Abstract
The presently licensed meningococcal vaccine is a tetravalent capsular polysaccharide vaccine that induces immunity to serogroups A, C, Y, and W-135 but not to group B, which causes nearly half of the meningitis cases in the United States. The purpose of this study was to evaluate the safety and immunogenicity of an intranasal native outer membrane vesicle (NOMV) vaccine prepared from a capsule negative strain of group B of Neisseria meningitidis. In this study all volunteers received the same dose of vaccine, but we evaluated two different immunization schedules and the oropharyngeal and intranasal routes of vaccine delivery, assessed nasal cytology for cellular infiltration, and measured antibody-secreting cells (enzyme-linked immunospot assay [ELISPOT]) as an early marker for systemic immune response. Additionally, both intranasal and serum vaccine-specific antibodies were measured as well as serum bactericidal activity. Four groups with a total of 42 subjects were immunized on days 0, 28, and 56. Group 3 received an additional dose on day 7. Group 2 subjects were immunized both intranasally and oropharyngeally. Group 4 received a different lot of vaccine. All groups received approximately 1,200 μg of vaccine per subject. Patients were evaluated for side effects. The vaccine was well tolerated without evidence of inflammation on nasal cytology. The group receiving the extra vaccine dose showed the maximum increase in bactericidal activity. Thirty of 42 subjects demonstrated an increase in meningococcus-specific intranasal immunoglobulin A (IgA) titers, while 23 of 42 demonstrated an increase in specific IgG titers. The group receiving vaccine intranasally and oropharyngeally showed the highest rise in intranasal titers for both IgA and IgG. Groups 1, 3, and 4 showed a significant increase in antibody-secreting cells on ELISPOT. Eighteen of 42 volunteers demonstrated a fourfold or greater rise in bactericidal titers, with 81% showing an increase over baseline. We have demonstrated the immunogenicity and safety of a group B lipopolysaccharide-containing, intranasal, NOMV vaccine.
Meningitis and septicemia from Neisseria meningitidis continue to represent a major worldwide threat. In the United States 2,500 to 3,000 cases of meningococcal disease occur each year. This is associated with significant morbidity, with up to 19% of survivors being left with neurologic sequelae (3). Most of the outbreaks in the United States are caused by serogroups B, C, and Y, with the predominance of cases occurring in young adults and infants. Multistate surveillance conducted between 1992 and 1996 reported 35% serogroup C cases, 32% B cases, and 26% Y cases (12). Serogroup C is responsible for the majority of cases in the adolescent population, whereas cases in infants less than 1 year old are more often due to group B.
Presently licensed vaccines are available to immunize against serogroups A, C, Y, and W-135. Unfortunately, a licensed vaccine is not available against group B meningococci. The difficulties in developing a group B vaccine have included the lack of immunogenicity of the purified capsular polysaccharide (10, 17). Attempts at improving the immunogenicity have included noncovalent complexing and covalent conjugating of the polysaccharide to proteins. Zollinger et al. demonstrated transient increases in specific immunoglobulin M (IgM) antibodies after noncovalent complexing, but these antibodies were not bactericidal with human complement (18). The covalent conjugate vaccines using unmodified B capsular polysaccharide did not yield any better results and were basically not protective or immunogenic in animals. Chemically modified B polysaccharide conjugated to recombinant meningococcal PorB is immunogenic in animals and induces a relatively high-quality antibody response, including IgG antibodies that are bactericidal with homologous complement, but safety and immunogenicity in humans have not been demonstrated (6).
The approach shifted to developing lipopolysaccharide (LPS)-depleted outer membrane protein (OMP) vaccines because of the demonstration of bactericidal antibodies against both LPS and OMP in human sera following group B carriage (9). Meningococcal group B vaccine trials of parenteral vaccines demonstrated that vaccines based on OMPs can induce protective antibody responses. Several trials conducted in Cuba, Brazil, and Europe have demonstrated efficacy in the range of 50 to 80% (1, 4, 15). Similar findings were reported for a Chilean trial showing 51% efficacy (2).
Another novel approach to developing a group B vaccine came from using the naturally occurring outer membrane blebs known as native outer membrane vesicles (NOMV). The previously studied LPS-depleted OMP vaccines had been modified and possibly exposed epitopes which induced high levels of nonbactericidal antibody. The NOMV contain unmodified OMPs in a natural lipid environment so that important epitopes may be presented to the immune system in a native conformation and environment. Additionally, such formulations were administered intranasally in order to prevent pyrogenic reactions while mimicking nasopharyngeal colonization by wild-type strains. Drabick et al. demonstrated the successful induction of bactericidal antibodies against PorA and L3,7,9 LPS in humans by using an intranasal NOMV vaccine (5). The latter trial used two different doses of vaccine all delivered intranasally. The authors measured systemic response with bactericidal assays and enzyme-linked immunosorbent assays (ELISA) and did not assay cellular changes in the nose.
