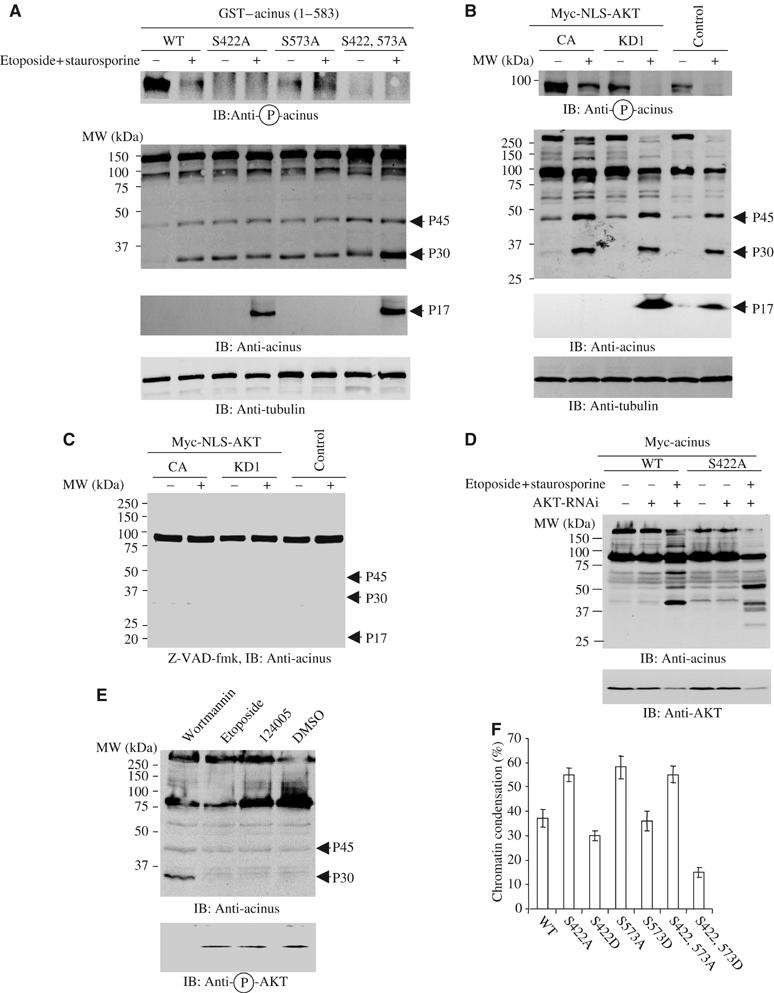
Akt phosphorylation prevents in vivo acinus proteolytic cleavage. (A) S422A and S422, 573A mutants generate p17 active form in transfected cells. GST–acinus wild-type and mutants were transfected into HEK 293 cells, and treated with 100 μM etoposide for 2 h and followed by 1 μM staurosporine for 7 h. Eto/STS treatment substantially decreased wild-type acinus phosphorylation. As expected, no S422 phosphorylation was detected in S422A or S422, 573A mutant (top panel). Both p45 and p30 bands were produced in acinus-S mutant-transfected cells. S422, 573A exhibited the most abundant p30 form, while no p30 was detected in wild-type transfected cells before apoptotic stimulation. Remarkably, the active p17 form was selectively generated in S422A and S422, 573A mutant cells, but not in wild-type or S573A mutant cells (middle panels). Equal amount of cell lysate was employed (bottom panel). (B) Active nuclear Akt phosphorylates acinus-S and prevents its apoptotic cleavage. PC12 cells were stably transfected with inducible form of Myc-NLS-Akt. Upon induction, cells were subjected to drug treatment. Eto/STS treatment substantially decreased the robust acinus-S S422 phosphorylation in CA cells. By contrast, the faint acinus-S phosphorylation was completely eliminated in both KD and EV cells (top panel). Both p45 and p30 bands were produced in all cells. However, the active p17 form was selectively appeared in KD and EV cells, but not in CA cells (second and third panels). Equal amount of cell lysates was employed (bottom panel). (C) A pan-caspase inhibitor z-VAD-fmk prevents acinus degradation. NLS-Akt stably transfected PC12 cells were pretreated with a pan-caspase inhibitor z-VAD-fmk (20 μM) for 30 min, and followed by the drug treatment. Acinus apoptotic cleavage was almost completely blocked. (D) Depletion of Akt enhances acinus wild-type apoptotic cleavage. PC12 cells were stably transfected with inducible form of Myc-tagged acinus wild-type and S422A. Cells were induced and infected with adenovirus expressing shRNAi of rat Akt1, and pretreated with 50 ng/ml NGF for 1 h, followed by Eto/STS treatment. Knocking down of Akt elicited wild-type acinus cleavage as S422A mutant (upper panel). Endogenous Akt was markedly diminished by its RNAi (lower panel). (E) Inhibition of Akt by wortmannin selectively triggers acinus cleavage. A variety of pharmacological agents were incubated with PC12 cells for 24 h, and the cell lysate was analyzed by immunoblotting with anti-acinus and anti-phospho-Akt antibodies. A phosphoinositol ether analog, a putative Akt inhibitor, fails to incur p30 formation, whereas wortmannin potently provokes acinus degradation. Akt activation was blocked by wortmannin but not by other agents. (F) Acinus stable cells were infected with Akt1 shRNAi for 36 h, then treated with Eto/STS. Depletion of Akt in acinus (S422A), (S573A) and (S422, 573A) cells elicits stronger chromatin condensation than wild-type cells. Chromatin condensation in (S422, 573D) cells is significantly less than that in (S422D) and (S573D) cells.
