Abstract
We investigated the phagocytosis of Haemophilus ducreyi both in vitro and in vivo. Human granulocyte and monocyte phagocytosis of opsonized and nonopsonized, fluorescence-labeled H. ducreyi was assessed by flow cytometry. Both Escherichia coli and noncapsulated H. influenzae were included as controls. The maximal percentage of granulocytes taken up by H. ducreyi was 35% after 90 min. In contrast, 95% of H. influenzae bacteria were phagocytosed by granulocytes after 30 min. These results indicated that H. ducreyi phagocytosis was slow and inefficient. Bacterial opsonization by using specific antibodies increased the percentage of granulocytes phagocytosing H. ducreyi from 24 to 49%. The nonphagocytosed bacteria were completely resistant to phagocytosis even when reexposed to granulocytes, indicating that the H. ducreyi culture comprised a mixture of phenotypes. The intracellular survival of H. ducreyi in granulocytes, in monocytes/macrophages, and in a monocyte cell line (THP-1) was quantified after application of gentamicin treatment to kill extracellular bacteria. H. ducreyi survival within phagocytes was poor; approximately 11 and <0.1% of the added bacteria survived intracellularly after 2 and 20 h of incubation, respectively, while no intracellular H. influenzae bacteria were recovered after 2 h of incubation with phagocytes. The role of phagocytes in the development of skin lesions due to H. ducreyi was also studied in vivo. Mice that were depleted of granulocytes and/or monocytes and SCID mice, which lacked T and B cells, were injected intradermally with approximately 106 CFU of H. ducreyi. Within 4 days of inoculation, the granulocyte-depleted mice developed lesions that persisted throughout the experimental period. This result reinforces the importance of granulocytes in the early innate defense against H. ducreyi infection. In conclusion, H. ducreyi is insufficiently phagocytosed to achieve complete eradication of the bacteria. Indeed, H. ducreyi has the ability to survive intracellularly for short periods within phagocytic cells in vitro. Since granulocytes play a major role in the innate defense against H. ducreyi infection in vivo, bacterial resistance to phagocytosis probably plays a crucial role in the pathogenesis of chancroid.
Haemophilus ducreyi is the causative agent of chancroid, a sexually transmitted disease that is characterized by mucocutaneous ulcerative lesions on the external genitals and occasionally painful swollen regional lymph nodes. The disease is common in developing countries, particularly in Africa and Southeast Asia, but localized outbreaks have been reported in Canada, the United States, and Europe (10, 25). The disease has received renewed attention following reports that genital ulcers facilitate the transmission of human immunodeficiency virus (HIV) in populations in which genital ulcers are endemic (17, 36, 40).
The pathogenesis of chancroid is not well understood, and information concerning essential H. ducreyi virulence components is scarce. The known H. ducreyi virulence factors include lipooligosaccharide (LOS), which may contribute to ulcer formation by inducing the migration of inflammatory cells (7, 18). H. ducreyi also expresses cell surface components that are similar to human cell receptors and that may prevent host immune recognition of the bacteria (22). In addition, the bacteria have the ability to bind to extracellular matrix protein (5). Other identified virulence factors include pilus-like structures; heat shock proteins (8, 11, 27); and components that enable the bacteria to survive within the host, including outer membrane proteins, hemoglobin-binding proteins, and periplasmic superoxide dismutase (6, 30, 37). Two H. ducreyi toxins, the cytolethal distending toxin (CDT) and hemolysin, have been shown to destroy various human cells (9, 20, 28, 34, 38, 39).
Previous results showed that the bactericidal activities of specific antibodies, including antibodies to LOS, are insufficient for the killing of H. ducreyi, although many other gram-negative bacteria can be killed in this manner (12). It has also been suggested that virulent, in contrast to avirulent, H. ducreyi strains are relatively resistant to killing by human neutrophils in vitro (12, 19).
Several animal models, including rabbits, pigs, and mice, have been used to study the pathogenesis of and host immune responses involved in H. ducreyi infection (15, 16, 18, 31). All these models of infection result in intraepidermal lesions containing infiltrates of polymorphonuclear leukocytes (PMNL), monocytes, macrophages, and T cells. Similar cell populations were described for early-stage disease in humans; in these studies, bacteria were injected intradermally into the forearm skin of volunteers (26, 33, 37). In human infection studies such as these, although H. ducreyi was found to be associated with fibrin and collagen in the dermis of the pustule, no intracellular bacteria were observed, suggesting that H. ducreyi remains extracellular in the early stages of infection (3, 4). Examination of Wright-stained smears of a lesion from a laboratory-acquired H. ducreyi infection showed the presence of PMNL and both intracellular and extracellular rod-shaped bacteria (41). In clinical cases of chancroid, where smears of the lesion exudate were examined by using fluorescence microscopy and a specific monoclonal antibody (MAb) against H. ducreyi LOS, both phagocyte-engulfed and extracellular H. ducreyi bacteria were observed (2).
Neutrophils and monocytes/macrophages are the earliest types of leukocytes entering the tissue in response to invading pathogens. The major role of neutrophils in inflammatory and immune responses is thought to be bacterial phagocytosis, followed by the killing of bacteria via the generation of reactive oxygen intermediates and the release of lytic enzymes. Neutrophils play a crucial protective role in the early phases of many infectious diseases, such as Staphylococcus aureus skin infection, and are important for a beneficial outcome (24, 43). The monocytes/macrophages phagocytose bacteria, act as antigen-presenting cells, and secrete large numbers of regulatory products. A common detrimental outcome of infection is that of macrophage-mediated tissue destruction (44). However, the role of phagocytic cells in host-parasite interactions in chancroid is poorly understood and requires further investigation.
The aims of this study were to elucidate the kinetics and efficiency in vitro of the phagocytosis of opsonized and nonopsonized H. ducreyi strains by human phagocytes and the capacity of the bacteria to survive within phagocytic cells. In addition, we investigated the role of granulocytes and monocytes in the early defense against intradermally injected H. ducreyi in a mouse model.
MATERIALS AND METHODS
Mice.
Female BALB/c mice 5 to 6 weeks old and SCID mice were obtained from B&K Universal AB (Stockholm, Sweden). They were housed in the Experimental Biomedicine Animal Facility of the University of Göteborg. Mice were maintained under standard conditions of light and temperature and fed standard laboratory chow and water ad libitum. All animal experiments were approved by the Animal Ethics Committee of the University of Göteborg.
Bacterial strains and cultivation.