The purpose of our study was to (i) use a fixed dose of vaccine but study two different immunization schedules, (ii) compare both intranasal and oropharyngeal routes of immunization, (iii) evaluate systemic immunogenicity using ELISPOT to measure trafficking antibody-secreting cells while assessing nasal cellular response by performing cytologic analysis on nasal secretions, (iv) compare the effectiveness of a new lot of NOMV vaccine derived from the same strain as lot no. 0123, and (v) corroborate the safety and degree of immunogenicity noted in the previous NOMV trial, which was performed with a small number of volunteers.
MATERIALS AND METHODS
Volunteer characteristics.
Forty-four subjects, 21 men and 23 women, were recruited from the Washington, D.C., area. Forty-two volunteers completed the study. One male volunteer dropped out from the study at the outset, and one other did not return after receiving the day 28 vaccination (second dose). The dropouts were not due to any vaccine-associated adverse outcome but for personal reasons. The ages of the participants ranged from 21 to 47 years with a median age of 36 years. The exclusion criteria included (i) history of severe organ-system disease, (ii) history of allergy to any vaccine, (iii) history of allergic or nonallergic rhinitis or chronic sinusitis, (iv) presence of clinically significant abnormalities on screening laboratory tests, (v) use of any nasal spray, including over-the-counter preparations, on a regular basis, (vi) fever greater than 38°C or upper respiratory tract infection (i.e., common cold) on the day of immunization, (vii) human immunodeficiency virus seropositivity or any other immunosuppressive state, (viii) pregnancy (positive serum β-human chorionic gonadotropin (β-HCG) on the day prior to each immunization), (ix) prior receipt of any group B meningococcal OMP vaccine or vaccines with meningococcal OMP, (x) high levels of baseline bactericidal antibodies on screening (≥1:16), and (xi) nasopharyngeal carriage of meningococcus at the time of screening. Volunteers were not excluded based on having received the licensed meningococcal tetravalent polysaccharide vaccine. Prior to inclusion in the study, all volunteers had screening complete blood count, serum chemistry profile, liver function tests, human immunodeficiency virus antibody, and group B meningococcal bactericidal titers. A nasopharyngeal swab for culture was performed on days 0, 14, 28, 42, 56, 70, and 98 in order to assess for meningococcal colonization. One day prior to each immunization, women had a β-HCG pregnancy test. The study was conducted at the Walter Reed Army Institute of Research, which follows Good Clinical Practices guidelines. Informed consent was obtained from all volunteers, and the protocol was approved by both the U.S. Food and Drug Administration (BB-IND 6993) and the local institutional review boards.
Vaccine description.
Vaccine lot no. 0123 was produced in March 1995 and lot no. 0471 was produced in September 1997 under current good manufacturing practice conditions at the Walter Reed Army Institute of Research in the Forest Glen Pilot Vaccine Production Facility (Forest Glen, Md.). The intranasal vaccines used in this study were from a seed strain derived from N. meningitidis 9162 (B:15:P1.3), which was a clinical isolate from a patient in Iquique, Chile. The parent strain was genetically modified by partially deleting synX. The latter is essential for biosynthesis of sialic acid, which is required for sialylation of the LPS and capsule formation. Thus, the above modification resulted in a capsule negative strain, 9162 synX-negative, with nonsialylated LPS.
The vaccine consisted of NOMV. These were extracted from strain 9162 synX-negative without the use of detergents or denaturing agents. The basic method of preparing the NOMV vaccine has been described elsewhere (13, 16, 19). Briefly, previously frozen cells grown under iron-limiting conditions were thawed and suspended in Tris-buffered saline with 0.01 M EDTA at a pH of 7.5. The suspension was warmed to 56°C for 30 min, sheared in a blender (Waring, Inc., New Hartford, Conn.) for 3 min, and centrifuged to remove cells and debris. The supernatant was removed and ultracentrifuged to harvest the NOMV, which were washed in distilled water. The ratio of protein to LPS in the NOMV was about 4:1 (wt/vol). The vaccines were bottled in sterile normal saline without preservative at the following concentrations: lot no. 0123, 0.8 mg of protein/ml; and lot no. 0471, 1.0 mg of protein/ml. Vaccines were stored at 5°C prior to use. Both lots of vaccine were previously tested intranasally with animals without any pyrogenic effects, and lot no. 0123 was previously studied with 32 healthy volunteers without any significant side effects or febrile reactions (5). All lots passed sterility testing.
Study design.