To further evaluate the effect of acinus phosphorylation by Akt, we employed stably transfected PC12 cells with an inducible form of NLS-tagged Akt constructs (Ahn et al, 2004). Markedly phosphorylated acinus-S is observed in CA Akt cells, but not in kinase-dead (KD) or control empty vector (EV)-transfected cells. Etoposide/staurosporine treatment partially decreases phosphorylation of acinus in CA cells, whereas it completely abrogates S422 phosphorylation in KD and EV cells (Figure 3B, top panel). Although both p45 and p30 fragments are observed in all three cell lines upon apoptotic stimulation, acinus-L in CA cells is only partially degraded, compared with its absolute absence in KD and EV cells. Consistently, similar cleavage pattern also occurs to acinus-S, which is markedly decreased in KD and EV cells; in contrast, substantial level of acinus-S remained in CA cells. P17 is evidently produced in KD and control EV cells but not CA cells, underscoring that Akt phosphorylation prevents acinus apoptotic cleavage (Figure 3B, second and third panels). A pan-caspase inhibitor such as z-VAD-fmk robustly inhibits acinus-S cleavage (Figure 3C). Depletion of Akt1 using shRNAi adenovirus significantly increases wild-type acinus cleavage, which is comparable to acinus S422A mutant (Figure 3D, upper panel). The slightly different cleavage patterns of wild-type and S422A acinus might be due to the compensation of other Akt isoforms. Presumably, not only Akt-mediated acinus phosphorylation but also amino-acid substitution-incurred structural alteration implicate in protecting acinus-S from cleavage. To explore whether different cell death-inducing agents blocking Akt could elicit acinus degradation, we employed PI3K inhibitor wortmannin and a putative Akt inhibitor, a phosphatidylinositol ether analogy (Hu et al, 2000). Inhibition of Akt by wortmannin robustly triggers p30 acinus formation, whereas phosphatidylinositol analogy and etoposide control fail. The acinus cleavage activity couples to Akt phosphorylation status, suggesting that Akt activation is indispensable for protecting acinus from apoptotic degradation (Figure 3E). Knocking down of Akt1 in acinus stably transfected cells reveals stronger chromatin condensation in phosphorylation-crippled mutant cells than wild-type cells, consistent with the extent of acinus cleavage in Akt knocked-down cells (Figure 3D). The least abundant chromatin condensation occurred in phosphorylation mimetic S422, 573D cells, followed by S422D and S573D cells, underscoring that Akt phosphorylation on acinus is essential for preventing chromatin condensation (Figure 3F). Therefore, our experiments demonstrate that active nuclear Akt phosphorylates acinus and protects its proteolytic cleavage during apoptosis.
Akt binds to acinus
To explore whether acinus-S binds to Akt, we transfected RFP-Akt into HEK 293 cells with various GST–acinus-S constructs, and treated transfected cells with EGF for 20 min. GST pulldown reveals that wild-type acinus-S potently associates with Akt, and EGF treatment enhances the binding. Strikingly, the interaction is almost completely disrupted by either S422A or S573A mutation. EGF treatment displays a modest effect on Akt association with acinus mutants. S422 is potently phosphorylated in acinus-S S573A, but the binding by S573A mutant to Akt is also crippled. Mapping experiment reveals that the C-terminus of acinus is implicated in binding Akt (data not shown). Presumably, C-terminal S573 is directly involved in interacting with Akt or critical for maintaining the conformation of acinus, albeit it is less important for preventing acinus apoptotic degradation than S422. Interestingly, S422D acinus mutant binds to Akt, though it is weaker than the wild-type counterpart, and this interaction is not regulated by EGF (Figure 4A, top panel).
Figure 4.