Four H. ducreyi strains obtained from the Culture Collection, University of Göteborg (CCUG), and one strain obtained from the American Type Culture Collection (ATCC) were included in this study. Strains CCUG 7470, CCUG 27022, and ATCC 35000 have a nonasaccharide LOS structure (long LOS) and produce CDT, except for strain CCUG 27022, which is not a toxin producer (1, 23, 28). Strain CCUG 4438 has a hexasaccharide LOS structure (short LOS) and does not produce toxin (1, 28). The noncapsulated H. influenzae strain, CCUG 7566, which has been shown to be 100% sensitive to phagocytic killing by PMNL, was used as a control (12).
H. ducreyi was cultivated on chocolate agar plates as previously described (28). Since H. ducreyi aggregates on solid medium, bacteria were further cultured in liquid medium and incubated in an anaerobic jar with rotation at 100 rpm for 15 to 16 h at 33°C as previously described (28). The liquid medium used for the cultivation of H. ducreyi was brain heart infusion broth supplemented with 1% hemin-histidine (Sigma Chemical Company, St. Louis, Mo.), 0.04% l-histidine (Fluka Chemie AG, Buchs, Switzerland), 10% fetal calf serum (FCS), 1% IsoVitaleX, and 3 μg of vancomycin (Department of Bacteriology, Sahlgrenska Hospital, Göteborg, Sweden)/ml. H. influenzae was cultivated on chocolate agar plates for 24 h at 37°C in a CO2-enriched atmosphere.
Animal experiments.
Six groups of mice, with 10 to 15 mice/group, were used in this study. One group of BALB/c mice was depleted of granulocytes by intraperitoneal injections of partially purified MAb RB6-8C5 (DNAX, Research Institute of Molecular and Cellular Microbiology, Palo Alto, Calif.). The MAb was administered at 1 mg of protein/ml 1 day before challenge with bacteria and on days 2 and 4 postchallenge (24, 43). A control group of 15 mice was injected with an unrelated MAb, CT17/13 (specific for cholera toxin), in the same manner. In order to deplete peripheral monocytes, a group of BALB/c mice was treated with etoposide (VePesid; Bristol-Mayers SQUIBB, Bromma, Sweden) (44). This cytostatic drug leads to a selective decrease in monocyte numbers in peripheral blood (44). Etoposide was diluted to 2 mg/ml in phosphate-buffered saline (PBS), and 200 μl was injected subcutaneously once a day throughout the experimental period. Another group of mice was depleted of both granulocytes and monocytes. The efficacies of those treatments were investigated by flow cytometric analysis of peripheral blood cells. Treated mice showed 95 and 50% fewer granulocytes and monocytes, respectively, than control mice. The extent of leukocytopenia was similar to that described in previous studies (24, 43, 44). Groups of T- and B-cell-deficient mice (SCID) and naive BALB/c mice (control) were also included in this study. All mice were injected intradermally with 106 CFU of H. ducreyi strain CCUG 7470 that had been cultured in liquid medium for 15 h. Lesion development was examined over a period of 10 days (18). Bacteria were cultivated from lesions at days 2, 5, and 10.
In addition, four groups of mice (five mice per group) that were (i) granulocyte depleted, (ii) monocyte depleted, (iii) SCID, or (iv) untreated and immunocompetent (naive) were injected intradermally with two doses, 150 and 15 μg, of purified LOS from H. ducreyi strain CCUG 7470 (1, 18). Lesion development was monitored as described above.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed as described previously (12). Briefly, the H. ducreyi bacteria were suspended in PBS to an optical density at 600 nm of 0.3. Heat-killed (65°C for 80 min) or non-heat-killed bacteria were used to coat microtitration plates (Greiner, Labortechnik GmbH, Frichenhausen, Germany) overnight at room temperature (23°C). Plates were blocked with 1% (wt/vol) bovine serum albumin (Sigma) in PBS. A 100-μl quantity of rabbit immune serum against surface antigens (24-kDa protein, 60-kDa heat shock protein, and LOS) was added per well (starting with a dilution of 1:100 and then diluted 10-fold in dilution buffer [0.1% bovine serum albumin-PBS-Tween 20]) and incubated overnight at room temperature. Nonimmunized rabbit serum and antiserum to H. ducreyi LOS were used as reference sera. The conjugate used was alkaline phosphatase-labeled anti-rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories); for reaction development, 1 mg of disodium p-nitrophenyl phosphate (Sigma) per ml in 1 M diethanolamine-0.5 mM MgCl2 (pH 9.8) was used as a substrate. The dilution giving an absorbance value approximately 0.2 −log10 unit greater than the background value at A405 was determined as the endpoint titer. Anti-LOS serum was used as a positive control because the LOS structure is not affected by heat.
Fluorescence labeling and opsonization of bacteria.
Fluorescein isothiocyanate (FITC; Sigma) was used to label bacterial strains as described previously by Gentry et al. (14). Briefly, bacteria grown in liquid medium for 15 to 16 h were washed twice in PBS and heat killed for 80 min at 65°C in a water bath. In certain experiments, live H. ducreyi was used. Bacteria were pelleted, suspended in 2 ml of PBS containing 1 mg of FITC/ml, and incubated for 1 h at 4°C. After being washed in PBS, the labeled H. ducreyi and H. influenzae bacteria were blocked with 0.1% gelatin suspended in Hank’s balanced salt solution (GHBSS) (14).
For the opsonization of H. ducreyi strain CCUG 7470, homologous rabbit antisera were raised against (i) the oligosaccharide part of LOS conjugated to tetanus toxoid as described earlier (1), (ii) the 24-kDa surface protein, (iii) the 60-kDa heat shock protein (11), and (iv) whole-cell sonicate. The immunization procedure was described previously (12). The antibody titers are summarized in Table 1.
TABLE 1.
Reciprocal antibody titers of sera raised against purified homologous and surface antigens of H. ducreyi strain CCUG 7470 in an ELISA
| Antigen against which rabbit antiserum was raised | ELISA antibody titer (−log10) to:
|
|
|---|---|---|
| Homologous antigen | Whole cells (surface antigen) | |
| H. ducreyi bacteria | NAa | 7.2 |
| 24-kDa surface protein | 4.0 | 4.5 |
| 60-kDa heat shock protein | 4.0 | 6.8 |
| Oligosaccharide component of LOS | 3.5 | 3.8 |
| None (control: baby rabbit) | NAa | 2.4 |
NA, not applicable.
Bacteria suspended in PBS were incubated for 30 min at 37°C with heat-inactivated (56°C for 30 min) immune serum (12). As controls, inactivated baby rabbit serum and PBS were included. To reduce the cross-reactivity of natural rabbit antibodies with H. ducreyi antigens, all sera were diluted 1:400. The heat-inactivated serum was previously shown to lack complement activity but to possess functional antibody (12). Following opsonization, the bacteria were washed and resuspended in GBHSS to a final concentration of 108 CFU/ml and divided into aliquots. Labeled bacteria were examined by using fluorescence microscopy to confirm uniform staining.