Subjects were randomized into one of four groups in a manner to ensure gender equality among all groups. Groups 1 and 4 consisted of 12 volunteers each (one dropout from groups 1 and 4), while groups 2 and 3 had 10. Each group received a total dose of 1,248 μg of vaccine, except group 4, which received 1,200 μg, due to the difference in stock concentration of lot no. 0471 and the fixed volume delivered by the pump. Groups 1 to 3 received lot no. 0123, while group 4 received lot no. 0471. Group 2 was immunized both intranasally and oropharyngeally and group 3 received an extra dose on day 7 but maintained the same total dose (1,248 μg) as all other groups (Table 1).
TABLE 1.
| Group | Dose/immu- nization (μg) | Schedule |
|---|---|---|
| 1 | 416 ± 40 | 3 IN immunizations on days 0, 28, and 56 with NOMV lot no. 0123 |
| 2 | 416 ± 40 | 3 IN/OP immunizations on days 0, 28, and 56 with NOMV lot no. 0123 |
| 3 | 312 ± 30 | 4 IN immunizations on days 0, 7, 28, and 56 with NOMV lot no. 0123 |
| 4 | 400 ± 40 | 3 IN immunizations at days 0, 28, and 56 with NOMV lot no. 0471 |
IN, intranasal; OP, oropharyngeal.
Vaccination schedule, route of delivery, and dose per day of immunization for the four study groups are shown.
All intranasal vaccines were delivered using a fixed-volume atomized sprayer (Valois of America, Greenwich, Conn.) which delivered 100 μl (group 4) or 130 μl (groups 1 to 3). A different volume sprayer was used for group 4, since the concentration of lot no. 0471 was different from the concentration of lot no. 0123 used for groups 1 to 3. The volume of vaccine given was approximately 0.5 ml (four sprays at 130 μl each) for groups 1 to 3 and 0.4 ml (four sprays at 100 μl each) for group 4. The vaccine to be used for group 3 was diluted with 3 ml of sterile saline per vial to give a concentration of 0.6 mg of protein/ml in order to adjust for the extra day 7 dose. This insured that the total dose remained the same for group 3 (1,248 μg). The oropharyngeal immunizations were performed using a pump sprayer which also delivered 130 μl. The intranasal immunization was performed with the subjects' heads tilted forward prior to spraying and then tilted backward after receiving the vaccine. All immunizations were delivered by the investigators using the same delivery technique. To ensure the accuracy of volume delivery, the vaccine spray bottles were weighed before and after each immunization.
Nasal cytology.
Before the study and 5 days after each immunization, nasal secretions from beneath the inferior turbinate were collected using a disposable plastic scoop (Rhinoprobe; Synbiotics, San Diego, Calif.) for cytologic examination under direct visualization. The procedure involved collecting the specimen (mucus) from beneath the inferior turbinates. The specimen was transferred to a glass microscope slide, fixed, and stained using methodology described by Hansel (8). Slides were examined initially under low power (magnification, ×100) and were then graded by evaluating 10 fields at high power (oil immersion; ×1,000). Cell counts (eosinophils, mononuclear cells, and polymorphonuclear cells) were expressed in a semiquantitative fashion using a scale previously described (Alfredo Jalowayski, 1991, A practical guide for diagnostic nasal cytology, p. 1-8, Synbiotics Corp., San Diego, Calif.): no cells, 0; few scattered cells or small clumps, 1+; moderate number of cells and larger clumps, 2+; many cells, easily seen, do not cover entire field, 3+; larger number, covering entire field, 4+.
Nasal and serum antibody determinations.
IgA, IgM, and IgG ELISA with nasal and serum samples were performed with the NOMV vaccine as the solid-phase antigen. Serum specimens were drawn on days 0, 14, 28, 42, 56, 70, and 98. Serum antibody titers were measured in micrograms per milliliter, whereas the nasal secretion titers were normalized by dividing the vaccine-specific responses by the total isotype measured in the sample. The total IgG, IgM, and IgA in the secretions were also measured using an ELISA. The assay was performed as previously described except that protease inhibitors (aprotinin, 1 μg/ml; leupeptin, 10 μg/ml; Bestain, 3.25 μM; and Pefabloc, 0.2 mM) were added to the nasal wash fluid at the time of specimen collection (14). Nasal washes were obtained by having the volunteer hyperextend the neck with the glottis closed and by instilling 5 ml of normal saline into each nostril via pipette. The 10 ml of fluid was held for 10 s and was then forcibly expelled from the nares into a clean container. The secretions were aliquoted into a sterile cryogenic tube and frozen. The procedure was performed on each volunteer three times during the course of the study on days 0, 42, and 98.
ELISPOT.