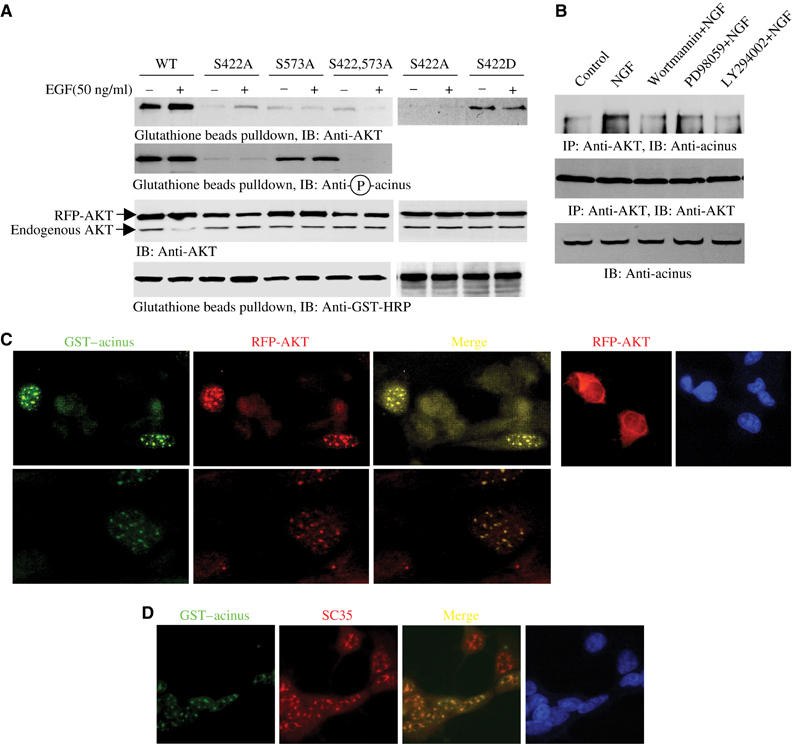
Akt binds to acinus. (A) Akt co-precipitates with GST–acinus-S. HEK 293 cells were cotransfected with RFP-Akt and GST–acinus-S wild type and mutants, followed by EGF stimulation. Akt strongly binds to acinus-S under basal condition. EGF enhanced the interaction. No significant interaction was observed between acinus mutants and Akt. Interestingly, S422D acinus binds to Akt, which was not regulated by EGF (top panel). S422 was potently phosphorylated in both wild-type and acinus (S573A) mutant (second panel). Equal amount of RFP-Akt and GST–acinus-S was employed (third and bottom panels). (B) Endogenous Akt binds to acinus-S. PC12 cells were respectively pretreated with various inhibitors for 30 min, then NGF was introduced. NGF elicited robust interaction between Akt and acinus-S, but PI3K inhibitors markedly blocked it. By contrast, MEK1 inhibitor had no effect (top panel). Equal amount of Akt was pulled down (middle panel). Equal level of acinus-S was employed (bottom panel). (C) Akt colocalizes with Acinus in the nucleus. HEK 293 cells were cotransfected with RFP-Akt and GST–acinus-S. The transfected cells were stained with anti-GST-FITC antibody. RFP-Akt colocalizes with GST–acinus in the nucleus (left three panels). RFP-Akt alone occurred in both the cytoplasm and the nucleus (right panels). (D) Akt and acinus-S colocalize in the nucleus with speckles. GST–acinus-transfected cells were stained with FITC-conjugated anti-GST antibody and anti-SC35, a specific marker for nuclear speckle.
To determine whether endogenous acinus-S binds to Akt in naïve PC12 cells, we conducted coimmunoprecipitation with Akt antibody-conjugated beads. Compared with control cells, NGF stimulates robust interaction between acinus-S and Akt. PI3K inhibitor pretreatment significantly decreases the binding; by contrast, MEK1 inhibitor PD98059 exhibits a negligible effect (Figure 4B, top panel). Thus, activation of PI3K pathway is indispensable for the interaction between Akt and acinus-S. To further explore the interaction between Akt and acinus-S, we performed immunohistochemistry on transfected HEK 293 cells. RFP-Akt alone predominantly occurs in the cytoplasm, but it also distributes in the nucleus as well. However, when it is cotransfected with GST–acinus-S, they both notably colocalize in the nucleus with speckles (Figure 4C). Immunofluorescent staining further demonstrates that acinus colocalizes with a nuclear speckle marker, SC35, confirming the previous finding (Schwerk et al, 2003) (Figure 4D).