Isolation of human phagocytic cells.
Granulocytes and monocytes were isolated from heparinized blood of healthy blood donors as described previously (34). Briefly, granulocytes were prepared by dextran sedimentation followed by Ficoll-Hypaque density centrifugation (Amersham Pharmacia Biotech AB, Uppsala, Sweden) and hypotonic lysis to remove erythrocytes as described previously (34). The granulocytes were washed twice with Krebs-Ringer phosphate buffer containing 1 mM Ca2+ (pH 7.2), resuspended in RPMI 1640 (Life Technologies, Täby, Sweden) to a concentration of 106 cells/ml, and kept on ice until used later on the same day.
Monocytes were selected from a fraction of peripheral blood mononuclear cells situated between the plasma and Ficoll-Hypaque and were suspended in RPMI 1640 containing 5% active human AB serum. Cells were seeded at a concentration of 106 per well in 96-well microtiter tissue culture plates to allow monocytes to adhere to the surface (34).
Monocytes were maintained in cell cultures in RPMI 1640 supplemented with 10% inactivated FCS and 1% l-glutamine for 5 to 6 days to allow differentiation into macrophages (13).
In addition, the human monocyte cell line THP-1 (ATCC T1B 202), derived from the peripheral blood of a 1-year-old boy with acute monocytic leukemia, was maintained in RPMI 1640 with 10% FCS (42). Cell viability, determined at the start of each experiment by the trypan blue exclusion test, was greater than 95%.
Phagocytosis assays.
The phagocytosis of opsonized and nonopsonized H. ducreyi was investigated by two methods in vitro.
(i) FACS assay.
The phagocytosis of opsonized and nonopsonized H. The phagocytosis of FITC-labeled H. ducreyi was measured by flow cytometry (fluorescence-activated cell sorting [FACS]; Becton Dickinson, San Jose, Calif.) (14). A commercial test kit (Phagotest; Orpegen Pharma, Heidelberg, Germany) was used, and test procedures were performed according to the manufacturer's instructions. Heparinized whole blood (100 μl; containing approximately 8,000 white cells/μl) was mixed with 20 μl of bacterial suspension (108 CFU/ml); the ratio of phagocytes to bacteria was approximately 1:25. Samples were mixed well and incubated for 10, 30, 60, and 90 min at 37°C in a water bath. Negative control samples were kept on ice. FITC-labeled opsonized Escherichia coli bacteria that were highly phagocytosed within a 10-min period were included in each assay as a test control. A noncapsulated strain of H. influenzae was used for comparison. Placing the samples on ice stopped phagocytosis. The quenching solution allowed the discrimination of attached and internalized bacteria by suppressing the fluorescent green color of surface-attached bacteria but not that of internalized remaining bacteria. DNA staining was used to distinguish live cells from bacterial aggregates. Samples were analyzed by flow cytometry with blue-green excitation at 488 nm by use of an argon ion laser and FACSCalibur CellQuest software. The cell populations were analyzed by means of forward light scatter and side light scatter. A live gate (gating for live cells) was set (red fluorescence), and a control marker containing a sample maintained at 0°C was used to exclude background cell autofluorescence (fluorescence intensity) in each of the histograms. The percentages of granulocytes and monocytes that ingested bacteria as well as their fluorescence intensity channel means were recorded. The mean and standard deviation (SD) for three independent experiments were calculated.
In separate FACS assays, 100 μl of bacteria (heat killed or live H. ducreyi or E. coli) at 6 × 107 CFU/ml was inoculated with 100 μl of heparinized whole blood and assayed as described above. Supernatants containing nonphagocytosed bacteria were subsequently reincubated with fresh phagocytes for 30 min at 37°C and analyzed by FACS.
(ii) Fluorescence microscopy.
To examine the locations of intracellular bacteria, isolated granulocytes (106 cells/ml) in RPMI 1640 were incubated with H. ducreyi (107 CFU/ml) for 60 min at 37°C with gentle shaking. The reaction was stopped by placing the samples on ice. Cytocentrifugation was used to transfer the samples to cytoslides (Shandon, Inc.). The cells were washed twice with PBS after every step. The cells were fixed in 2% paraformaldehyde in PBS for 30 min at 4°C and then pretreated with a 1:500 dilution of MAb MAHD7 (1 mg/ml), which is specific for H. ducreyi LOS, for 30 min at room temperature in a humid chamber. The cells were permeabilized with 0.5% Triton X-100 for 3 min at room temperature and incubated for 30 min at room temperature with a 1:100 dilution of a goat anti-mouse antibody conjugated to Rhodamine Red-X (Jackson ImmunoResearch Laboratories). The cells were again incubated with MAb MAHD7 for 60 min at 37°C. Finally, the cells were incubated for 30 min at 37°C with a 1:100 dilution of a goat anti-mouse antibody conjugated to FITC (Sigma). The glass slides were mounted, examined at a magnification of ×100 by using fluorescence microscopy (Zeiss, Oberkochen, Germany), and photographed.
Bacterial survival assays.
The survival in human phagocytic cells of H. ducreyi strains CCUG 7470, CCUG 27022, and CCUG 4438 and the noncapsulated H. influenzae strain was examined over a 20-h incubation period. The assay was carried out as previously described (29), with some modifications. Briefly, H. ducreyi strains grown in liquid medium (4 × 107 to 8 × 107 CFU/ml) were added to sterile plastic tubes containing approximately 1 × 106 to 3 × 106 granulocytes or THP-1 monocytes per ml or to 96-well plates containing adherent macrophages. The samples were incubated at 37°C in the presence of 5% CO2 for 1 h to allow phagocytosis. The samples were then washed twice with PBS to remove nonadherent bacteria; resuspended in fresh medium containing 200 μg of gentamicin (Gibco/BRL)/ml, i.e., a concentration of antibiotic sufficient to kill extracellular bacteria (35); and further incubated for 1 h. Subsequently, the samples were washed twice with PBS (for the 2-h survival assay), or the medium was replaced with RPMI 1640 containing 50 μg of gentamicin/ml to prevent the extracellular growth of any released bacteria (for the 20-h survival assay). At the end of the 2- and 20-h incubation periods, the samples were processed for viable count determinations.