An ELISPOT assay was performed on days 0, 7, 35, and 63 as previously described (13). Samples were collected in Vacutainer CPT tubes (Becton Dickinson, Franklin Lakes, N.J.) which were centrifuged, and the mononuclear cell layer was collected. The cells were washed twice with Dulbecco's phosphate-buffered saline (Life Technologies, Rockville, Md.) and suspended in cell culture medium (RPMI 1640 with 10% fetal bovine serum, gentamicin, and l-glutamine). Peripheral blood mononuclear cells were enumerated using a Coulter Counter, and the cell concentration was adjusted to 2.5 × 106 cells/ml. Cells were plated out in NOMV-coated 96-well microtiter plates at 100 μl/well and were incubated at 37°C for at least 3 h. The plates were washed four times, and the wells were filled with goat anti-human IgG, IgA, or IgM alkaline phosphatase conjugate. The plate contents were incubated overnight at room temperature and washed, and 100 μl of 5-bromo-4-chloro-3-indolylphosphate substrate in soft agar was added to each well. After overnight incubation at room temperature, the blue spots in the agar were counted and data were expressed as the number of spots per million cells.
Bactericidal assay.
Bactericidal assays were performed using the following wild-type clinical isolates from a group B epidemic in Chile: 9162 (B:15:P1.7-2, 3P:5.10,11:L3,7,9) and 8532 (B:15:P1.7-2,3:P5.10,11:L3,7,8,9). The two strains are closely related except for L8 LPS in strain 8532. The assays were performed as previously described (11). Briefly, 50 μl of test serum was mixed with 25 μl of bacterial suspension (containing viable organisms) and 25 μl of human complement (human serum without bactericidal activity against the test strain). Serial twofold dilutions of the test sera were treated similarly. All the mixtures were incubated and plated onto GC medium plus Kellog supplement agar. The following day a colony count was performed and the percentage of bacteria killed was computed by comparing the heat-inactivated complement control (zero kill control) to the test mixture. Serum samples were obtained on days 0, 14, 28, 42, 56, 70, and 98. The serum dilution yielding 50% killing was reported as the titer. Seroconversion was defined as a fourfold increase in titer.
Adverse reactions.
Reactogenicity to immunization was monitored at 1, 24, and 48 h after each vaccine by assessing volunteer symptoms and vital signs and by giving a physical exam. Additionally, the volunteers were given symptom diaries to be completed for the first 48 h. They were instructed to record any symptoms and to measure a temperature reading if they felt febrile. Reported reactions were graded according to severity on a scale of 1 to 4 with 1+ signifying barely noticeable symptoms (minimal), 2+ signifying noticeable symptoms that did not impair normal activities (mild), 3+ signifying symptoms severe enough to impair normal activities (moderate), and 4+ signifying life-threatening symptoms (severe).
Statistics.
For each group, changes in titer values over the entire study period were examined using Friedman's test, with pairwise comparisons made using the Wilcoxon signed-rank test. For comparisons between two time points, differences were considered statistically significant if the Bonferroni-adjusted P values were <0.05. Differences in the maximum changes in titers from the baseline were explored between groups with the Wilcoxon rank sum test. Data were analyzed using Statistical Package for the Social Sciences for Windows (version 10; SPSS Inc., Chicago, Ill.).
RESULTS
The vaccine was well tolerated as summarized in Table 2. Two patients reported transient fevers of 99.7 and 100°F, respectively. The following grade 3 symptoms were self reported: four subjects were tired, two had headaches, and one had nasal stuffiness. All of the symptoms were self limiting and resolved within 48 h; no one over the last year and a half has reported any long-term, adverse reactions. Two subjects developed short-lived illnesses which did not appear to be vaccine related. One developed a 30-s episode of wheezing 20 h after receiving an immunization. During a 24-h follow-up examination, no abnormal findings were noted. The remainder of the study was uneventful for this volunteer. The second participant developed bronchitis-pneumonia diagnosed by her primary care physician. This occurred 3 weeks after an immunization and therefore was very unlikely to be vaccine related. However, her last vaccine was not administered due to the underlying illness at the time of the third dose. Two weeks later, she reported resolution of all symptoms. All reported reactions did not predictably correlate with the immunizations, and none of the reactions were reproducible in any given volunteer.
TABLE 2.
| Symptom | No. of patients with subjective complaints and grade of complaint (X = 42) after dose:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4 (group 3 only)
|
||||||||||||||
| 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | ||
| Fatigue | 4 | 4 | 4 | 4 | 1 | 1 | 1 | 1 | |||||||||
| Headache | 2 | 4 | 2 | 2 | 1 | 1 | 3 | ||||||||||
| Muscle aches | 3 | 1 | 1 | 1 | 1 | ||||||||||||
| Runny nose | 4 | 2 | 1 | 1 | 2 | 1 | |||||||||||
| Nasal discharge; stuffiness | 2 | 1 | 2 | 1 | 4 | 1 | |||||||||||
| Sore throat | 1 | 1 | 1 | 1 | |||||||||||||
| Cough | 2 | 1 | 1 | 1 | 1 | ||||||||||||
| Nasal sting-burn | 1 | ||||||||||||||||
| Nasal itch | 2 | ||||||||||||||||
| Nosebleed | |||||||||||||||||
| Postnasal drip | 1 | 4 | |||||||||||||||
One patient had a 99.7°F fever after dose 1. One patient had a 100°F fever after dose 2.