Nuclear Akt regulates chromatin condensation and DNA fragmentation
The active form of acinus is sufficient and necessary for triggering chromatin condensation (Sahara et al, 1999). Akt phosphorylates acinus and suppresses the active fragment formation from apoptotic degradation (Figure 3). To address whether nuclear Akt mediates chromatin condensation, we induced Myc-NLS-Akt in PC12 cells and treated the cells with etoposide and staurosporine. Enormous chromatin condensation is observed in both KD and control EV cells. In contrast, substantially less chromatin condensation in CA cells (Figure 5A). These observations precisely correlate with acinus apoptotic degradation and active form production (Figure 3B). The induction of Myc-NLS-tagged Akt and its kinase activity are confirmed (Figure 5B). Consistently, evident DNA fragmentation occurs in KD cells, while demonstrable but less extensive DNA fragmentation is also observed in EV cells; by contrast, no DNA fragmentation is detected in CA cells (Figure 5C, upper panel). In addition, caspase-3 is completely cleaved in KD and EV cells, whereas marked caspase-3 remains intact in CA cells (Figure 5C, lower panel), which couples to nuclear Akt kinase activity, indicating that nuclear Akt could somehow also regulate cytoplasmic upstream apoptotic machinery. To further explore whether nuclear Akt regulates chromatin condensation, we infected PC12 cells with adenovirus expressing Myr-Akt (myristolated Akt) and NLS-Akt, respectively. Acinus degradation is substantially blocked in NLS-Akt-infected cells compared with control and Myr-Akt-infected cells (Figure 5D, top panel). Chromatin condensation couples to acinus apoptotic cleavage pattern (Figure 5D, bottom panel). Therefore, nuclear, but not cytoplasmic, Akt plays an essential role in mediating chromatin condensation, probably through suppressing acinus from apoptotic degradation by phosphorylation.
Figure 5.

Nuclear Akt regulates chromatin condensation and DNA fragmentation. (A) Chromatin condensation assay with DAPI. Myc-NLS-Akt-transfected PC12 cells were induced and stimulated with NGF for 30 min, then treated with Eto/STS. The nuclei with condensed chromatin are indicated by white arrowheads (left panels). Quantitative analysis of chromatin condensation (right panel). Numbers of nuclei with condensed chromatin morphology were calculated as means (±s.d.) of five determinations and are representative of three experiments. (B) Characterization of induced nuclear Akt cell lines. Robust induction of CA and KD Akt was detected in the corresponding cell lines (upper panel). In vitro Akt kinase assay demonstrated that Akt in CA cells possesses much higher kinase activity than those in EV and KD cells (lower panel). (C) DNA fragmentation and caspase-3 cleavage. Drug treatment triggered evident DNA fragmentation in KD cells, while less extensive DNA fragmentation was also observed in EV cells. By contrast, no significant DNA fragmentation occurred in CA cells (upper panel). Immunoblotting of caspase-3 (lower panel). (D) Nuclear Akt prevents chromatin condensation and acinus cleavage. PC12 cells were infected with control adenovirus and adenovirus expressing Myr-Akt and NLS-Akt, followed by Eto/STS treatment. NLS-Akt substantially suppressed acinus apoptotic cleavage, but the cytoplasmic Akt failed (top panel). The expression of cytoplasmic and nuclear Akt was verified (middle panel). Compared with control and Myr-Akt-infected cells, chromatin condensation was decreased in NLS-Akt cells (bottom panel).
Acinus-mediated chromatin condensation is blocked by Akt phosphorylation
To further investigate whether acinus's apoptotic cleavage and subsequent chromatin condensation is regulated by Akt, we established stably transfected PC12 cell lines with inducible form of Myc-acinus-S. The induced cells were pretreated with NGF, followed by etoposide/staurosporine treatment. If p17 from acinus-S proteolytic fragmentation is blocked by Akt phosphorylation, then, upon NGF treatment, wild-type acinus-S-transfected cells should reveal significantly less cleavage and chromatin condensation than Akt phosphorylation sites mutated cell lines. As predicted, the active form p17 is selectively generated in S422A and S422, 573A cells but not in wild-type or S573A cells after drug treatment. The p30 form is evident in all cells regardless of drug treatment, but it was increased after stimulation. Basal level of p45 form is also detected in all cells, but it was robustly enhanced after drug treatment (Figure 6A). Chromatin condensation assay shows that about 50% nuclei in both S422A and S422, 573A cells displayed condensed chromatin morphology with less than 10% in S573A and wild-type acinus-S-transfected cells, coupling to p17 formation from acinus-S apoptotic degradation. The nuclei with condensed chromatin morphology are indicated by white arrowheads (Figure 6B). We also investigated acinus degradation and chromatin condensation in the absence of NGF. Demonstrable acinus degradation occurred in wild-type, S to A and S to D mutant cells. By contrast, acinus degradation was substantially decreased in S422, 573D cells. Moreover, the active p17 form was almost not detectable in S422D or S422, 573D cells, underscoring that S422 phosphorylation is essential for suppressing its apoptotic cleavage in vivo (Figure 6C, top and middle panel). Quantitative analysis of chromatin condensation fits with this finding (Figure 6C, bottom panel).
Figure 6.
