Abstract
Vaccination of mice with the 42-kDa region of Plasmodium yoelii merozoite surface protein 1 (MSP142) or its 19-kDa C-terminal processing product (MSP119) can elicit protective antibody responses in mice. To investigate if the 33-kDa N-terminal fragment (MSP133) of MSP142 also induces protection, the gene segment encoding MSP133 was expressed as a glutathione S-transferase (GST) fusion protein. C57BL/6 and BALB/c mice were immunized with GST-MSP133 and subsequently challenged with the lethal P. yoelii YM blood stage parasite. GST-MSP133 failed to induce protection, and all mice developed patent parasitemia at a level similar to that in naive or control (GST-immunized) mice; mice immunized with GST-MSP119 were protected, as has been shown previously. Specific prechallenge immunoglobulin G (IgG) antibody responses to MSP1 were analyzed by enzyme-linked immunosorbent assay and immunofluorescence. Despite being unprotected, several mice immunized with MSP133 had antibody titers (of all IgG subclasses) that were comparable to or higher than those in mice that were protected following immunization with MSP119. The finding that P. yoelii MSP133 elicits strong but nonprotective antibody responses may have implications for the design of vaccines for humans based on Plasmodium falciparum or Plasmodium vivax MSP142.
The Plasmodium falciparum merozoite surface protein 1 (MSP1) is one of the major surface proteins of the merozoite, and immunization with full-length, native MSP1 protein can protect monkeys from otherwise lethal infection with parasites (29). MSP1 is processed into a complex of polypeptides on the merozoite surface, including N-terminal and central regions of 82, 30, and 38 kDa, as well as the C-terminal region of 42 kDa (18, 24). At the time of invasion of red blood cells (RBC), MSP142 is further processed by proteolytic cleavage into a 33-kDa fragment (MSP133), which is shed with the rest of the complex, and a C-terminal 19-kDa fragment (MSP119) (3). Only the C-terminal MSP119 remains on the merozoite surface and is carried into parasitized RBC (pRBC) (2). In monkeys, immunization with recombinant MSP142 and MSP119 has been shown to elicit various degrees of protection against P. falciparum challenge (6, 11, 19). The importance of the P. falciparum MSP133 region for induction of protective immunity is not known.
MSP1 from the rodent malaria parasite Plasmodium yoelii appears to be processed in a manner similar to that of P. falciparum MSP1 (17), and recombinant P. yoelii MSP119 can elicit protective immunity in mice (8, 15, 21). The immunity induced by MSP119 in mice is antibody dependent (1, 7, 15, 22) and may, to a large extent, be mediated by antibodies that act directly on the parasite (21, 27). A possible mechanism could be interference with the processing of MSP142, resulting in inhibition of merozoite invasion; this has been shown in vitro with antibodies to P. falciparum MSP119 (4). While immunization with recombinant P. yoelii MSP142, which contains both MSP133 and MSP119, protects mice, immunization with recombinant proteins corresponding to N-terminal or central regions of MSP1 has little if any protective effect (34).
P. yoelii MSP133 has not previously been evaluated for its ability to elicit protection. Antibodies to MSP133 could be potentially protective—for example, if they interfere with processing of MSP142, as has been shown for antibodies to P. falciparum MSP119. Alternatively, antibodies to MSP133 may hinder the binding of protective antibodies induced by MSP119 and thus compromise immunity induced by MSP119 alone (14).
In the present study, mice were immunized with P. yoelii MSP133 and challenged with a virulent clone of P. yoelii (YM) to assess the protective capacity of MSP133. Although MSP133 was found to be highly immunogenic and elicited levels of antibodies to native MSP1 that were comparable to the levels induced in mice immunized with MSP119, no protection of MSP133-immunized mice was observed, suggesting that antibody responses to recombinant P. yoelii MSP133 are not protective.
MATERIALS AND METHODS
Cloning of P. yoelii MSP133 and expression of recombinant GST fusion proteins.