Shown are reported side effects per dosing day for all 42 volunteers. The scoring system is described in “Adverse reactions.”
The nasal cytology is presented in Table 3 and reveals sparse cellularity on the majority of smears. Of the 168 smears, 31 had positive findings, and of these, 19 were 1+ (few scattered cells), 9 were 2+ (moderate cells), and 3 were 3+ (many cells).
TABLE 3.
Summary of nasal cytologya
| Day that smear was obtained and group no. | No. of volunteers and semiquan- titative cell count for:
|
||
|---|---|---|---|
| Polymorpho- nuclear cells | Eosinophils | Monocytes | |
| Day 0 | |||
| 1 | 1 (1+) | 1 (1+) | |
| 2 | |||
| 3 | 1 (1+) | ||
| 4 | 1 (1+) | ||
| Day 7 | |||
| 1 | |||
| 2 | 4 (1+, 1+, 2+, 3+) | (1+) | |
| 3 | 1 (1+) | 1 (1+) | |
| 4 | 1 (2+) | 2 (2+, 2+) | |
| Day 14 | |||
| 3 (only) | 2 (1+, 2+) | ||
| Day 35 | |||
| 1 | 1 (1+) | ||
| 2 | 1 (1+) | ||
| 3 | 1 (1+) | ||
| 4 | 1 (3+) | 1 (1+) | |
| Day 63 | |||
| 1 | 2 (1+, 1+) | ||
| 2 | 1 (1+) | 1 (1+) | |
| 3 | 2 (1+, 2+) | ||
| 4 | 3 (2+, 2+, 3+) | 1 (2+) | |
Cell counts are given in parentheses. Shown are nasal cytology results from nasal smears performed on all volunteers. Scores are given according to the semiquantitative criteria described in text.
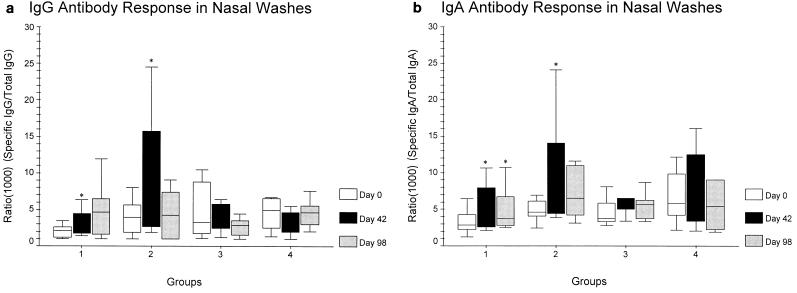
Nasal wash ELISA data revealed an increase in titer over baseline for IgG and IgA in 23 of 42 (55%) and 30 of 42 (71%) volunteers, respectively. However, only 10 of 42 (4 in group 1, 4 in group 2, and 2 in group 4) had at least a twofold rise in IgG titers, while 14 of 42 (5 in group 1, 5 in group 2, 2 in group 3, and 2 in group 4) demonstrated a similar increase in IgA. Figure 1a and b show the mean intranasal IgG and IgA titers by group. Group 2 had the largest increase at day 42 in both isotypes (IgA, 12.2; IgG, 10.9). Groups 1 and 2 had a statistically significant increase over baseline for IgA on day 42 (P < 0.027), while only group 1 had such an increase on day 98 (P = 0.034). Nasal IgG revealed statistical significance only on day 42 for groups 1 and 2. Group 3 had an increase in IgA from 4.6 to 7.0, although this did not reach statistical significance. Neither group 3 nor 4 demonstrated a significant increase in either isotype on days 42 and 98.
FIG. 1.
(a) Intranasal NOMV-specific IgG antibody titer normalized to the total IgG measured in the nasal secretion specimen. The asterisk indicates statistical significance; P < 0.05. (b) Intranasal NOMV-specific IgA antibody titer normalized to the total IgA measured in the nasal secretion specimen. The asterisk indicates statistical significance; P < 0.05. Interquartile ranges and maximum and minimum excluding outliers are shown.
The total serum antibody levels measured by ELISA did not show a significant increase over preimmunization levels. A few individuals demonstrated a transient increase in serum titer, but this was not sustained over the course of the trial.
The antibody-secreting cells counted for each group and isotype are shown in Table 4. IgA-secreting lymphocytes increased significantly on day 7 for groups 1, 3, and 4 (P < 0.037). On day 35 the increase in IgA cells was statistically significant for groups 1 and 3 (P = 0.024). On day 63 none of the groups showed statistical significance for IgA. The IgG assay revealed a significant increase on day 7 for groups 1 and 4 (P < 0.025), on day 35 for groups 1, 3, and 4 (P < 0.037), and on day 63 for group 1 (P = 0.036). The IgM antibody-secreting cells were statistically significant only for group 1 on day 7 (P = 0.027).