The cell-bacterium mixtures were washed three times with PBS. The cells were lysed by adding 1 ml of ice-cold distilled water to each sample, followed by vigorous shaking, and were allowed to stand for 10 min. The cell lysates were serially diluted 10-fold in PBS, and aliquots were plated on chocolate agar to assess the numbers of viable intracellular bacteria. Two controls for each strain were included in the assay. The first control served to estimate the total numbers of viable bacteria per tube or well at the start of the experiment (time zero), and in the second control, gentamicin was added to the bacterial culture in order to estimate the efficacy of antibiotic killing. Results are expressed as the number of bacteria recovered from the bacterium-cell mixture exposed to gentamicin (viable intracellular bacteria) divided by the number of bacteria in the inoculum at time zero (initial inoculum).
Statistics.
The two-tailed Student t test was used to assess differences between paired means. Results showing P values of <0.05 were considered statistically significant.
RESULTS
H. ducreyi interactions with human phagocytic cells.
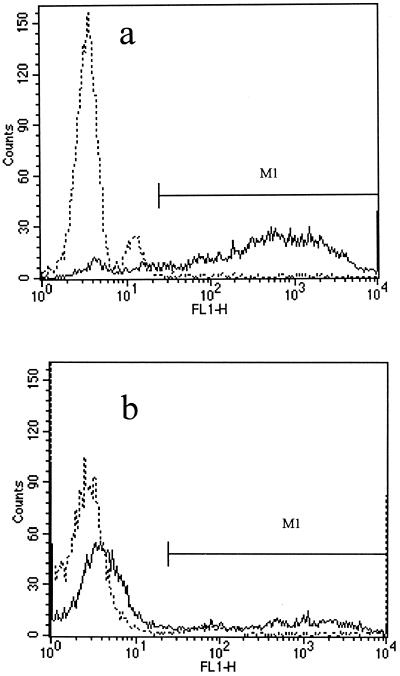
The kinetics of granulocyte and monocyte phagocytosis of H. ducreyi were studied at different times by using flow cytometry. Examples of flow cytometric histograms obtained following phagocytosis of either opsonized E. coli (test control) or opsonized H. ducreyi for 30 min are shown in Fig. 1a and b,respectively. After 10 min of incubation, 85% ± 4.2% of the granulocytes ingested E. coli, whereas 14% ± 2.3% to 18% ± 2.3% of the granulocytes ingested different H. ducreyi strains. After 1 h of incubation, the corresponding numbers were 97% ± 7.0% and 30% ± 4.7%, respectively.
FIG. 1.
Phagocytic activity of gated granulocyte populations analyzed by using histograms of cell number versus fluorescence intensity (FL1-H). Cells exposed to FITC-labeled, opsonized bacteria at 0°C (broken line) were used as a control marker (M1) to exclude the background. The percentage of granulocytes phagocytosing at 37°C (solid line) over 30 min (M1 area) was determined by FACS analysis. (a) E. coli (test control). (b) H. ducreyi. All values are expressed as the mean and SD for at least three independent experiments. Note that more than half of the granulocytes do not ingest H. ducreyi.
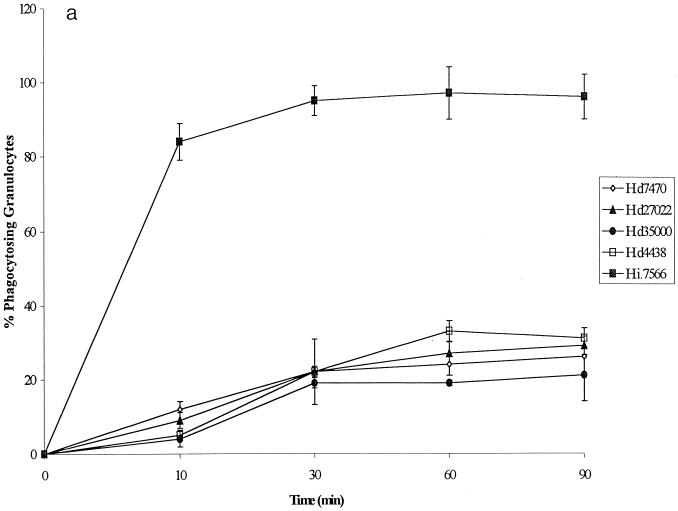
The kinetics of phagocytosis of the four nonopsonized H. ducreyi strains and the noncapsulated H. influenzae strain (control) are shown in Fig. 2a.A striking difference was observed between the H. influenzae and H. ducreyi strains with regard to the outcome of phagocytosis. After 30 min, 95% ± 1.5% of granulocytes had taken up H. influenzae; in comparison, 19% ± 1.4% to 22% ± 1.4% of granulocytes had ingested the different H. ducreyi strains (P < 0.0002 ). These findings indicate that all four H. ducreyi strains were phagocytosed to some extent by granulocytes; however, the H. ducreyi phagocytosis was slow, requiring longer incubation times than E. coli and H. influenzae. There was no significant difference in the process of phagocytosis among the different H. ducreyi strains.
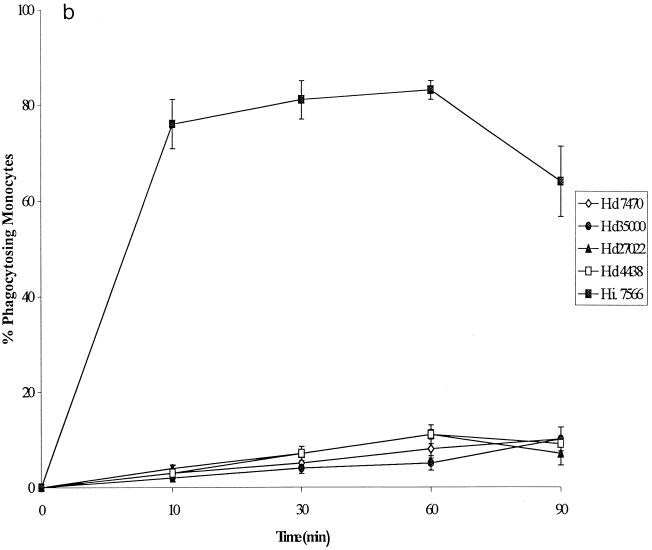
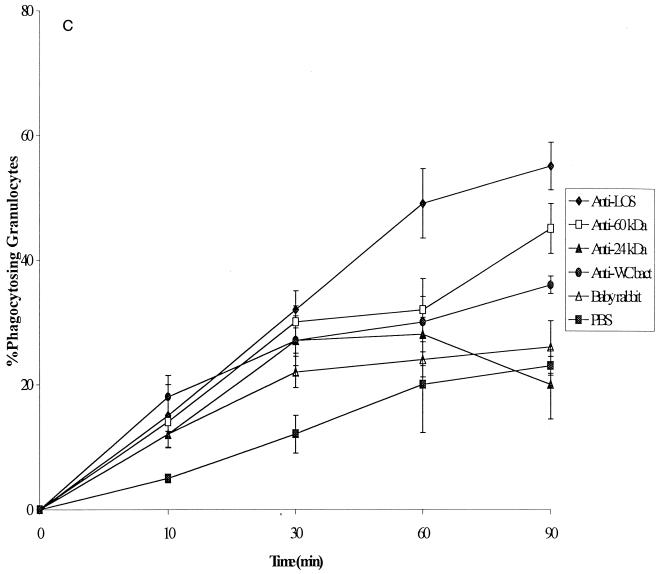
FIG. 2.