In order to express P. yoelii MSP133 as a glutathione S-transferase (GST) fusion protein, genomic DNA from P. yoelii YM was purified using Instagene according to the manufacturer’s instructions (Bio-Rad, Hemel Hempstead, United Kingdom). DNA encoding residues 1394 to 1655 of P. yoelii MSP1 (PyMSP1) (20) was PCR amplified using the sense primer 5′-GCG GAT CCA ATC CGA AGA TGC ACC AGA A-3′ and the antisense primer 5′-GCG AAT TCT AGC TGG AAG AAC TAC AGA A-3′ (VH BIO, Newcastle, United Kingdom). The PCR product was purified from a 1% agarose gel using Prep-a-gene (Bio-Rad), restricted with BamHI and EcoRI (Promega, Madison, Wis.), and then purified using a High Pure PCR kit (Boehringer GmbH, Mannheim, Germany).
The PCR product was ligated into linearized pGEX-3X (Pharmacia, Uppsala, Sweden), digested with the same restriction enzymes, using T4 DNA ligase (Promega). After transformation of Escherichia coli strain DH5α with ligated plasmid, positive clones were screened for protein expression after induction with isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma, Poole, United Kingdom). The plasmid from a clone expressing a protein of the expected size was purified using Wizard miniprep columns (Promega), and the complete insert was confirmed by DNA sequencing.
For production of recombinant GST fusion proteins, E. coli DH5α was transformed with plasmids encoding either GST-MSP133, GST-MSP119 (residues 1649 to 1754 [21]), or GST alone. The proteins were produced as described previously (30) with some modifications (1). Briefly, overnight cultures of transformed bacteria were induced with 1 mM IPTG. The cells were harvested and lysed in phosphate-buffered saline (PBS). The lysate was centrifuged and the supernatant was recovered. Recombinant proteins were purified by affinity chromatography on glutathione-agarose (Sigma) using 10 mM reduced glutathione (Sigma) for elution. Free glutathione was removed by dialysis against PBS.
Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) indicated that GST-MSP119, GST-MSP133, and GST were predominantly full length and had relative molecular masses of 42, 65, and 26 kDa, respectively. The concentrations of purified proteins were determined by multiplying the absorbance at 280 nm with factors calculated for each protein based on size and content of aromatic amino acids (13).
For production of GST-free MSP119 and MSP133, glutathione-agarose was incubated with supernatants from bacterial culture (21). After extensive washing, the agarose beads were treated with factor Xa (Sigma) for 12 h at 22°C. Factor Xa was removed from the solution containing the GST-free proteins by incubation with p-aminobenzamidine bound to agarose (Sigma). Simultaneous incubation with glutathione-agarose ensured that any contaminating GST was removed. The proteins were dialyzed against PBS and analyzed by SDS-PAGE, and the concentrations were determined as above. Only one band was observed by SDS-PAGE for purified GST-free MSP119 as well as MSP133 and corresponded to relative molecular masses of 20 and 34 kDa, respectively.
P. yoelii parasites.
P. yoelii parasites of the lethal strain YM (38), kindly provided by David Walliker, Edinburgh University, were kept frozen at −70°C or by regular passage in mice. Infected mice were given drinking water supplemented with 2.5 g of para-benzoaminoic acid/liter (25).
Immunization and challenge of mice.
Female C57BL/6 and BALB/c mice (Harlan, Oxford, United Kingdom) were used at 7 to 10 weeks of age. Groups of five mice were injected intraperitoneally with 40 μg of GST-MSP119, GST-MSP133, or GST in complete Freund’s adjuvant (Sigma). Booster injections were given 3 and 6 weeks later using the same amount of antigen but in incomplete Freund’s adjuvant (Sigma). Prechallenge sera were drawn 2 weeks after the last booster injection. Three weeks after the last immunization, mice were inoculated intravenously with 104 pRBC. Giemsa-stained blood smears were made daily from day 3 of infection onwards. Parasitemia was assessed by counting the proportion of pRBC by light microscopy.
ELISA.
GST-free MSP119 or MSP133 was adsorbed to Immunolon 4 plates (Dynatech Laboratories Inc., Billingshurst, United Kingdom) at a concentration of 2 μg/ml in PBS overnight at 8°C. The wells were blocked with 1% bovine serum albumin in PBS for 1 h at room temperature, and then diluted sera were added and incubated for 1 h at 37°C. Bound antibodies were detected by subsequent incubation with goat anti-mouse immunoglobulin G1 (IgG1), IgG2b, or IgG3 (Southern Biotechnology, Birmingham, Ala.) or sheep anti-mouse IgG2a (The Binding Site, Birmingham, United Kingdom) conjugated to horseradish peroxidase. As the substrate, o-phenylenediamine (Sigma) was used. Endpoint serum titers were estimated by analyzing sera at consecutive twofold dilutions, starting at 1:64, using an absorbance value of 0.3 as the cutoff for positive sera. The mean + 2 standard deviations absorbance for normal mouse serum, diluted 1:64, was lower than the cutoff for all antigens analyzed. A high-titer serum pool was included in all assays as an internal standard.