TABLE 4.
Serum ELISPOT
| Group | Day of test | Median (range) of spots/106 PBMCa for:
|
||
|---|---|---|---|---|
| IgA | IgG | IgM | ||
| 1 | 0 | 0 (0-1) | 0 (0-3) | 0 (0-1) |
| 7 | 6 (0-163) | 10.5 (0-127) | 2 (0-47) | |
| 35 | 10 (0-93) | 15.5 (0-61) | 0.5 (0-29) | |
| 63 | 2 (0-48) | 13.5 (0-28) | 0 (0-6) | |
| 2 | 0 | 0 (0-35) | 0 (0-6) | 0 (0-1) |
| 7 | 2.5 (0-101) | 1 (0-146) | 0 (0-0) | |
| 35 | 1.5 (0-30) | 1.5 (0-40) | 0.5 (0-2) | |
| 63 | 0.5 (0-10) | 2.0 (0-7) | 0 (0-4) | |
| 3 | 0 | 0 (0-1) | 0 (0-1) | 0 (0-0) |
| 7 | 8.5 (0-46) | 10.5 (0-72) | 0 (0-0) | |
| 35 | 9 (0-15) | 4 (0-12) | 0 (0-4) | |
| 63 | 1 (0-4) | 1 (0-6) | 0 (0-0) | |
| 4 | 0 | 0 (0-6) | 0 (0-2) | 0 (0-0) |
| 7 | 4 (0-39) | 3 (0-25) | 0 (0-6) | |
| 35 | 3 (0-13) | 3 (0-54) | 0 (0-9) | |
| 63 | 1 (0-26) | 1.5 (0-74) | 0.5 (0-8) | |
PBMC, peripheral blood mononuclear cells. Shown are the median and range of antibody-secreting cells for IgA, IgG, and IgM, measured using an ELISPOT for the four groups. Values represent the number of spots counted per 106 peripheral blood mononuclear cells.
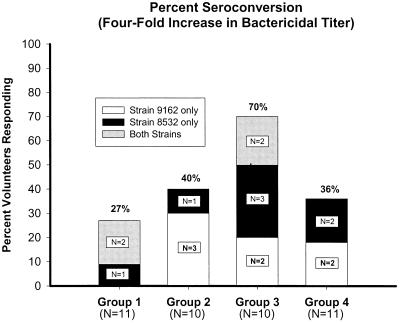
The peak serum bactericidal activity per volunteer against strains 9162 and 8532 is shown in Fig. 2. The seroconversion (>4-fold increase) rate ranged from 18 to 50% against strain 8532 and 18 to 40% against strain 9162. However, the conversion rate with either strain ranged from 27 to 70%. The highest number of seroconversions was seen in group 3, with 7 of 10 reacting to one strain or the other. Fifteen of 42 volunteers had a twofold increase in bactericidal titers against strain 9162, while 6 of 42 demonstrated an increase in bactericidal killing but did not reach a 50% kill rate. Similar analysis against strain 8532 yielded 15 of 42 volunteers with a twofold increase and 8 of 42 with a detectable increase in killing but less than the 50% killing threshold. The increase for group 2 and group 3 bactericidal antibody over time against strain 9162 was statistically significant (P = 0.006 and P = 0.005 for each group, respectively). However, against strain 8532, only the increase over time by group 3 was significant (P = 0.03). The maximum increase in titer against strain 8532, when comparing groups, was significantly higher in group 3 than in group 2 (P = 0.029). The comparisons of maximal change with the other groups did not reach statistical significance.
FIG. 2.
Percentage of volunteers per group who mounted a ≥4-fold increase in bactericidal antibody titer from baseline when sera were assayed against bacterial strains 9162 and 8532. Group 3 showed statistical significance (P = 0.029) when the maximal increase in bactericidal titer over the baseline was compared between groups.
DISCUSSION
The mechanisms producing infection secondary to Neisseria meningitis involve upper respiratory tract invasion, transgression into the bloodstream, and potential seeding of the meninges. Although abnormalities in serum bactericidal activity have definitely been associated with an increased risk of disease, the correlation of protection with mucosal secretory antibody is less clear. Some have postulated that secretory antibody may provide protection by preventing epithelial attachment of the organism. Therefore, due to poor efficacy of the parenteral group B vaccines, development shifted to mimicking natural exposure and immunizing intranasally in hopes of inducing not only serum bactericidal activity but also local secretory antibody. A previous trial performed by our group using an NOMV vaccine used 160 μg (total dose of 480 μg) and 416 μg (total dose of 1,248 μg) of vaccine per dose. The data showed a slightly better bactericidal response with the higher dose. In our study we chose to fix the total dose at approximately 1,200 μg and study two dosing schedules, two routes of administration (oropharyngeal and intranasal), and two additional assays (ELISPOT and nasal cytology) to assess immunogenicity. Additionally, we wanted to corroborate the findings of the original trial with additional volunteers, since only 32 were studied by Drabick et al. (5).