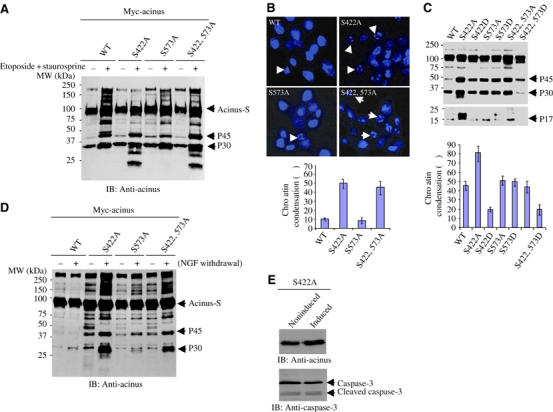
Acinus-mediated chromatin condensation is Akt-phosphorylation dependent. (A) Acinus cleavage in stably transfected PC12 cell lines. Myc-acinus stably transfected PC12 cells were induced and pretreated with NGF, followed by Eto/STS treatment. The p30 form was evident in all cells, but it was increased after stimulation. P45 form was substantially enhanced after drug treatment. Notably, p17 was selectively generated in S422A or S422, 573A cells after apoptotic stimulation (top panel). (B) Chromatin condensation analysis. The nuclei with condensed chromatin were labeled with white arrowhead (upper panel). About 50% nuclei in S422A and S422, 573A cells displayed condense chromatin morphology, with less than 10% in S573A and wild-type acinus-transfected cells (lower panel). (C) S422, 573D cells resist caspase-induced degradation and chromatin condensation. Stably transfected PC12 cells were induced and followed by Eto/STS treatment in the absence of NGF. Acinus apoptotic cleavage and p17 were substantially decreased in S422D and S422, 573D cells compared with other cell lines (top and middle panels). Chromatin condensation correlated with acinus degradation (lower panel). (D) NGF withdrawal provokes acinus cleavage and chromatin condensation. Stably transfected PC12 cells were incubated with NGF for 5 days. The differentiated cells were cultured in medium without NGF. In 24 h, markedly cleaved p45 and p30 fragments of acinus occurred in S422A and S422AS573A cells, and S573A cells revealed less extent of p45 and p30 fragments. (E) Induction of S422A in PC12 cells does not affect caspase-3 activation. PC12 cells were stably transfected with inducible form of Myc-tagged acinus (S422A). Cells were induced. Immunoblotting analysis was conducted with anti-caspase-3 antibody.
To explore further acinus cleavage under more physiological condition, we differentiated stably transfected PC12 cells with NGF for 5 days, followed by NGF withdrawal. Both S422A and S422, 573A cells reveal robust acinus degradation. However, S573A cells exhibit decreased level of acinus degradation; by contrast, wild-type cells display negligible acinus cleavage (Figure 6D). Chromatin condensation results couple to acinus degradation (data not shown). The prominent effect in S422A cells raises the possibility that the mutation might cause a structural alteration, and somehow is toxic to the cells, leading to acting as a dominant-negative form, thereby enhancing apoptosis. To assess this possibility, we monitor caspase-3 activation before and after acinus S422A induction. Immunoblotting analysis shows that caspase-3 activity remains the same (Figure 6E). Therefore, Akt-mediated acinus phosphorylation is essential for preventing its cleavage and chromatin condensation.
Knocking down of acinus diminishes chromatin condensation
To examine whether depletion of endogenous acinus affects chromatin condensation, we infected NLS-Akt cells with control adenovirus or adenovirus expressing shRNAi of acinus. In 36 h, we treated the infected cells with Eto/STS. Control immunoblotting reveals that acinus was markedly depleted by its RNAi (Figure 7A). Under control condition, most evident acinus degradation occurs in KD cells, followed by control EV cells, and most acinus-S remains intact in CA cells. Knocking down of acinus significantly abolishes acinus-S in all cells. P30 is substantially diminished in CA cells, but it is demonstrable in both KD and EV cells. Compared with control, p17 is almost completely depleted after acinus RNAi treatment (Figure 7B, left panel). Depletion of acinus substantially decreases chromatin condensation in all cells compared with control adenovirus, suggesting that acinus is essential for this event. Surprisingly, more chromatin condensation occurs in EV cells than KD cells (Figure 7B, right panel). Presumably, nuclear Akt has numerous downstream effectors, contributing to prevention of chromatin condensation. Hence, when Akt is overexpressed, the substrate effectors might be activated and suppress apoptosis. The nonsubstrate-binding targets including JNK interacting protein-1 (JIP1) might also be sequestered from death machinery and block apoptosis. Together, these experiments thus establish that acinus-S is a novel nuclear substrate of Akt. Acinus phosphorylation by Akt prevents its apoptotic cleavage and active form production, leading to suppression of chromatin condensation.
Figure 7.