IFAT.
For the indirect immunofluorescent antibody test (IFAT), blood was drawn from P. yoelii YM-infected mice into heparinized tubes. The blood was washed and diluted 1:200 in phosphate-saline buffer containing 0.2% glucose. Then 10 μl of cell suspension was added to wells of 15-well multitest slides (ICN Biomedicals Inc., Aurora, Ohio) that were air dried before storage at −20°C. Prior to staining, the slides were fixed with acetone for 2 min and blocked in PBS with 1% bovine serum albumin for 30 min at room temperature. The wells were incubated for 1 h at 37°C with serum in consecutive twofold dilutions in PBS. Specific antibodies were detected by incubation for 1 h at 37°C with fluorescein isothiocyanate-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotechnology). Between incubations, the slides were washed in PBS. Titers were determined as the reciprocal of the last positive dilution when analyzed by UV microscopy.
RESULTS
Challenge of immunized mice with P. yoelii parasites.
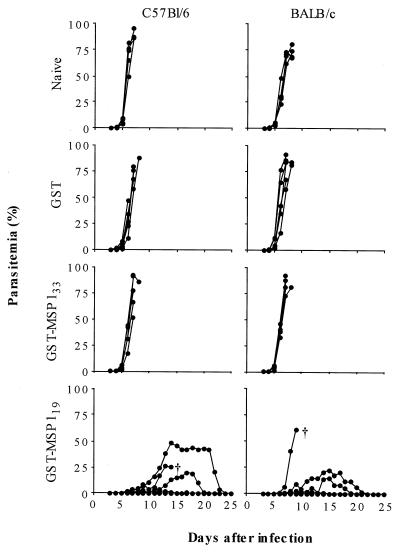
BALB/c and C57BL/6 mice were immunized three times with GST-MSP119, GST-MSP133, or GST. Following immunization, the mice were challenged with 104 parasites of the lethal strain P. yoelii YM; naive mice were included for comparison (Fig. 1). In naive mice and GST-immunized control mice, the course of infection was rapid. All mice died between days 7 and 9 with parasitemias above 60%. In BALB/c and C57BL/6 mice immunized with GST-MSP119, protection was observed in four of five mice in each group, with one mouse (BALB/c) being fully protected (i.e., not having any detectable parasitemia). One BALB/c mouse died with fulminant infection at day 10 and thus showed only a minor delay in the course of infection. One C57BL/6 mouse died despite a delayed course of infection (day 15) and a maximal parasitemia of 26%. In mice immunized with GST-MSP133, no protection was observed; all mice died at day 8 or 9, and there was no difference between the two mouse strains.
FIG. 1.
Challenge infection with P. yoelii YM of mice immunized with MSP133 or MSP119. Three weeks after the third immunization with GST-MSP133 or -MSP119, groups of four or five C57BL/6 and BALB/c mice were inoculated intravenously with 104 P. yoelii YM. Parasitemia was monitored daily from day 3. Control groups consisted of mice immunized with GST alone and naive mice. Only mice in the GST-MSP119 groups were able to survive the challenge; mice that did not survive are indicated by †.
Antibody responses to GST-MSP1 fusion proteins as measured by ELISA.
To evaluate the predictive value of prechallenge IgG antibody levels on protection, sera from all mice immunized with GST-MSP119 and GST-MSP133 were analyzed for IgG subclass reactivity by ELISA and by IFAT. Direct comparison of ELISA IgG titers to MSP119 and MSP133 is not possible because different coating antigens were used in the ELISAs. Nevertheless, the results suggest that BALB/c mice responded with roughly comparable antibody titers to MSP119 and MSP133, although there was some difference in the subclass of the antibodies, with mean titers in the GST-MSP119 group being higher for IgG1 and lower for IgG2a and IgG2b compared with the GST-MSP133 group (Table 1). A similar result was observed for the C57BL/6 mice, although the differences between the groups were more pronounced, with mean IgG1 titers being twice as high for MSP119 as for MSP133 and mean IgG2a titers being threefold higher for MSP133 than for MSP119 (Table 1).