In this phase 1 trial we demonstrated the safety of mucosal immunization with a NOMV formulation containing up to 25% LPS relative to protein. The vaccine was well tolerated with only minimal complaints (Table 2), which were not reproducible from dose to dose. There were no 4+ symptoms. Our data did not show any significant cellular infiltration on nasal cytology. This suggests that the vaccine did not induce a detectable nasal inflammatory response. These findings, along with only two self-limiting, low-grade fevers, suggest that an LPS-containing vaccine can be safely administered mucosally without inducing pyrogenic side effects.
The increase in secretory IgA and IgG levels reached statistical significance for groups 1 and 2. Group 3 did have a slight increase in titer, although not statistically significant, and group 4 was essentially unchanged. The magnitude of the response in the first group was similar to that seen in our previous study. The higher levels noted in group 2 could be due to the fact that this group received the vaccine both intranasally and oropharyngeally. The increased mucosal exposure may have resulted in a higher level of response. Individuals in group 4 had higher baseline titers, which may account for their lack of response. The absence of a booster response was reported by Haneberg et al. (7). They postulated that the immune response to mucosally delivered antigen could be negatively affected by preexisting antibodies.
The poor total serum antibody levels found in this trial are consistent with our previous findings. The mucosal vaccine does seem to increase total IgG levels in certain individuals, but the increases are not sustained. These findings could be due to the lack of an adjuvant. Even though the total antibody levels did not change, some level of bactericidal killing was evident in most volunteers. Therefore, the low level of antibody that was formed systemically was of good functional quality.
The ELISPOT data corroborate the findings of the bactericidal assay in that a significant systemic effect was noted by the marked increase in vaccine-specific antibody secreting lymphocytes after nasal immunization. Many of the volunteers who had an increase in the ELISPOT also are the ones who demonstrated positive findings on the bactericidal testing. The study by Haneberg et al. showed discordant specificity of the IgA antibody between serum and secretions, suggesting the independent functioning of the mucosal and systemic immune systems (7). However, based on the ELISPOT, serum bactericidal, and secretory antibody data, it is evident that mucosally presented antigen results in lymphocyte trafficking between the mucosal and systemic immune systems, thus inducing both local and serum immunogenicity.
The functional bactericidal response against two similar strains of N. meningitidis was not as high as in our previous study (60 to 70% seroconverted with a fourfold increase in titer) (5). One explanation could be that vaccine lot no. 0123 had some degradation over time and that the new lot tested was not identical to the old lot. The recent data by Fischer et al. showed a seroconversion rate of 26% (against strain B:15:P1.7,16), similar to what we have reported (18 to 40% against homologous vaccine strain) in this study (M. Fischer, J. Holst, I. S. Aaberge, E. A. Hoiby, I. L. Haugen, J. L. Burns, B. A. Perkins, and B. Haneberg, Abstr. 12th Int. Pathog. Neisseria Conf., abstr. 113, 2000). Even though the seroconversion rate was as low as 18%, there were additional volunteers who had an increase in bactericidal titer, albeit less than fourfold. This suggests that the vaccine was immunogenic in a large proportion of individuals and that, with vaccine modifications such as adding an adjuvant, these individuals could be seroconverted. The highest-responding group was 3. These individuals received an extra dose of vaccine on day 7, thus suggesting that a closer dosing interval may confer better immunogenicity when a vaccine is mucosally delivered. This finding was corroborated by the two Norwegian studies performed with intranasal vaccines, where the investigators reported higher seroconversion rates in the first trial with weekly immunization than in the second study, where vaccine was delivered at a 6-week interval (Fischer et al., Abstr. 12th Int. Pathog. Neisseria Conf., 2000).
Although we have noted many similarities and some differences in results between our trial and the two studies with the Norwegian vaccine, it is important to appreciate that the two vaccines differ in formulation. The OMV vaccine used in Norway was extracted in deoxycholate, which leads to removal of the majority of LPS and phospholipids (7). Thus, those researchers have been able to administer the vaccine both intramuscularly and intranasally without noting any pyrogenic side effects. The NOMV vaccine that we studied has been uniquely developed by our group and was not exposed to detergent, thereby preserving the phospholipids and LPS in their native conformation. Additionally, the Norwegian vaccine contained Opc protein, which the investigators suggest is important in generating a bactericidal response. Our formulation does not have expression of this protein. The OMV and NOMV vaccines were produced from different seed strains, thus adding further to the differences, which explains some discordance in results. Finally, the NOMV was manufactured from cells grown under iron-limiting conditions in order to induce iron-regulated proteins, which may contribute to the immune response. This is in contrast to the OMV (Norwegian) vaccine, which did not express iron uptake proteins and adds an additional variable when the two vaccines are compared. Direct comparisons between the studies need to be interpreted in light of the differences discussed above.