(a) Kinetics of phagocytosis by human granulocytes of four nonopsonized H. ducreyi strains and one noncapsulated H. influenzae strain. FACS was used to determine the percentage of phagocytosing granulocytes. Hd, H. ducreyi; Hi., H. influenzae. (b) Kinetics of phagocytosis by monocytes of four nonopsonized H. ducreyi strains and one noncapsulated H. influenzae strain. The percentage of phagocytosing monocytes was calculated from the experiments shown in panel a. (c) Phagocytosis by human granulocytes of H. ducreyi strain CCUG 7470 opsonized with sera specific for different antigens. The percentage of phagocytosing granulocytes was determined by FACS. WCbact, whole-cell sonicate. All values are expressed as the mean and SD for at least three independent experiments.
The mean fluorescence intensity of bacterium/cell mixtures in FACS analyses was used as a measure of the relative number of bacteria per cell. The mean mean fluorescence intensities for granulocytes that had phagocytosed H. ducreyi and H. influenzae were 781.5 ± 236.8 and 1,339 ± 196.5, respectively. After 60 min, granulocytes containing ingested H. ducreyi exhibited significantly lower mean mean fluorescence intensities than granulocytes containing ingested H. influenzae (P = 0.032). These results indicate less efficient granulocyte phagocytosis of H. ducreyi than of H. influenzae.
The kinetics of phagocytosis of the four H. ducreyi strains and the noncapsulated H. influenzae strain by monocytes were similar to those seen with granulocytes and showed that a higher percentage of monocytes ingested H. influenzae than H. ducreyi (Fig. 2b). Twice as many granulocytes as monocytes phagocytosed H. ducreyi.
Furthermore, we used fluorescence microscopy to confirm the FACS results. The features of H. influenzae and H. ducreyi phagocytosis differed with regard to the number of cells having ingested bacteria and the number of bacteria in each cell. After 30 min of phagocytosis, few phagocytes contained H. ducreyi, and there were many clumped bacteria, whereas at this time almost 80% of the cells were filled with H. influenzae (data not shown). Bacterial aggregation occurred even though the H. ducreyi strains were grown in liquid medium and the cultures were vortexed vigorously with glass beads in order to disrupt clumps and guarantee single-bacterium suspensions. We cannot exclude the possibility that bacterial clumping might have had some influence on the phagocytosis of H. ducreyi.
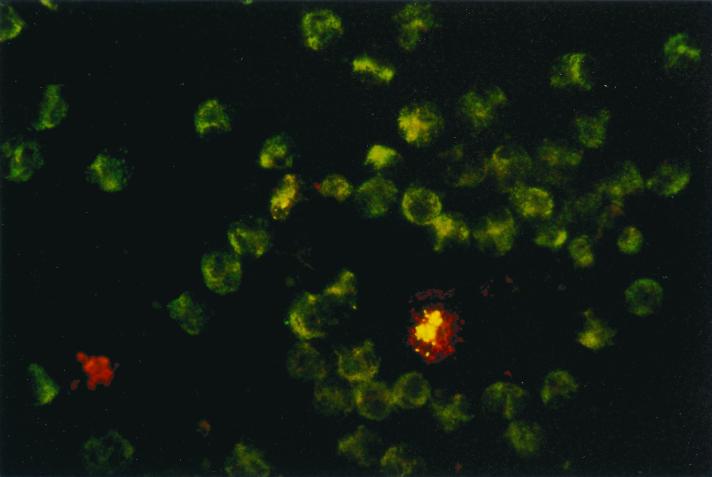
In addition, we used a double-immunofluorescence method to determine the intracellular location of H. ducreyi in granulocytes. The extracellular bacteria stained red, whereas the intracellular bacteria stained green or yellow-green (Fig. 3).
FIG. 3.
Localization of H. ducreyi in isolated granulocytes. Cells were processed for double-immunofluorescence staining after 30 min of exposure to H. ducreyi and examined by fluorescence microscopy. The extracellular bacteria stained red, whereas the intracellular bacteria stained green or yellow-green.
In order to study the antigen specificity of opsonizing antibodies, antisera directed against different bacterial surface components were used (Table 1). The results obtained for bacterial phagocytosis by granulocytes are summarized in Fig. 2c. Phagocytosis was enhanced by the opsonization of bacteria with specific antibodies. The highest level of opsonization was noted for antiserum to the O-side component of LOS (P < 0.003). For example, after 60 min of incubation, 49% ± 5.6% of granulocytes had ingested bacteria opsonized with antibody to LOS; in contrast, 28% ± 2.8% of granulocytes had taken up bacteria coated with antibody against the 24-kDa protein. The surface antibody titers in sera tested against heat-killed and live bacteria showed no significant statistical difference when analyzed by an ELISA. The results indicate that specific antibodies promote phagocytosis and that anti-LOS antibodies have the highest opsonizing capacity compared with antibodies to other surface antigens. By FACS analysis, when the phagocytosis of opsonized heat-killed H. ducreyi was compared with that of opsonized live H. ducreyi, a slightly but not statistically significantly higher percentage (approximately 5%) of granulocytes phagocytosed the live bacteria.
Further experiments were performed to investigate whether certain subpopulations of heat-killed or live H. ducreyi had pronounced antiphagocytic activity. Nonphagocytosed bacteria were reexposed to phagocytes, and the efficacy of phagocytosis was examined by FACS. The results showed that previously nonphagocytosed bacteria were not phagocytosed during a subsequent exposure to phagocytes (Table 2). It is noteworthy that even when high concentrations of H. ducreyi and E. coli were used, the resistance to phagocytosis of previously nonphagocytosed H. ducreyi bacteria was significantly higher than that of previously nonphagocytosed E. coli bacteria. These results indicated that a subpopulation of H. ducreyi was resistant to ingestion by granulocytes and that bacterial components other than LOS might mediate this resistance.
TABLE 2.