TABLE 1.
IgG subclass responses to GST-MSP119 and GST-MSP133 in C57BL/6 and BALB/c mice
| Mouse strain | Immunogen | Mean ELISA titera (103) ± SD
|
|||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | ||
| C57BL/6 | GST-MSP119 | 120 ± 29 | 0.18 ± 0.070 | 21 ± 11 | 0.076 ± 0.028 |
| GST-MSP133 | 41 ± 25 | 0.74 ± 0.81 | 56 ± 46 | 0.44 ± 0.36 | |
| BALB/c | GST-MSP119 | 260 ± 160 | 2.8 ± 1.8 | 15 ± 11 | 0.47 ± 0.88 |
| GST-MSP133 | 147 ± 82 | 4.4 ± 2.9 | 25 ± 9.5 | 0.45 ± 0.38 | |
For specific detection of antibodies to MSP1 proteins, sera from mice immunized with GST-MSP119 and GST-MSP133 were tested in ELISA for reactivity with GST-free MSP119 and MSP133, respectively.
Antibody responses to GST-MSP1 fusion proteins as measured by IFAT.
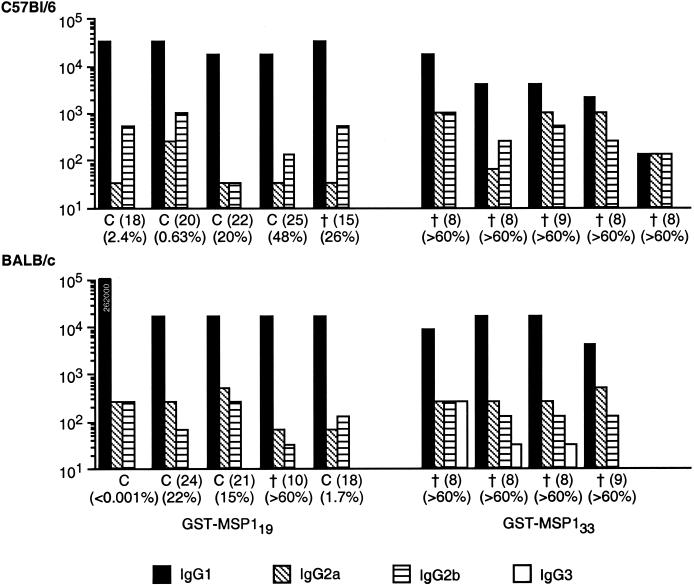
To compare antibody responses to native MSP1 in mice immunized with GST-MSP119 or GST-MSP133, individual sera from mice in each group were tested by IFAT to determine the endpoint antibody titer and antibody subclass by using acetone-fixed blood from P. yoelii-infected naive mice. Although absolute titers determined by IFAT were lower than those measured by ELISA, IFAT titers correlated well with the ELISA data; the individual IFAT titers for each group of mice are shown in Fig. 2.
FIG. 2.
IgG subclass of antibodies to native P. yoelii MSP1 in mice immunized with GST-MSP133 or -MSP119, as measured by IFAT. Sera from C57BL/6 and BALB/c mice immunized three times with either GST-MSP133 or -MSP119 were analyzed by IFAT for reactivity with mouse RBC infected with P. yoelii YM. IFAT reactivity for all four IgG subclasses was measured and is shown as titers, with different bar patterns representing different subclasses. One of the GST-MSP119-immunized BALB/c mice had an IgG1 titer in excess of 100,000 (262,000). IgG3 was only detected in BALB/c mice immunized with GST-MSP133. C indicates mice that cleared their infection; the number in parentheses indicates the day on which the infection was cleared. † indicates mice that died or were humanely killed, with the day of death in parentheses. Percentages indicate the highest parasitemia detected during the course of infection.
Despite the small number of mice included in the study, there was a clear trend towards an association between antibody responses and protection in the GST-MSP119-vaccinated mice, as described previously (1). The fully protected BALB/c mouse had an extremely high IgG1 response in comparison with the other mice, whereas the BALB/c mouse that did not survive had the lowest antibody titer. Also, the C57BL/6 mice with the lowest maximal parasitemias had the highest overall antibody titers in that group. However, the C57BL/6 mouse that died on day 15 with a parasitemia of 26% did not stand out as a low responder in that group.