In conclusion, we have demonstrated the safety of intranasal meningococcal vaccination, particularly showing the absence of a nasal inflammatory response. We have corroborated the findings of our previous trial which studied only 32 volunteers and have shown that NOMV vaccine presented mucosally possesses the structures to induce both serum bactericidal and local secretory antibody. This trial suggests that a narrower dosing interval and increased surface exposure (intranasal and oropharyngeal) may be important when administering vaccine mucosally. The use of the ELISPOT and bactericidal assays can serve as sensitive markers of systemic immunogenicity in light of a low total serum antibody response. The rate of seroconversion is inadequate but may be increased by modifying the vaccine structure, adding an adjuvant, and altering the dosing schedule as discussed.
Acknowledgments
We thank Moshe Shumlarsky and the rest of the clinical trials section of Walter Reed Army Institute of Research for their professional assistance with all aspects of the conduction of this study. We also thank Robin Howard and Joyce Hershey for their help with the statistical analysis and manuscript preparation.
Editor: R. N. Moore
REFERENCES
- 1.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, H. H. Fredriksen, A. Halstensen, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 2.Boslego, J., J. Garcia, C. Cruz, W. D. Zollinger, B. Brandt, S. Ruiz, et al. 1995. Efficacy, safety and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49(RR-7):1-2. [Google Scholar]
- 4.De Moraes, J. C., B. A. Perkins, M. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, et al. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 5.Drabick, J. J., B. L. Brandt, E. E. Moran, N. B. Saunders, D. R. Shoemaker, and W. D. Zollinger. 2000. Safety and immunogenicity testing of an intranasal group B meningococcal native outer membrane vesicle vaccine in healthy volunteers. Vaccine 18:160-172. [DOI] [PubMed] [Google Scholar]
- 6.Fusco, P. C., F. Michon, J. Y. Tai, and M. S. Blake. 1997. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and non-human primates. J. Infect. Dis. 175:364-372. [DOI] [PubMed] [Google Scholar]
- 7.Haneberg, B., R. Dalseg, E. Wedege, E. A. Høiby, I. L. Haugen, F. Oftung, S. R. Andersen, L. M. Næss, A. Aase, T. E. Michaelsen, and J. Holst. 1998. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect. Immun. 66:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansel, F. K. 1966. Cytologic diagnosis in respiratory allergy and infection. Ann. Allergy 24:564-569. [PubMed] [Google Scholar]
- 9.Jones, D. M., and J. Eldridge. 1979. Development of antibodies to meningococcal proteins and lipopolysaccharide antigens in healthy carriers. J. Med. Microbiol. 12:107-111. [DOI] [PubMed] [Google Scholar]
- 10.Mandrell, R. E., and W. D. Zollinger. 1982. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J. Immunol. 129:2172-2178. [PubMed] [Google Scholar]
- 11.Moran, E. E., B. L. Brandt, and W. D. Zollinger. 1994. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect. Immun. 62:5290-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 13.Saunders, N. B., D. R. Shoemaker, B. L. Brandt, E. E. Moran, T. Larsen, and W. D. Zollinger. 1999. Immunogenicity of intranasally administered meningococcal native outer membrane vesicle in mice. Infect. Immun. 67:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders, N. B., D. R. Shoemaker, B. L. Brandt, and W. D. Zollinger. 1997. Confirmation of suspicious cases of meningococcal meningitis by PCR and enzyme-linked immunosorbent assay. J. Clin. Microbiol. 35:3215-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra, G. V. G., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-210. [PubMed] [Google Scholar]
- 16.Zollinger, W. D., D. L. Kasper, B. J. Veltri, and M. S. Artenstein. 1972. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect. Immun. 6:835-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of the complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zollinger, W. D., R. E. Mandrell, and J. M. Griffiss. 1980. Enhancement of immunological activity by noncovalent complexing of meningococcal group B polysaccharide and outer membrane proteins, p. 254-262. In J. B. Robbins, J. C. Hill, and J. Sadoff (ed.), Seminars in infectious disease, vol. 4. Bacterial vaccines. Thieme-Stratton, Inc., New York, N.Y. [Google Scholar]
- 19.Zollinger, W. D., R. E. Mandrell, J. M. Griffiss, P. Altieri, and S. Berman. 1979. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J. Clin. Investig. 63:836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]