Resistance of the nonphagocytosed H. ducreyi population to repeated phagocytosis by granulocytes, as measured by FACS
| Bacterial population and incubationa | % Phagocytosisb after the following min:
|
||
|---|---|---|---|
| 10 | 30 | 60 | |
| H. ducreyi | |||
| First | 28 ± 0.7 (26.8 ± 4.9) | 36 ± 1.4 (40 ± 5.6) | 44 ± 0.7 (53 ± 1.4) |
| Second (supernatant of the first incubation) | 4 ± 5.0 (5 ± 1.4) | 3 ± 2.1 (7 ± 2.8) | 2 ± 1.4 (3 ± 2.2) |
| E. coli (control) | |||
| First | 90 ± 1.3 | 91 ± 3.0 | 93 ± 2.1 |
| Second (supernatant of the first incubation) | 67 ± 0.8 | 41 ± 7.0 | 38 ± 1.5 |
See Materials and Methods for bacterial concentration and procedures for the phagocytosis of bacteria.
Results are reported as the mean and SD for three experiments. For H. ducreyi, values are for heat-killed (live) bacteria tested with antiserum to LOS.
Survival in human phagocytes.
The survival of H. ducreyi strains in granulocytes, monocytes/macrophages, and monocytic cell line THP-1 was assessed. The H. influenzae strain was used as a control. H. ducreyi strains survived in phagocytic cells while noncapsulated H. influenzae was completely killed within 2 h (Table 3) . H. ducreyi survival in granulocytes and macrophages was low, 11 and 2.7% of the inoculum, respectively, after 2 h of incubation. Similarly, up to 6.6% of the H. ducreyi inoculum was found to have survived in THP-1 monocytes after 2 h of incubation (Table 3). After 20 h of incubation, H. ducreyi survival in all the phagocytic cells was extremely low, i.e., <0.1%. Interstrain differences in the ability of H. ducreyi to survive in phagocytes were slight; however, strain CCUG 4438 was recovered in small numbers, possibly reflecting its slower growth compared with that of the other strains (data not shown).
TABLE 3.
Survival of H. ducreyi in phagocytic cells and in the monocyte cell line THP-1
| Straina | % of the initial inoculum recovered from the following cellsb after the indicated h:
|
||||
|---|---|---|---|---|---|
| Granulocytes (macrophages)
|
THP-1 cells
|
||||
| 2 | 20 | 2 | 20 | ||
| H. ducreyi | |||||
| CCUG 7470 (CDT+, long LOS) | 11.00 ± 1.4 (2.71 ± 1.1) | 0.03 ± 0.2 (0.09 ± 0.01) | 6.46 ± 0.28 | 0.02 ± 0.00 | |
| CCUG 27022 (CDT−, long LOS) | 7.50 ± 0.1 (1.66 ± 0.6) | 0.03 ± 0.0 (0.08 ± 0.001) | 5.13 ± 0.48 | 0.02 ± 0.001 | |
| CCUG 4438 (CDT−, short LOS) | 2.00 ± 0.2 (1.10 ± 0.2) | 0.001 ± 0.0 (0.004 ± 0.00) | 1.51 ± 0.18 | 0.001 ± 0.002 | |
| H. influenzae | 0 | 0 | 0 | 0 | |
CDT+ and CDT−, presence or absence of CDT production.
Results are reported as the mean and SD for three independent experiments.
Role of phagocytic cells in ulcer development following intradermal injection of H.
ducreyi in the mouse model. We also evaluated the importance of phagocytic cells in early host defense against H. ducreyi in vivo. Mice that were selectively depleted of granulocytes and/or monocytes or that lacked T and B cells were injected intradermally with about 106 CFU of live bacteria, and the development of skin lesions was monitored. Mice that were granulocyte depleted or granulocyte plus monocyte depleted developed hemorrhagic nodules 2 days after bacterial inoculation; in almost all cases, these nodules ruptured on day 4 (Table 4). In the monocyte-depleted group, 2 of 15 mice developed slight skin disruptions at day 4. The control groups (untreated mice and mice injected with an unrelated MAb) as well as SCID mice developed a nodule within 2 days; in about 50% of cases, the nodule healed completely within 10 days. Bacteria were recovered from the skin lesions of granulocyte- and/or monocyte-depleted mice up to day 5 postinfection. Examples of skin lesions that developed in different groups of mice are presented in Fig. 4.These findings demonstrate the crucial role of granulocytes in inhibiting ulcer development early in H. ducreyi infection.
TABLE 4.
Skin lesions in mice after intradermal injection of 106 CFU of H. ducreyi
| Mouse group<1> d0150 X: C S: ©<1> d0150 X: C S: ©<1> d0150 X: C S: © | No. of lesions/no. of mice tested at day 4 postinfection |
|---|---|
| Granulocyte depleted | 14/15 |
| Monocyte depleted | 2/15 |
| Granulocyte plus monocyte depleted | 12/15 |
| SCID (T- and B-cell deficient) | 0/10 |
| Control (treated with an unrelated MAb) | 0/15 |
| Control (untreated) | 0/10 |
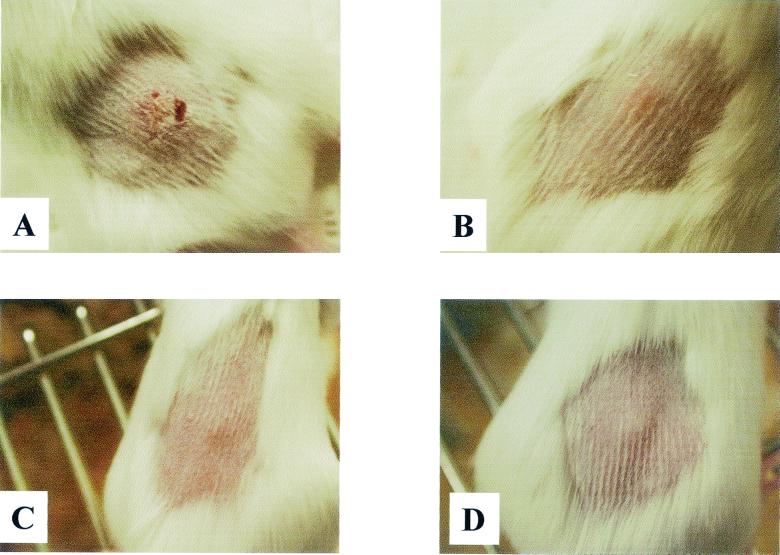
FIG. 4.
Lesion formation at 4 days postinoculation in mice injected with 106 CFU of H. ducreyi. (A) Granulocyte-depleted mouse showing a developed skin lesion. (B) Monocyte-depleted mouse showing slight inflammation. (C and D) SCID (C) and control (untreated) (D) mice showing nodule development.