The mice immunized with GST-MSP133 had, as also observed by ELISA, lower mean titers of IgG1 but higher titers of IgG2a and IgG2b than mice immunized with GST-MSP119. In BALB/c mice, the IgG3 response to GST-MSP133 was also higher than that to GST-MSP119.
Despite the fact that IgG1 responses to GST-MSP133 were generally lower than those to GST-MSP119, some of the nonprotected GST-MSP133-immunized mice had antibody levels that were comparable to or higher than those of several mice that were protected by immunization with GST-MSP119. Two BALB/c mice immunized with GST-MSP133 had IgG1 levels that were comparable to those of three of the protected mice in the GST-MSP119-immunized group. These GST-MSP133-immunized mice also had comparable or higher titers of the other IgG subclasses. Of the C57BL/6 mice immunized with GST-MSP133, one mouse had comparable IgG1 and higher IgG2a and IgG2b titers than two of the mice protected by immunization with GST-MSP119.
It was clear from the IFAT analysis that the specificity of antibodies raised by GST-MSP133 differed from the specificity of antibodies to GST-MSP119. Antisera to GST-MSP119 stained both pigmented schizonts and ring stages, whereas antisera to GST-MSP133 only stained pigmented schizonts.
DISCUSSION
Immunization of BALB/c mice with P. yoelii MSP142, which comprises both MSP133 and MSP119 polypeptides, elicits protective immune responses (34), but similar levels of protection can be achieved in both BALB/c and C57BL/6 mice by immunization with the MSP119 polypeptide alone (8, 15, 21, 34). The extent to which the MSP133 region contributes to the protective immune response in MSP142-immunized mice is thus rather unclear. The data presented here show that epitopes contained entirely within the MSP133 polypeptide do not mediate protection. This finding is in agreement with the finding that a monoclonal antibody to P. yoelii MSP133 is unable to passively protect mice; this monoclonal antibody binds to an epitope located some distance from the cleavage site within MSP142 (31).
However, another monoclonal antibody, D3, which does passively protect mice against infection, recognizes an epitope in MSP142 that is not present in either MSP119 or MSP133 (31) and may therefore recognize an epitope formed by the physical interaction of the two polypeptides. As the protective capacity of antibodies against P. falciparum MSP119 (PfMSP119) is, at least in part, related to the ability of the antibodies to inhibit proteolytic processing of PfMSP142 (4, 14), antibodies binding to an epitope formed by sequences from both MSP133 and MSP119 and lying close to the cleavage site could inhibit processing and thus contribute to protection. A monoclonal antibody that inhibits P. falciparum invasion may also bind to epitopes formed from both PfMSP133 and MSP119 (37).
In order to examine the protective effect of MSP133, it was expressed as a GST fusion protein (GST-MSP133) and used, in parallel with GST-MSP119, to immunize BALB/c and C57BL/6 mice. The results confirmed the protective effects of vaccination with GST-MSP119; in contrast, no protection was observed in mice immunized with MSP133. Protective immunity in mice immunized with recombinant P. yoelii MSP119 is largely dependent on antibodies; passive transfer of antiserum to MSP119 can confer partial protection on naive recipients, whereas immunoglobulin μ-chain-deficient mice cannot be immunized with MSP119 (7, 15, 22, 23). We therefore investigated whether the lack of protection following immunization with MSP133 was due to an insufficient antibody response.
Although the antibody response to MSP133 was, on average, lower than the response to MSP119, several mice immunized with MSP133 developed an anti-P. yoelii MSP1 antibody response comparable to or higher than the response in mice that were well protected following immunization with GST-MSP119. We therefore conclude that the lack of a sufficient antibody titer is, in itself, not responsible for the failure of GST-MSP133 to induce protection, and we assume that antibodies elicited by recombinant MSP133 are of a specificity that fails to inhibit the growth of parasites.
It has been shown that folding of the MSP119 recombinant protein into the correct three-dimensional conformation is important for its ability to elicit protective antibodies (21). The importance of correct folding of MSP119 is probably related to the high number of cysteine residues, which form multiple disulfide bonds; this is less likely to present problems for expression of MSP133 because it does not contain any disulfide bonds (20). Indeed, antibodies induced by GST-MSP133 reacted strongly with native, parasite-derived antigen in IFAT. It is thus likely that the antibodies to MSP133 are specific for nonprotective epitopes.