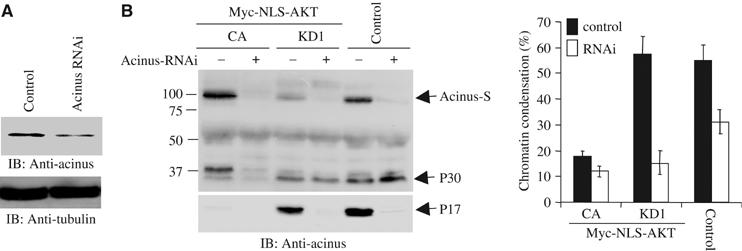
Knocking down of acinus decreases chromatin condensation. (A) Endogenous acinus was decreased upon shRNAi of acinus treatment but not in the control sample, while tubulin control is not affected. (B) NLS-Akt cells were infected with control adenovirus and adenovirus expressing shRNAi to knock down acinus. The infected cells were then treated with Eto/STS. Depletion of acinus abolished p17 formation (left panel). Compared with control cells, depletion of acinus decreased chromatin condensation (right panel).
Discussion
In this report, we have identified and characterized a novel nuclear substrate for Akt, acinus. Akt phosphorylates acinus on both S422 and S573 in vitro and in vivo. Acinus undergoes systematically apoptotic degradation during programmed cell death. However, S422 phosphorylation plays a more critical role than S573 in preventing active form p17 production from acinus-S. Further, we have demonstrated that nuclear Akt blocks chromatin condensation through preventing acinus from apoptotic cleavage.
Acinus-S possesses three Akt substrate consensus motifs, but aa 398–404 RERTRSE, and cannot be phosphorylated by active Akt in vitro (Figure 1B). Both aa 417–423, RSRSRSR and aa 568–574, RSRSRST can be robustly phosphorylated by Akt, but they display different activities in preventing acinus-S cleavage. When both residues serine 422 and 573 are mutated into alanine, acinus-S is extremely sensitive to in vitro apoptotic cleavage. Cell-free apoptotic assay reveals that a 30 kDa fragment yielded from GST–acinus-S recombinant proteins, but p45 or p17 form was not detected, suggesting that D335 site is the major cutting site in GST–acinus fusion protein. Wild-type acinus strongly binds to Akt, but it was substantially cleaved (Figure 2C), suggesting that Akt binding cannot shield acinus from caspase cleavage. However, apoptotic cleavage with in vitro transcription and translation 35S-labeled acinus displays multiple bands in S422A and S422, 573A mutants. As fragment 228–335 does not contain any methionine, p17 is not radiolabeled; therefore, it is invisible (Figure 2D). These results demonstrate that 228 site can also be cleaved. In the absence of Akt treatment, both S422D and S422, 573D resist to apoptotic cleavage; however, S573D is somehow degraded (Figure 2E). The findings demonstrate that S422 phosphorylation plays a more important role in suppressing proteolytic cleavage of acinus than S573 does.
The different acinus degradation patterns in Figure 2C and D suggest that acinus-S expressed in mammalian system possesses distinct activities from bacterial expressed recombinant proteins in resisting against apoptotic cleavage, presumably due to different post-translational modification. It is also possible that GST tag protein might shield some cleavage sites in acinus from caspases. Upon staurosporine treatment, p30 fragment is evidently augmented in GST–acinus-S-transfected HEK 293 cells or PC12 cells (Figures 3, 5 and 6), fitting with in vitro cleavage results with recombinant GST-fusion proteins (Figure 2C). In alignment with this observation, p17 active form is robustly generated in GST–acinus-S (S422A) and (S422, 573A) but not GST–acinus-S (S573A) mutant- or wild-type-transfected cells (Figure 3A), and our results with chromatin condensation assay demonstrate that p17 but not p30 or 45 form accounts for the activity, consistent with previous report (Sahara et al, 1999). Induction of S422A resulted in production of p17 and more chromatin condensation in acinus S422 mutant cells than wild-type cells. It is possible that not only Akt-mediated acinus phosphorylation but also amino-acid substitution-incurred structural change contributes to protect acinus-S from cleavage, albeit the latter is less likely. Nevertheless, both S422D and S422, 573D mutants resist to caspase-dependent degradation even in the absence of Akt treatment (Figure 2E), supporting that acinus phosphorylation by Akt is essential for preventing acinus from apoptotic degradation. Since the C-terminus of acinus is implicated in binding Akt (data not shown), S573 might be directly involved in binding to Akt, resulting in S573A mutant failing to associate with Akt (Figure 4A). However, the possibility that S573 mutation might incur acinus conformational change cannot be absolutely excluded either. In absence of NGF, acinus degradation also occurs in S422D and S422, 573D stable cells; however, the extent is less than other mutant cell lines. The active p17 form is absent in S422, 573D cells and decreased in S422D cells (Figure 6C), suggesting that coordinate S422 and S573 phosphorylation is essential for Akt to prevent acinus-S from apoptotic degradation in vivo, though S422 phosphorylation appears more critical than that of S573.