In a previous study, LOS was implicated in lesion development in mice; however, high doses of intradermally injected purified LOS were used (18). In the present study, injections of high doses of an LOS preparation resulted in the development of lesions in two of five naive, control mice after 4 days. Only one of five SCID mice showed slight skin disruptions. The granulocyte- and/or monocyte-depleted mice did not show any skin lesions. The results indicate that bacterial LOS, even in high doses, is not responsible for the development of skin disruptions in leukopenic mice.
DISCUSSION
Human phagocytic cells, such as granulocytes, monocytes, and macrophages, defend against different pathogens by ingesting and killing the invaders. The effectiveness of phagocytic cells in eliminating H. ducreyi bacteria was addressed in this study.
Previously reported in vitro studies showed that H. ducreyi was localized both inside and outside of PMNL (19). Similarly, in patients with chancroid, bacteria were seen to be either engulfed by phagocytic cells or localized extracellularly (2). In a recent study with a human model of early-stage chancroid, no intracellular H. ducreyi bacteria were found, although the bacteria were associated with professional phagocytes (3).
In the present study, phagocytosis and intracellular killing of different H. ducreyi strains by human phagocytes were determined in vitro by using FACS analysis, microscopy, and a survival assay with gentamicin treatment. We found that H. ducreyi bacteria were, in general, poorly phagocytosed by both human granulocytes and human monocytes compared to H. influenzae and E. coli. There were no significant differences in the ingestion of different H. ducreyi strains, despite interstrain differences in LOS structures and the production of CDT. Opsonization with antibodies to surface components increased the uptake of H. ducreyi; however, phagocytosis was not complete, since less than half of the granulocyte population ingested bacteria. It is noteworthy that serum with moderate levels of antibodies specific for the O-side component of LOS was far more effective in bacterial opsonization than sera specific for other surface-localized antigens with high homologous antibody titers. However, no marked differences in phagocytosis were observed when live bacteria or heat-killed bacteria were analyzed. It is also possible that the bacteria express other structures that influence their resistance to phagocytosis. We also discovered that a subpopulation of the H. ducreyi culture used in the phagocytosis assay was completely resistant to phagocytosis by granulocytes. This was true for both heat-killed and live bacteria. The reason for the insufficient phagocytosis of H. ducreyi observed here and in other studies (19, 45) is so far unclear. One possible explanation is that bacterial aggregation impairs the ingestive capacity of granulocytes. Alternatively, H. ducreyi may express different surface structures, such as those deployed by gonococci, e.g., pili, that promote attachment to but impede engulfment by professional phagocytes (32).
Another bacterial strategy for avoiding host defense mechanisms is the capacity to survive the microbicidal milieu in the developing phagolysosome (21). We observed that only a small but reproducible number of H. ducreyi bacteria survived in human phagocytic cells, while noncapsulated H. influenzae was rapidly and completely killed. Our results indicate that a fraction of the bacteria can survive for only a short period of time after phagocytosis in human phagocytic cells, in the absence of any apparent intracellular multiplication. Similar findings were reported by Wood et al., who showed that H. ducreyi did not survive in the human macrophage-like cell line U-937 after 24 h of incubation (45). Since neither the bacterial population nor the phagocytosis conditions resemble those in vivo, these results need to be assessed with caution. Therefore, H. ducreyi probably can be considered a transiently intracellular pathogen but not a typical intracellular pathogen, such as Mycobacterium tuberculosis (21).
The present in vitro results indicate the ability of H. ducreyi to avoid phagocytosis by both granulocytes and monocytes and the capacity to survive for a limited time in phagocytic cells. To study the role of phagocytic cells in lesion development in vivo, infection studies with H. ducreyi were carried out with mice depleted of granulocytes and/or monocytes and with mice that lacked T and B cells. Mice were previously used to study H. ducreyi pathogenesis. However, in the earlier study, large bacterial numbers (more than 107 CFU) were needed to elicit dermonecrotic lesions, and intradermal injections of high doses of LOS resulted in the development of similar lesions (18). In this study, in order to study the role of neutrophil leukocytes, mice were depleted of granulocytes by injections of MAb RB6-8C5, which is directed against differentiation antigens on myeloid cells (24). This treatment has been shown to cause pronounced early neutropenia, with depletion of at least 90% of peripheral blood granulocytes (24). All granulocyte-depleted mice injected with 105 CFU of H. ducreyi developed skin lesions within 4 days, and the lesions persisted for 10 days after infection. In contrast, naive and SCID mice developed transient nodules only. Importantly, lesion development in granulocyte-depleted mice was not due to the presence of LOS, since even a high dose of LOS (150 μg) did not cause lesions in these mice. These results emphasize the crucial and independent role of neutrophils in the early defense against H. ducreyi bacteria. As shown in the present study, monocytes play a minor role, since monocyte-depleted mice with an intact granulocyte population only occasionally developed skin lesions. The recruitment of neutrophils during the initial stages of H. ducreyi infection is likely to be critical for the development of ulcers in chancroid.
In conclusion, the experiments described in this study demonstrate that (i) H. ducreyi is weakly phagocytosed by granulocytes and monocytes, as a subpopulation of the bacteria resist phagocytic ingestion; (ii) H. ducreyi is capable of short-term survival inside human phagocytic cells; (iii) granulocytes play a very important role in the defense against H. ducreyi infection; and (iv) variations in the chemical structure of LOS and in CDT production by H. ducreyi may not play major roles in the processes of phagocytosis and survival.
Phagocytic killing seems to be an important host defense mechanism against H. ducreyi, and bacterial resistance to phagocytosis may represent a pathogenic strategy for successfully establishing disease. This characteristic of H. ducreyi may be relevant in vivo for the maintenance of chancroid and bacterial persistence in tissues or ulcers. Further studies are needed to define the components and mechanisms by which H. ducreyi inhibits effective phagocytosis.
Acknowledgments
This work was supported by the Swedish Agency for Research Co-operation with Developing Countries (SIDA/SAREC) and the Swedish Medical Research Council (grant 12630).
We thank Vincent Collins for revising the English text of the manuscript.