A relevant question is whether or not a vaccine based on MSP119 is affected by the inclusion of MSP133. Comparisons of the immunogenicity and protective capacity of P. yoelii MSP119 and MSP142 in BALB/c and A/J mice did not show any difference between the constructs (34). However, it is possible that in some mouse strains which respond less well to MSP119 (33), the addition of MSP133 could provide additional T-cell epitopes to enhance the antibody response to MSP119. T-cell responses are known to contribute to protection induced by MSP119 vaccination and are essential for immune memory (16). The linkage of exogenous T-cell epitopes to MSP119 has been shown to improve protective antibody responses in mouse strains that are poorly protected by immunization with MSP119 alone (1).
The importance of increasing the number of T-helper-cell epitopes in immunogens based on P. falciparum MSP119, intended for use in humans, is not known. Individuals from areas where malaria is endemic who have been immunized by natural infection with P. falciparum are frequently seronegative for antibodies to MSP119 (9, 12, 28, 35), and T-cell proliferative responses to peptides derived from PfMSP119 tend to be infrequent and relatively weak (10, 26, 36). Importantly, however, T-cell proliferative and cytokine responses to recombinant proteins, including the 33-kDa region of PfMSP1, have been associated with protective immunity to malaria in epidemiological studies (26).
Vaccination of nonhuman primates with MSP19 or MSP42 has produced inconsistent and sometimes contradictory results. In one primate immunization study, vaccination with bacterium-derived MSP119 induced only relatively low-titer antibody responses which were not protective (5), whereas in a similar study using a different adjuvant, vaccination with baculovirus-derived PfMSP142 induced strong lymphoproliferative responses and high antibody titers to PfMSP119; vaccine-induced antibodies effectively inhibited parasite growth in vitro, and all immunized animals were protected against challenge infection (6).
In a direct comparison of the efficacy of PfMSP142 and PfMSP119 as immunogens, monkeys immunized with PfMSP119 appeared to be better protected than those immunized with PfMSP142 (19), but the number of animals tested was small and the two antigens were produced in different expression systems (Saccharomyces cerevisiae and E. coli, respectively), which may have contributed to differences in immunogenicity.
In another study, monkeys vaccinated with yeast-derived PfMSP119 were significantly less well protected than monkeys immunized with baculovirus-derived PfMSP142 (32). Inclusion of exogenous T-cell epitopes in the MSP119 construct does not necessarily enhance protection to immediate challenge, although such vaccines seem to induce strong memory responses (11). Further studies are therefore justified to determine the importance of MSP133-derived T helper epitopes in contributing to natural and vaccine-induced immunity to MSP1 in animal models and in human vaccination studies.
Acknowledgments
This work was funded by a Research Training Grant to N. Ahlborg from the Life Sciences and Technologies Directorate of the European Commission (contract number BMH4-CT97-5037) and by the Wellcome Trust.
We thank S. Haley for technical support and D. Cavanagh and S. Franks for technical advice.