Akt binds numerous proteins. Some of the binding partners are the substrates, whereas some proteins do not serve as Akt substrates, but rather seem to play a modulatory role or be regulated by Akt. For example, JIP1 directly binds to the PH domain of Akt. Overexpression of Akt1, but not Akt2, inhibits the ability of JIP1 to potentiate JNK1 activation in cells, protecting neurons from apoptosis triggered by excitotoxins (Kim et al, 2002). Moreover, this inhibition is independent of Akt kinase activity. Therefore, Akt may act as a ‘steric hindrance' to prevent assembly of the JNK signaling cassette around the scaffold JIP1. Hence, Akt may contribute to prevent apoptosis independent of its kinase activity. A number of nuclear proteins including acinus, MST1 and PKC-δ are implicated in triggering chromatin condensation. Knocking down of acinus alone substantially decreases chromatin condensation in control cells, suggesting that it is essential for this event. In addition, nuclear Akt might have numerous downstream effectors, contributing to prevention of chromatin condensation. Conceivably, when nuclear Akt (CA or KD) is overexpressed, the substrate effectors will be phosphorylated and inhibit apoptosis. The non-substrate-binding partners including JIP1 might be caged away from cell death machinery and suppress apoptosis, which might explain why chromatin condensation in NLS-Akt-KD cells is less than control EV cells, when acinus was knocked out by its RNAi (Figure 7).
Internucleosomal chromatin fragmentation is a biochemical hallmark of apoptosis. One of the nucleases primarily responsible for genomic DNA fragmentation during apoptosis is DFF40 or CAD. DFF40/CAD is activated by caspase-3 that cleaves the nuclease's inhibitor DFF45/ICAD (Widlak, 2000). In addition, apoptosis-inducing factor (AIF) provokes chromatin condensation in a caspase-3-independent pathway, which leads to large-scale DNA fragmentation without internucleosomal DNA cleavage (Lorenzo et al, 1999). Nevertheless, acinus triggers chromatin condensation without DNA fragmentation (Sahara et al, 1999). However, upon etoposide/staurosporine treatment, evident DNA fragmentation, which coincides with the appearance of active form of p17, was observed in KD and EV cells, but not CA cells. Clearly, different effectors regulate nuclear changes in apoptotic cells through distinct downstream targets.
Active nuclear Akt inhibits staurosporine or hypoxia-induced apoptosis in cardiomyocytes without affecting cytoplasmic Akt substrate phosphorylation (Shiraishi et al, 2004), arguing that nuclear Akt activation alone might be sufficient to suppress the apoptotic machinery. Here, we show that nuclear Akt mediates chromatin condensation through regulating acinus phosphorylation. Employing stably transfected PC12 cells with inducible Myc-NLS-Akt and NLS-Akt and Myr-Akt adenovirus, we demonstrate that nuclear, but not cytoplasmic, Akt prevents acinus apoptotic cleavage and chromatin condensation (Figure 5). Therefore, our finding provides further evidence that nuclear Akt inhibits apoptosis not only through phosphorylating transcription factors but also other apoptotic effectors in the nucleus.
Materials and methods
Cells and reagents
PC12 cells were maintained in medium A (DMEM with 10% fetal bovine serum (FBS), 5% horse serum and 100 U penicillin–streptomycin) at 37°C with 5% CO2 atmosphere in a humidified incubator. The Myc-acinus and Myc-NLS-Akt stably transfected PC12 cells (Tet-off cell line) were cultured in medium B (85% DMEM, 10% horse serum, 5% FBS, 100 μg/ml G418, 100 μg/ml hygromycin B, 2 μg/ml tetracycline and 100 U penicillin–streptomycin). The transfected genes were induced by culturing in medium B without tetracycline for 24 h. NGF was from Roche. Anti-caspase-3 and α-tubulin antibodies were from Santa Cruz Biotechnology, Inc. Anti-Myc, acinus, phospho-Akt-473 and Akt antibodies were from Cell Signaling. Active Akt protein was from Upstate Biotechnology, Inc. Akt inhibitor 124005 was from Calbiochem. All the chemicals not included above were from Sigma.
Generation of anti-phospho-acinus S422-specific antibody
Synthesized S422 phosphorylated peptide (CRSRS(PO3)RDRRRKERAKS-COOH) was conjugated to KLH and injected into rabbits. Antibodies were affinity purified from serum by use of unphosphorylated peptide crosslinked to Affi-Gel 10 or Affi-Gel 15 according to the manufacturer's protocol (Bio-Rad).
Generation of adenovirus expressing acinus and Akt RNAi
The short hairpin acinus-L (nucleotides: 3058–3086 GTT CGT CCC TTC ACT CTA GGC CAG CTA AA) and Akt1 (nucleotides: 511–539 GCC ACA GGT CGC TAC TAT GCC ATG AAG AT) RNAi were subcloned into pGE1 (Stratagene) between BamHI and XbaI sites, respectively, to transfect these constructs into HEK 293 cells and verify whether the RNAi is able to knock down the corresponding gene. Then the insert is cut by XbaI and XhoI and religated into pAdTrack. Adenovirus expressing shRNAi was prepared. The virus was purified by CsCl banding with 1011–1012 plaque-forming units, and introduced into PC12 cells and cultured for 36 h. GFP was monitored with a fluorescent microscope.