Editor: E. I. Tuomanen
REFERENCES
- 1.Ahmed, H. J., A. Frisk, J.-E. Månsson, E. K. Schweda, and T. Lagergård. 1997. Structurally defined epitopes of Haemophilus ducreyi lipooligosaccharides recognized by monoclonal antibodies. Infect. Immun. 65:3151-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, H. J., S. Borrelli, J. Jonasson, L. Eriksson, S. Hanson, B. Höjer, M. Sunkuntu, E. Musaba, E. L. Roggen, T. Lagergård, and A. A. Lindberg. 1995. Monoclonal antibodies against Haemophilus ducreyi lipooligosaccharide and their diagnostic usefulness. Eur. J. Clin. Microbiol. Infect. Dis. 14:892-898. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer, M. E., and S. M. Spinola. 1999. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bong, C. T. H., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellazzo, A., M. Shero, M. A. Apicella, and S. M. Spinola. 1992. Expression of pili by Haemophilus ducreyi. J. Infect. Dis. 165(Suppl. 1):S198-S199. [DOI] [PubMed]
- 9.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purvén, R. S. Munson, Jr., T. Lagergård, J. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Schryver, S. A., and A. Meheus. 1990. Epidemiology of sexually transmitted diseases: the global picture. Bull. W. H. O. 68:639-654. [PMC free article] [PubMed] [Google Scholar]
- 11.Frisk, A., C. A. Ison, and T. Lagergård. 1998. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect. Immun. 66:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisk, A., H. J. Ahmed, E. van Dyck, and T. Lagergård. 1998. Antibodies specific to surface antigens are not effective in complement-mediated killing of Haemophilus ducreyi. Microb. Pathog. 25:67-75. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor, C. D., F. X. McCormack, D. R. Voelker, S. E. McGowan, and L. S. Schlesinger. 1995. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 155:5343-5351. [PubMed] [Google Scholar]
- 14.Gentry, M. J., M. U. Snitily, and L. C. Preheim. 1995. Phagocytosis of Streptococcus pneumonia measured in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J. Infect. Dis. 171:350-355. [DOI] [PubMed] [Google Scholar]
- 15.Hansen, E. J., S. R. Lumbley, J. A. Richarson, B. K. Purcell, M. K. Stevens, L. D. Cope, J. Datte, and J. D. Radolf. 1994. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J. Immunol. 152:184-192. [PubMed] [Google Scholar]
- 16.Hobbs, M. M., L. R. San Mateo, P. E. Orndorff, G. Almond, and T. H. Kawula. 1995. Swine model of Haemophilus ducreyi infection. Infect. Immun. 63:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessamine, P. G., and A. R. Ronald. 1990. Chancroid and the role of genital ulcer disease in the spread of human retroviruses. Med. Clin. N. Am. 74:1417-1431. [DOI] [PubMed] [Google Scholar]
- 18.Lagergård, T. 1992. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb. Pathog. 13:203-217. [DOI] [PubMed] [Google Scholar]
- 19.Lagergård, T., A. Frisk, M. Purvén, and L.-Å. Nilsson. 1995. Serum bactericidal activity and phagocytosis in host defense against Haemophilus ducreyi. Microb. Pathog. 18:37-51. [PubMed] [Google Scholar]
- 20.Lagergård, T., M. Purvén, and A. Frisk. 1993. Evidence of Haemophilus ducreyi adherence to and destruction of human epithelial cells. Microb. Pathog. 14:417-431. [DOI] [PubMed] [Google Scholar]
- 21.Manca, C., S. Paul, C. E. Barry III, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect. Immun. 67:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandrell, R. E., R. McLaughlin, Y. Abu Kwaik, A. Lesse, R. Yamasaki, B. Gibson, M. S. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus ducreyi species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melaugh, W., N. J. Phillips, A. A. Campagnari, M. V. Tullius, and B. W. Gibson. 1994. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyi, strain 35000, and evidence for additional glycoforms. Biochemistry 33:13070-13078. [DOI] [PubMed] [Google Scholar]
- 24.Mölne, L., M. Verdrengh, and A. Tarkowski. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68:6162-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse, S. A. 1989. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 2:137-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer, K. L., C. T. Schnizlein-Bick, A. Orazi, K. John, C. Y. Chen, A. F. Hood, and S. M. Spinola. 1998. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J. Infect. Dis. 178:1688-1697. [DOI] [PubMed] [Google Scholar]
- 27.Parson, L. M., R. J. Limberger, and M. Shayegani. 1997. Alternations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect. Immun. 65:2413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purvén, M., and T. Lagergård. 1992. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect. Immun. 60:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raybourne, R. B., and V. K. Bunning. 1994. Bacterium-host cell interaction at the cellular level: fluorescent labeling of bacteria and analysis of short-term bacterium-phagocyte interaction by flow cytometry. Infect. Immun. 62:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Mateo, L. R., M. M. Hobbs, and H. T. Kawula. 1998. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol. Microbiol. 27:391-404. [DOI] [PubMed] [Google Scholar]
- 31.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Immune cells are required for cutaneous ulceration in a swine model of chancroid. Infect. Immun. 67:4963-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer, W. M., and R. F. Rest. 1989. Interactions of gonococci with phagocytic cells. Annu. Rev. Microbiol. 43:121-145. [DOI] [PubMed] [Google Scholar]
- 33.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 34.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergård. 2001. The impact of Haemophilus ducreyi cytholethal distending toxin on cells involved in immune response. Microb. Pathog. 30:157-166. [DOI] [PubMed] [Google Scholar]
- 35.Tabrizi, S. N., and R. M. Robins-Browne. 1993. Elimination of extracellular bacteria by antibiotics in quantitative assays of bacterial ingestion and killing by phagocytes. J. Immunol. Methods 158:201-206. [DOI] [PubMed] [Google Scholar]
- 36.Telzak, E. E., M. A. Chiasson, P. J. Bevier. R. L. Stoneburner, K. G. Castro, and H. W. Jaffe. 1993. HIV-1 seroconversion in patients with and without genital ulcer disease. Ann. Intern. Med. 119:1181-1186. [DOI] [PubMed] [Google Scholar]
- 37.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Totten, P. A., J. C. Lara, D. V. Norn, and W. E. Stamm. 1994. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect. Immun. 62:5632-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Totten, P. A., D. V. Norn, and W. E. Stamm. 1995. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect. Immun. 63:4409-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trees, D. L., R. J. Arko, G. D. Hill, and S. A. Morse. 1992. Laboratory-acquired infection with Haemophilus ducreyi type strain CIP 542. Med. Microbiol. Lett. 1:330-337. [Google Scholar]
- 42.Tsuchiya, S. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 43.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdrengh, M., and A. Tarkowski. 2000. Role of macrophages in Staphylococcus aureus-induced arthritis and sepsis. Arthritis Rheum. 43:2276-2282. [DOI] [PubMed] [Google Scholar]
- 45.Wood, G. E., S. M. Dutro, and P. A. Totten. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]