Editor: R. N. Moore
REFERENCES
- 1.Ahlborg, N., I. T. Ling, A. A. Holder, and E. M. Riley. 2000. Linkage of universal T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP-119) can enhance protective immunity against malaria and modulates the immunoglobulin subclass response to MSP-119. Infect. Immun. 68:2102–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H.-G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-blocking antibodies. J. Exp. Med. 172:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307–316. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burghaus, P. A., B. T. Wellde, T. Hall, R. L. Richards, A. F. Egan, E. M. Riley, W. R. Ballou, and A. A. Holder. 1996. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect. Immun. 64:3614–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. N. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236–243. [PubMed] [Google Scholar]
- 8.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodoo, D., T. G. Theander, J. A. L. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Antibody levels to conserved parts of Plasmodium falciparum merozoite surface protein 1 (PfMSP1) in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, A., M. Waterfall, M. Pinder, A. Holder, and E. Riley. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, A. F., M. J. Blackman, and D. C. Kaslow. 2000. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed and/or -rechallenged Aotus vociferans monkeys. Infect. Immun. 68:1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319–326. (Erratum, 189:283.) [DOI] [PubMed] [Google Scholar]
- 14.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400–3411. [PubMed] [Google Scholar]
- 16.Hirunpetcharat, C., P. Vukovic, X. Liu, D. Kaslow, L. Miller, and M. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309–7314. [PubMed] [Google Scholar]
- 17.Holder, A. A., and R. R. Freeman. 1984. Characterization of a high molecular weight protective antigen of Plasmodium yoelii. Parasitology 88:211–219. [PubMed] [Google Scholar]
- 18.Holder, A. A., J. S. Sandhu, Y. Hillman, L. S. Davey, S. C. Nicholls, H. Cooper, and M. J. Lockyer. 1987. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology 94:199–208. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, S., A. Yadava, D. Keister, J. Tian, M. Ohl, K. Perdue-Greenfield, L. Miller, and D. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis, A. P. 1989. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol. Biochem. Parasitol. 36:271–282. [DOI] [PubMed] [Google Scholar]
- 21.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63–67. [DOI] [PubMed] [Google Scholar]
- 22.Ling, I. T., S. A. Ogun, P. Momin, R. L. Richards, N. Garcon, J. Cohen, W. R. Ballou, and A. A. Holder. 1997. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine 15:1562–1567. [DOI] [PubMed] [Google Scholar]
- 23.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131–3137. [PubMed] [Google Scholar]
- 24.McBride, J. S., and H.-G. Heidrich. 1987. Fragments of the polymorphic Mr185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71–84. [DOI] [PubMed] [Google Scholar]
- 25.Peters, W. 1967. Chemotherapy of Plasmodium chabaudi infection in albino mice. Ann. Trop. Med. Parasitol. 61:52–56. [DOI] [PubMed] [Google Scholar]
- 26.Riley, E. M., S. Morris-Jones, M. J. Blackman, B. M. Greenwood, and A. A. Holder. 1993. A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol. 15:513–524. [DOI] [PubMed] [Google Scholar]
- 27.Rotman, H. L., T. M. Daly, R. Clynes, and C. A. Long. 1998. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J. Immunol. 161:1908–1912. [PubMed] [Google Scholar]
- 28.Shi, Y., U. Sayed, S. Qari, J. Roberts, V. Udhayakumar, A. Oloo, W. Hawley, D. Kaslow, B. Nahlen, and A. Lal. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect. Immun. 64:2716–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. N. Hui, S. E. Case, K. M. Yamaga, S. P. Chang, E. B. T. Chan, and S.-C. Kan. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc. Nat. Acad. Sci. USA 84:3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, D. B., and K. S. Johnson. 1988. Single step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–40. [DOI] [PubMed] [Google Scholar]
- 31.Spencer Valero, L. M., S. A. Ogun, S. L. Fleck, I. T. Ling, T. J. Scott-Finnigan, M. J. Blackman, and A. A. Holder. 1998. Passive immunization with antibodies against three distinct epitopes on Plasmodium yoelii merozoite surface protein 1 suppresses parasitemia. Infect. Immun. 66:3925–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stowers, A., V. Cioce, R. Shimp, M. Lawson, G. Hui, O. Muratova, D. Kaslow, R. Robinson, C. Long, and L. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian, J., L. Miller, D. Kaslow, J. Ahlers, M. Good, D. Alling, J. Berzofsky, and S. Kumar. 1996. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J. Immunol. 157:1176–1183. [PubMed] [Google Scholar]
- 34.Tian, J.-H., S. Kumar, D. C. Kaslow, and L. H. Miller. 1997. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect. Immun. 65:3032–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolle, R., K. Fruh, O. Doumbo, O. Koita, M. N′diaye, A. Fischer, K. Dietz, and H. A. Bujard. 1993. Prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malaria infections. Infect. Immun. 61:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udhayakumar, V., D. Anyona, S. Kariuki, Y. P. Shi, P. B. Bloland, O. H. Branch, W. Weiss, B. L. Nahlen, D. C. Kaslow, and A. A. Lal. 1995. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J. Immunol. 154:6022–6030. [PubMed] [Google Scholar]
- 37.Uthaipibull, C., B. Aufiero, S. E. H. Syed, B. Hansen, J. A. Guevara Patiño, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381–1394. [DOI] [PubMed] [Google Scholar]
- 38.Yoeli, M., B. Hargreaves, R. Carter, and D. Walliker. 1975. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann. Trop. Med. Parasitol. 69:173–178. [DOI] [PubMed] [Google Scholar]