Cytoplasmic and nuclear fractionation
HEK 293 cells and PC12 cells were collected and washed once with ice-cold 1 × PBS. The cell pellet was resuspended in CER I buffer. The cytoplasmic and nuclear fractions were prepared as described in manufacturer's protocol (PIERCE, NE-PER, nuclear and cytoplasmic extraction reagent).
Coimmunoprecipitation and in vitro binding assays
A 10-cm plate of HEK 293 cells or PC12 cells were washed once in PBS, and lysed in 1 ml lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride (PMSF)), and centrifuged for 10 min at 14 000 g at 4°C. The supernatant was transferred to a fresh tube. Experimental procedures for coimmunoprecipitation and in vitro binding assays are as described (Ye et al, 2000). After SDS–PAGE, the samples were transferred to a nitrocellular membrane. Western blotting analysis was performed with a variety of antibodies.
Cytochemical staining of apoptotic cells
Stably transfected PC12 cells were induced in tetracycline-free medium overnight. In the presence or absence of NGF, cells were treated with 10 μM etoposide for 24 h, followed by 1 μM staurosporine for another 5 h. Morphological changes in the nuclear chromatin of cells undergoing apoptosis were detected by staining with 4,6-diamidino-2-phenylindole (DAPI) as described (Ye et al, 1998). Totally, about 500 nuclei were counted under different fields.
Acknowledgments
This work is supported by grant from the National Institute of Health (RO1, NS045627) to K Ye. We are thankful to Dr Christian Schwerk for Flag-Acinus construct and Drs Mark Sussman and Kenneth Walsh for Akt adenovirus.
References
- Ahmed NN, Franke TF, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN (1993) The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene 8: 1957–1963 [PubMed] [Google Scholar]
- Ahn JY, Rong R, Liu X, Ye K (2004) PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J 23: 3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs WH III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Curr Opin Neurobiol 11: 297–305 [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241 [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689 [DOI] [PubMed] [Google Scholar]
- Du K, Montminy M (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379 [DOI] [PubMed] [Google Scholar]
- Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, Gallegos A, Powis G, Kozikowski AP (2000) 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem 43: 3045–3051 [DOI] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV (2002) Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron 35: 697–709 [DOI] [PubMed] [Google Scholar]
- Kim SJ (1998) Insulin rapidly induces nuclear translocation of PI3-kinase in HepG2 cells. Biochem Mol Biol Int 46: 187–196 [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM (1999) Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398: 630–634 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157 [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184 [DOI] [PubMed] [Google Scholar]
- Lorenzo HK, Susin SA, Penninger J, Kroemer G (1999) Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ 6: 516–524 [DOI] [PubMed] [Google Scholar]
- Lu PJ, Hsu AL, Wang DS, Yan HY, Yin HL, Chen CS (1998) Phosphoinositide 3-kinase in rat liver nuclei. Biochemistry 37: 5738–5745 [DOI] [PubMed] [Google Scholar]
- Marchisio M, Bertagnolo V, Colamussi ML, Capitani S, Neri LM (1998) Phosphatidylinositol 3-kinase in HL-60 nuclei is bound to the nuclear matrix and increases during granulocytic differentiation. Biochem Biophys Res Commun 253: 346–351 [DOI] [PubMed] [Google Scholar]
- Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA (1997) Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem 272: 30491–30497 [DOI] [PubMed] [Google Scholar]
- Neri LM, Milani D, Bertolaso L, Stroscio M, Bertagnolo V, Capitani S (1994) Nuclear translocation of phosphatidylinositol 3-kinase in rat pheochromocytoma PC 12 cells after treatment with nerve growth factor. Cell Mol Biol (Noisy-le-grand) 40: 619–626 [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 401: 82–85 [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ryder U, Lamond AI, Mann M (2002) Large-scale proteomic analysis of the human spliceosome. Genome Res 12: 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS (1999) NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401: 86–90 [DOI] [PubMed] [Google Scholar]
- Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y (1999) Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 401: 168–173 [DOI] [PubMed] [Google Scholar]
- Schwerk C, Prasad J, Degenhardt K, Erdjument-Bromage H, White E, Tempst P, Kidd VJ, Manley JL, Lahti JM, Reinberg D (2003) ASAP, a novel protein complex involved in RNA processing and apoptosis. Mol Cell Biol 23: 2981–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA (2004) Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 94: 884–891 [DOI] [PubMed] [Google Scholar]
- Widlak P (2000) The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim Pol 47: 1037–1044 [PubMed] [Google Scholar]
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (2000) Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103: 919–930 [DOI] [PubMed] [Google Scholar]
- Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC (1998) Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA 95: 1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407: 802–809 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, Reed R (2002) Comprehensive proteomic analysis of the human spliceosome. Nature 419: 182–185 [DOI] [PubMed] [Google Scholar]


